Published online Dec 14, 2009. doi: 10.3748/wjg.15.5821
Revised: September 14, 2009
Accepted: September 21, 2009
Published online: December 14, 2009
AIM: To investigate whether the secretion of phosphatidylcholine (PC) in intestinal mucus occurs by apical secretion or via basolateral excretion and to determine its subsequent passage across the tight junctions to the apical mucus.
METHODS: We addressed this question using the polarized intestinally differentiated tumor cell line CaCo-2 grown on filters to confluence in Transwell culture chambers. The released PC and sphingomyelin (Sph) from apical and basolateral media were analyzed by mass spectrometry.
RESULTS: The secreted PC species were identical in both compartments indicating the same intracellular origin of PC. However, PC secretion into the basolateral compartment was more effective, and the PC:Sph ratio in the basolateral compartment was significantly higher than that in the apical compartment (8.18 ± 1.84 vs 4.31 ± 1.22, P = 0.01). Both pathways were temperature sensitive and were unaltered in the presence of cyclosporine.
CONCLUSION: The data demonstrate the PC secretion capacity of CaCo-2 cells and indicate two separated apical and basolateral release mechanisms.
- Citation: Gotthardt D, Braun A, Tietje A, Weiss KH, Ehehalt R, Stremmel WR. Separate basolateral and apical phosphatidylcholine secretion routes in intestinally differentiated tumor cells. World J Gastroenterol 2009; 15(46): 5821-5826
- URL: https://www.wjgnet.com/1007-9327/full/v15/i46/5821.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.5821
The presence of phosphatidylcholine (PC) in intestinal mucus serves to protect the underlying mucosa from attack by the commensal bacterial flora which is of particular importance in the colon[1]. Recently it was shown that the mucus PC content is markedly reduced (up to 70%) in patients with ulcerative colitis (UC), irrespective of whether acute inflammation of the colon is present - when compared to controls or patients with Crohn’s disease[2,3]. This suggests its significance in the pathogenesis of UC. Accordingly, therapeutic replacement of PC in UC was shown to be very effective[4-6].
Furthermore, it was shown that apical PC secretion occurs mainly in the ileum, and is stimulated by bile acids[7]. It is assumed that luminally secreted PC -after absorption of bile acids in the terminal ileum- is attracted to the mucosal surface and moves along the colonic wall towards the rectum driven by colonic motility[1]. The exposure of PC to phospholipases of commensal colonic bacteria thins the PC layer towards the rectum, the “last lawn” with least PC content[1]. This could explain the continuous spread of inflammation starting from the rectum in UC where an intrinsic low PC content in the mucus is already present. Moreover, the fact that PC is mainly secreted in ileum[7] and that it is significantly impaired in UC[2,3] could explain the occurrence of pouchitis after colectomy due to the lack of protective PC in mucus. In contrast, pouchitis is not observed after colectomy for familial adenomatous polyposis (FAP).
PC within mucus is arranged as lamellar structures facing the luminal and mucosal surface of the mucus as well as liposomal structures within the layer. Of all phospholipids within the mucus, 60%-90% are reported to represent PC species[8,9]. This indicates a specific PC secretion mechanism. The conventional view of such a transport process would suggest apical secretion via vesicular excretion or by ABC-transporters similar to MDR 2/3 mediated translocation of PC at the apical pole of hepatocytes[10,11]. However, Alpers et al[12] claimed a predominant basolateral secretion mechanism with secondary back diffusion across the tight junctions and subsequent release into the mucus space.
To evaluate this secretion mechanism, we used the intestinally differentiated tumor cell line CaCo-2 which revealed a polarized growth in tissue culture. We addressed the following questions: (1) Is PC secretion detectable in CaCo-2 cells? (2) Does it occur apically, basolaterally or on both sides? (3) Are these secretion pathways identical? (4) What are the kinetic characteristics?
Caco-2 cells were cultured routinely at 37°C, 5% CO2 in DMEM (GIBCO Invitrogen, Karlsruhe, Germany), containing 10% fetal calf serum (FCS), 1% penicillin/streptomycin, 1% sodium pyruvate (Sigma-Aldrich, Munich, Germany) and 1% nonessential amino acids (Sigma-Aldrich, Munich, Germany). Cells were seeded in 6-well Corning® Transwell® polyester membrane inserts (pore size 3.0 μm, membrane diameter 24 mm) (Corning COSTAR, USA,) at a density of 105 cells/cm2. Cells were cultured for up to 21 d after reaching confluency to allow complete apical/basolateral polarization. Transepithelial electric resistance was checked regularly to verify appropriate polarization (World Precision Instruments, Berlin, Germany).
For measurement of PC secretion, polarized Caco-2 cells on filters were washed with FCS-free medium three times to remove extracellular lipids. They were then incubated in FCS-free medium containing 1% bovine serum albumin (BSA) (Sigma-Aldrich, Munich, Germany) for 16 h unless otherwise stated. Apical and basolateral media were collected and detached cells removed by centrifugation at 300 g for 5 min. All samples were stored at -80°C until further processing.
For experiments at different temperatures, CO2-independent medium (GIBCO Invitrogen, Karlsruhe, Germany) containing 1% w/v BSA was used. Where indicated, BSA was replaced by taurocholate (Sigma-Aldrich, Munich, Germany) at a concentration of 2.5 mmol/L. In addition, the following drugs were used at the following concentrations: (1) verapamil 500 μmol/L, (2) glibenclamide 50 μmol/L, (3) hydrocortisone 100 ng/mL, 500 ng/mL, and 1000 ng/mL, (4) cyclosporine 80 μmol/L (all Sigma-Aldrich, Munich, Germany). In experiments using hydrocortisone, cells were preincubated in regular medium containing the respective concentrations of hydrocortisone for 24 h to allow for changes in transcription. Experiments were performed in quadruplicate, except for the time-course experiments, which were performed in triplicate.
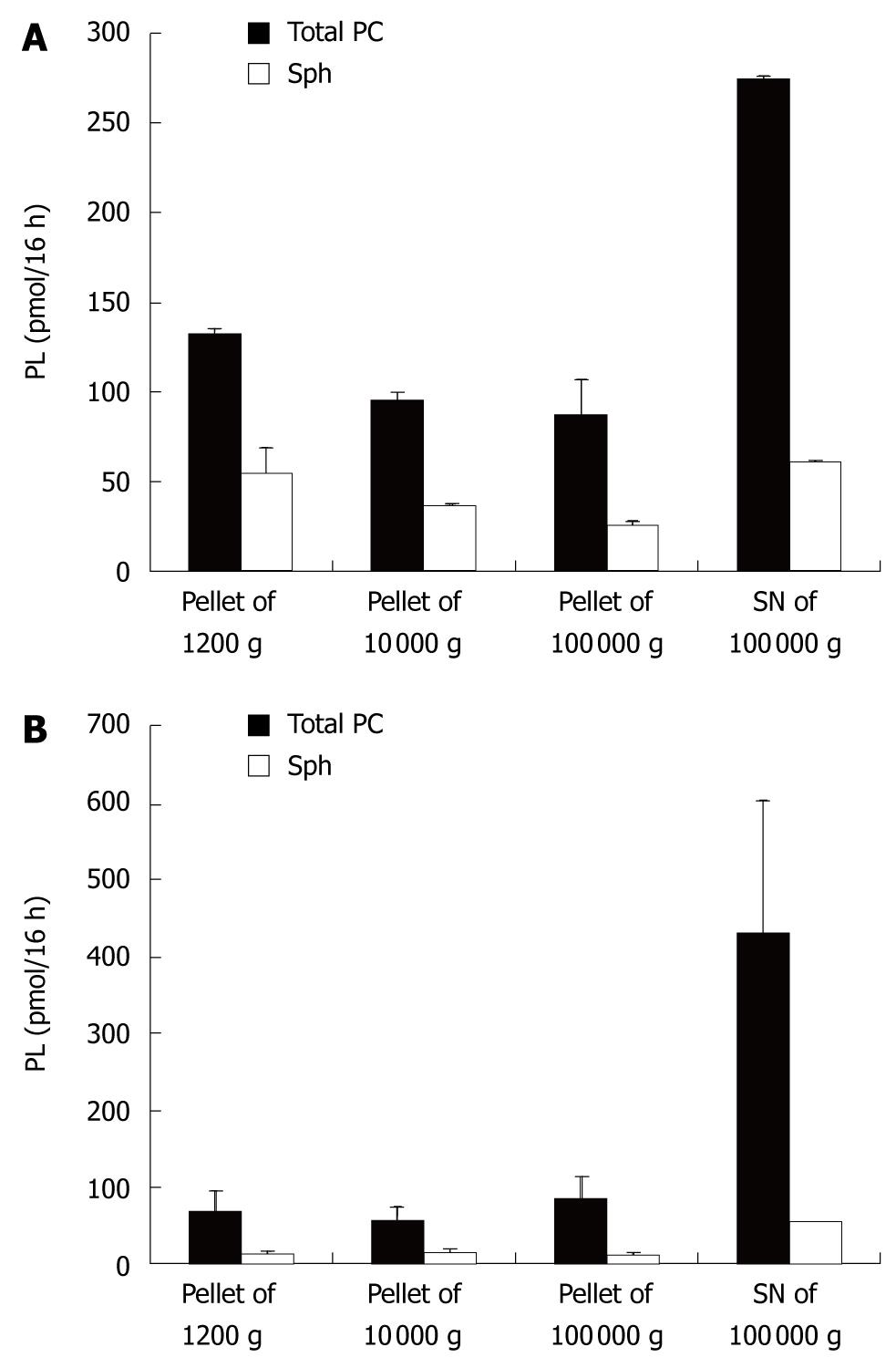
To retrieve particles of different sizes, the following standard setup was used. Cells were incubated as described above. The supernatant (SN) of the 300 g/5 min centrifugation was centrifuged at 1200 g for 20 min (P2). To obtain the next pellet the resulting SN was centrifuged at 10 000 g for 30 min (P3). This SN was then centrifuged at 100 000 g for 1 h (P4). Resulting pellets (P2-4) and the final supernatant were then subjected to lipid analysis.
Lipids from the respective medium samples were extracted according to Folch[13]. Before lipid extraction, non-physiologic 1,2-didodecanoyl-sn-glycero-3-PC, 1,2-tetradecanoyl-sn-glycero-3-PC, 1,2-dieicosanoyl-sn-glycero-3-PC, 1,2-dido-cosanoyl-sn-glycero-3-PC as well as 1-pentadecanoyl-2-hydroxy-sn-glycero-3-PC, 1-arachidonyl-2-hydroxy-sn-glycero-3-PC and sphingomyelin (Sph) were added for internal standardization. The apical and basolateral media were dried with a speed vac. In brief 75 μL aqua dest and 50 μL of the lipid standard were added to each sample. 500 μL of methanol were added and the sample was vortexed for 5 min. 1000 μL chloroform was added and vortexed for 5 min. The supernatant of the 5 min/17 000 g centrifugation was transferred to a new reaction tube. 300 μm aqua dest was added and vortexed again for 5 min. Phase separation was achieved by centrifugation at 500 g for 5 min. The lower organic phases were transferred to a separate glass tube and dried before resuspension for mass spectrometry.
Mass spectrometric analyses were performed with a triple quadrupole instrument [Finnegan MAT (San Jose, CA, USA) model TSQ 7000] equipped with a nano-electrospray source operating at a typical flow rate of 20 to 50 nL/min. The electrospray capillary was positioned at a distance of 0.5 to 1 mm away from the orifice of the heated transfer capillary which was maintained at 140°C. The instrument was used in the tandem MS mode. Argon was used as the collision gas at a nominal pressure of 2 mTorr. Crude lipid extracts, which had been dried completely, were resolved in 50 μL methanol/chloroform 2:1 (v/v), vortexed thoroughly, and infused into the capillary. The mass spectrometric resolution was set to about nominal mass resolution for the scan range of m/z 400 to 900. All specimens were analyzed in precursor ion scan mode for m/z 184. At least 150 consecutive scans, if possible more than 200 scans of 4-s duration, were averaged for each quantitative measurement.
For quantification of the physiological PC and sphingomyelin species, regression curves were determined from the non physiological standards. Due to a general loss in sensitivity with increasing molecular weight, a set of four PC species was added as internal standards to bracket the profile of naturally occurring PCs. For the quantitative determination of Sph, we used a single internal standard. For correction of the measured values to the molar abundances, we used a calibration curve with the slope corresponding to that of PC.
The most abundant physiological PC and Sph species were determined after comparison of all spectra. The molecular weights were related to the number of carbon atoms and double bonds with the help of a lipid data bank.
SPSS 16.0 was used for statistical analysis of the data. A Mann-Whitney-U-test was performed to analyze the differences between different conditions. Differences were considered significant, if P was < 0.05. In the time-course experiments a linear regression assuming a zero-crossing and applying the method of least squares was performed.
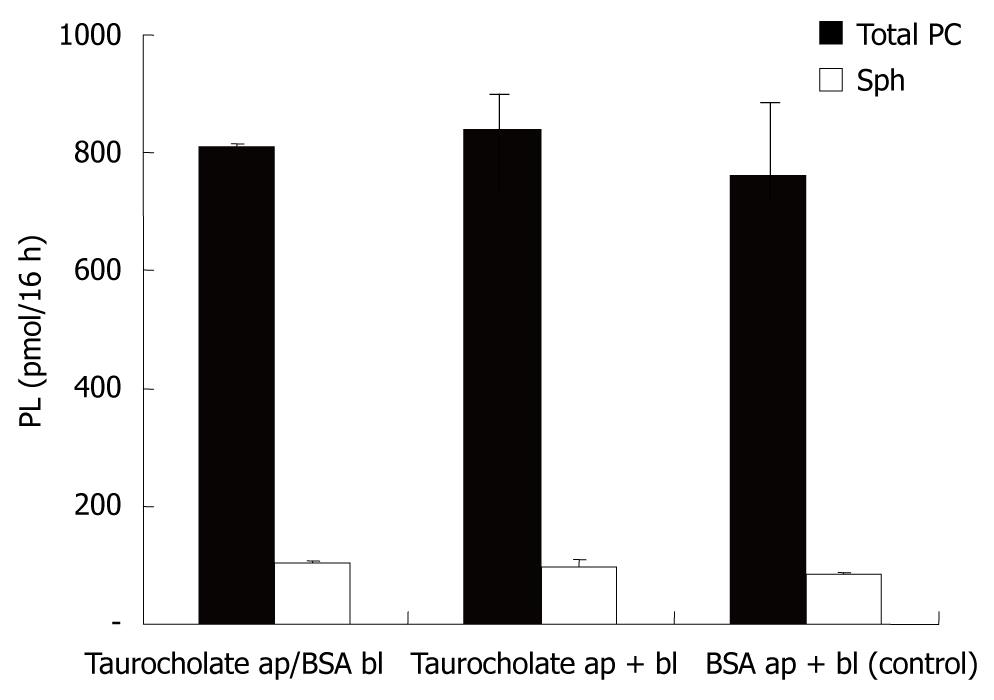
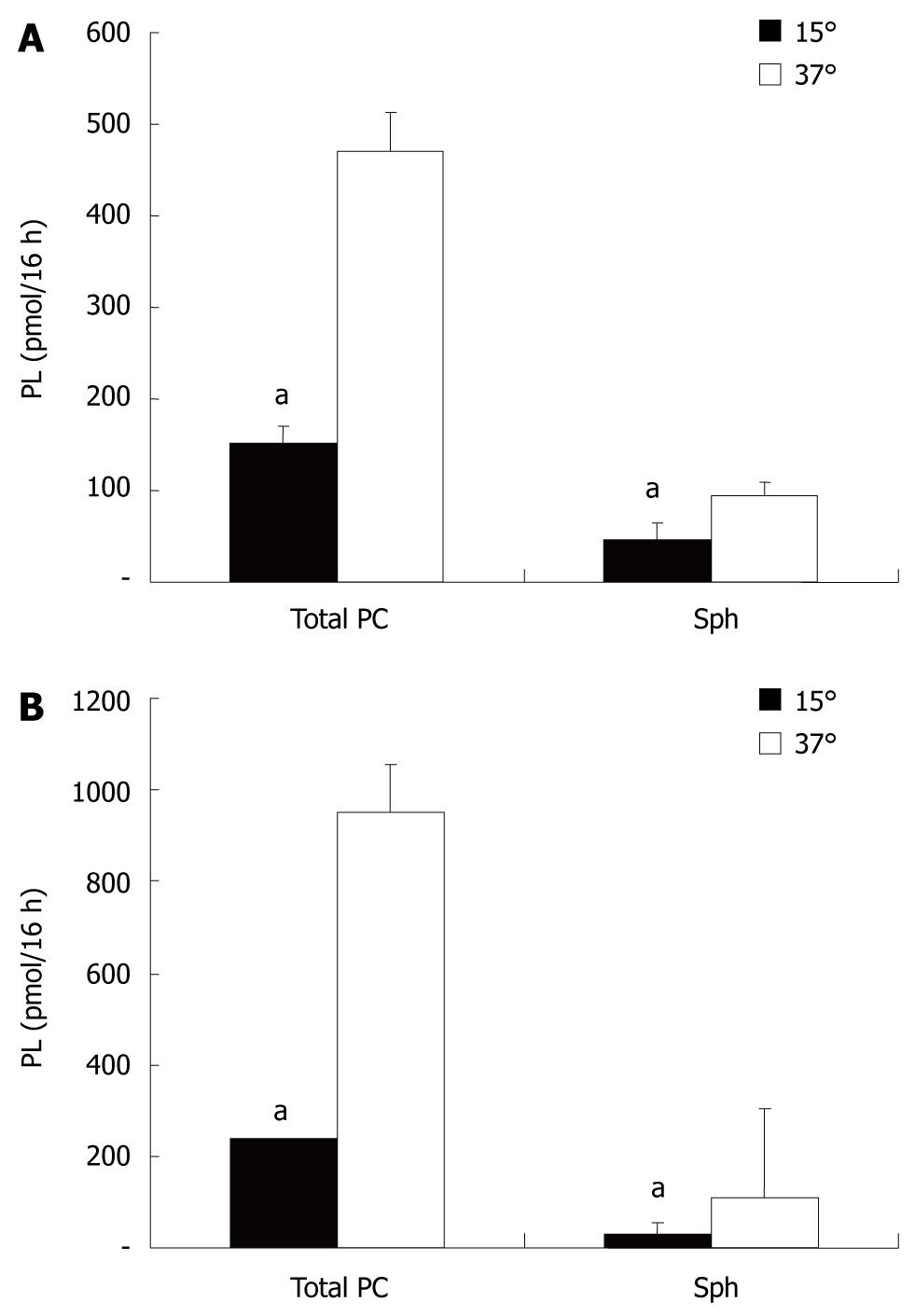
For polarization, CaCo-2 cells were grown on filters in Transwell culture dishes. Secretion of PC was examined in the apical and basolateral media containing, as lipid acceptors, taurocholate (2.5 mmol/L) or albumin (1% BSA). CaCo-2 cells showed PC secretion into both compartments. With regard to the acceptors of PC in both compartments (taurocholate or albumin), there was no difference recorded in the amount of PC or Sph released into the media (Figure 1). This indicated that under the experimental conditions employed, both acceptor molecules were present in excess and their capacity for PC and Sph solubilization was not compromised. The secretion of PC and Sph in both compartments was temperature sensitive with a significant reduction in the secretion rate at 15°C. The degree of temperature-induced acceleration/deceleration of the transport rates was comparable for PC and Sph at each side. However, the total secretion rates towards the basolateral side were significantly more inhibited at 15°C than those at the apical side. This observed drop in secretion is typical for vesicular transport, because much lower transport rates in membrane carrier systems are expected at 15°C (Figure 2).
At 37°C the rate of secretion was significantly higher at the basolateral side compared to the apical side - also an indication of two different secretion capacities (Figure 3).
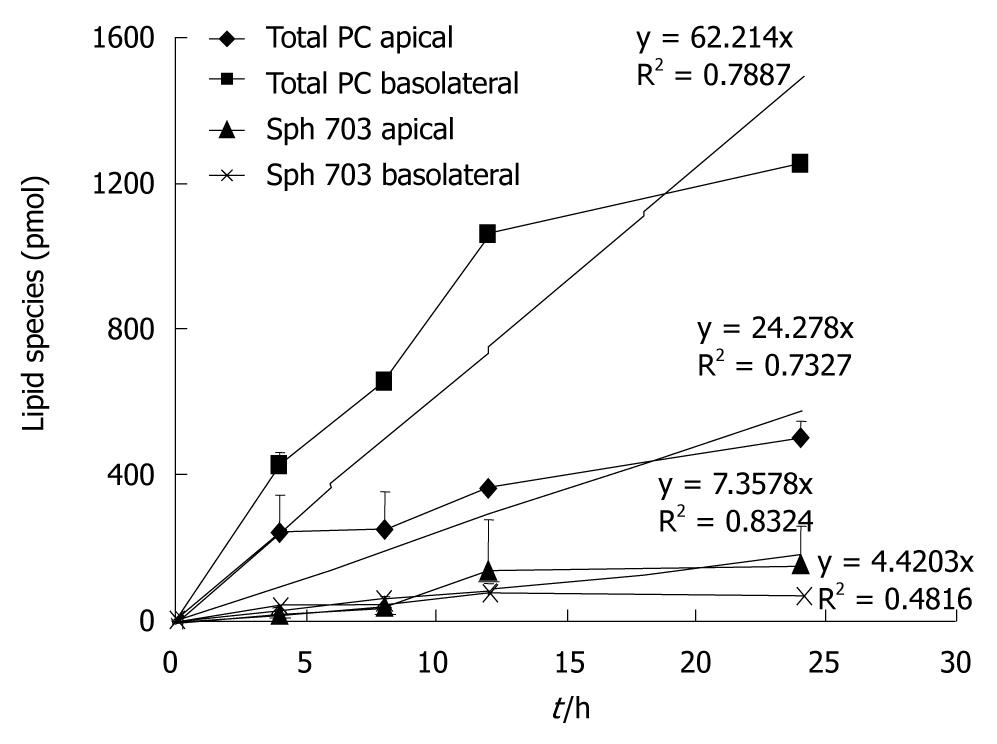
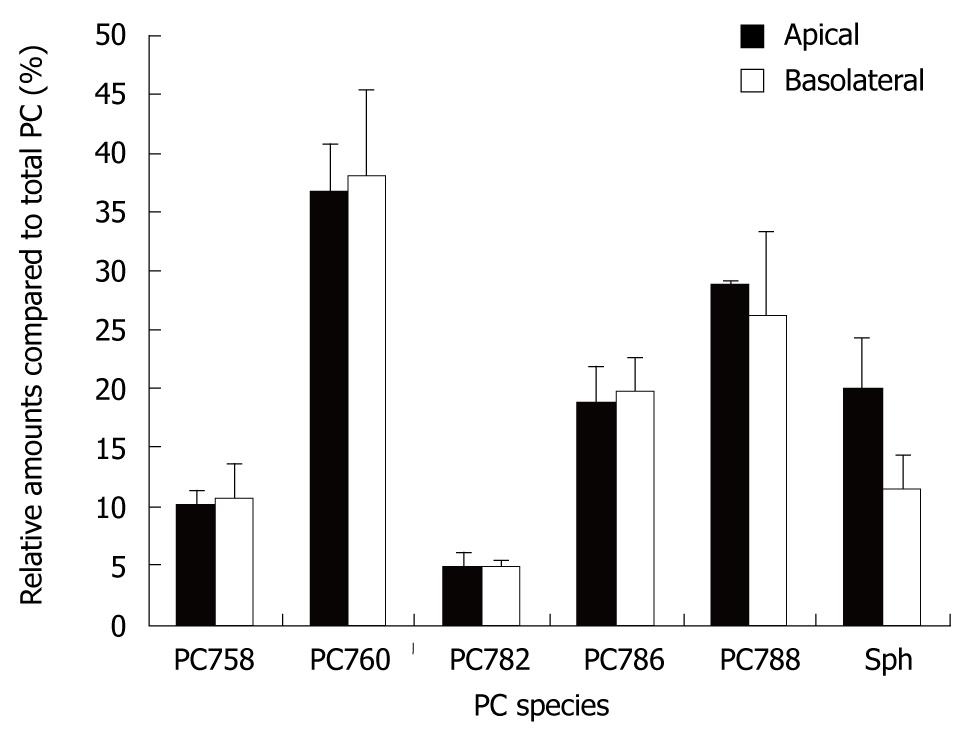
When the PC species and their secretion distribution on both sides were compared, they were virtually superimposable (Figure 4). This supports the concept that they originate from the same intracellular source. However, the relative fraction of Sph release compared to total PC release was significantly higher in the apical compartment. This corresponded to a higher level of Sph in the different pellets and the 100 000 g supernatant of the apical compartment vs the basolateral compartment (Figure 5). Sphingomyelins are typical phospholipids of vesicular membranes in the secretory pathway[14]. A higher proportion of Sph compared to the “cargo”-PC is suggestive of smaller sized secretory vesicles (“containers”). This is in line with the observation of a lower sensitivity towards temperature changes and a lower total apical PC secretion rate.
From a structural point of view, it would be easier for small sized vesicles to pass through the apical microvillous plasma membrane compared to large vesicles, which easily pass through the rather smooth basolateral side of the plasma membrane.
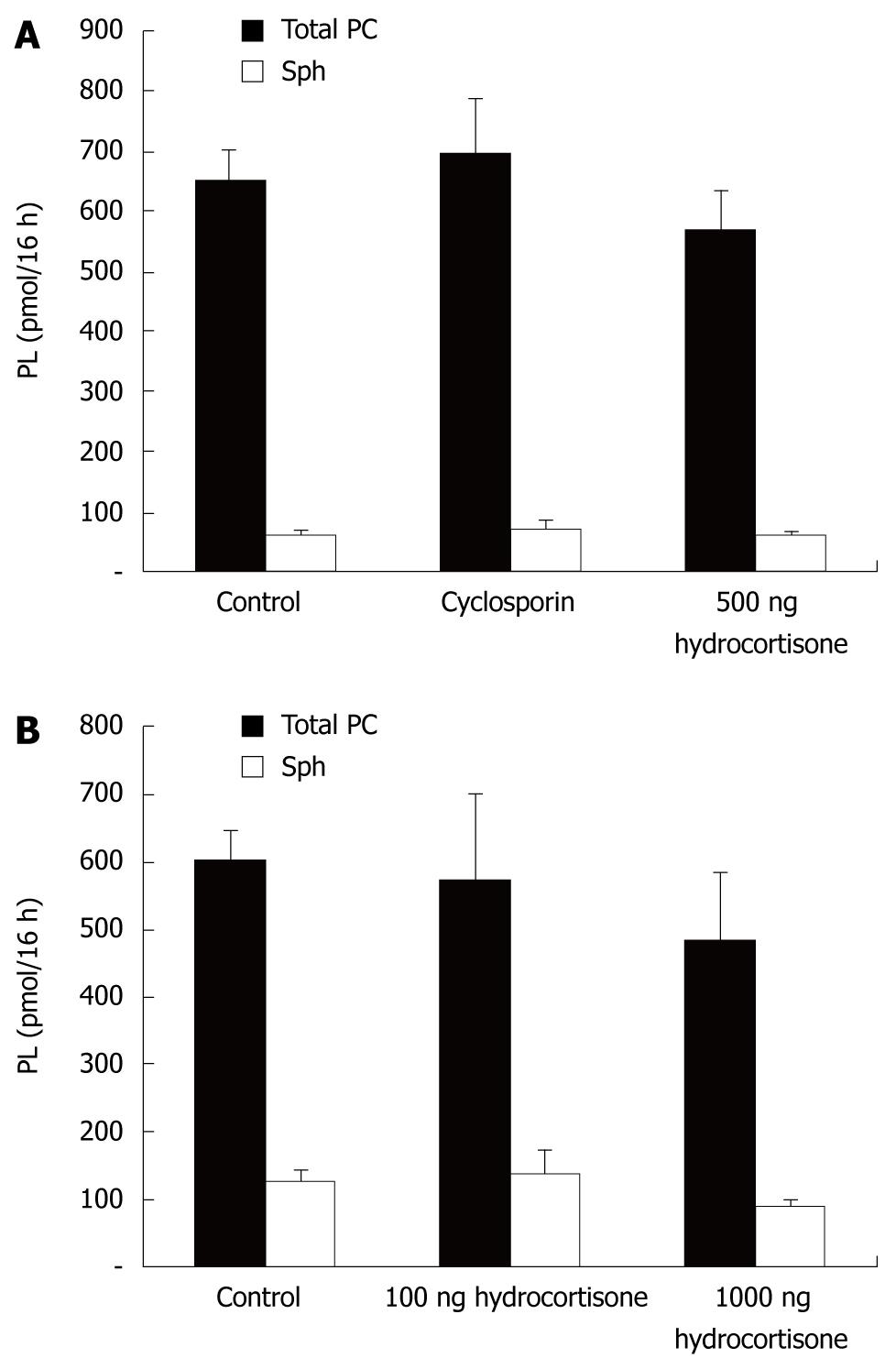
To further characterize the PC secretory pathway, we evaluated the effect of cyclosporine - a known inhibitor of ABC-transporters (Figure 6). No differences in transport rates were observed, underlying the hypothesis that PC secretion in intestinal mucosal cells more likely represents a vesicular excretion route. Similar results were obtained when other ABC-transport inhibitors (verapamil, glibenclamide and PSC833) were used (data not shown).
Since glucocorticoids are known to increase PC synthesis and excretion in the lung (surfactant), we evaluated their effects in CaCo-2 cells. Under the experimental conditions employed, no differences in secretion were detected (Figure 6). However, our experimental approach may have been too simple and unrefined to draw any further conclusions. It is possible that CaCo-2 cells do not express a glucocorticoid receptor.
The small intestine is an organ which is highly active in transport-mainly from the luminal to the basolateral side. It manages the complete absorption of all food constituents. The fact that the intestinal mucosa also has a secretory pathway towards the luminal site is a rather unrecognized feature. Thus, the high secretion rate at the basolateral side could be explained by the essential role of intestinal mucosal cells in the absorption process, in particular of lipids (high lipid throughput). After uptake of fatty acids, monoglycerides, lysophospholipids, and fat soluble vitamins into the mucosal cell, they are resynthesized to complex lipids (triglycerides, phospholipids), bound to apolipoproteins, and secreted at the basolateral side of mucosal cells via a vesicular pathway[14,15]. In contrast, at the microvillous side of the plasma membrane, it is sufficient to utilize a low capacity PC secretory pathway because mucus resembles a low PC-turnover compartment. Our experiments support the notion, that there are two separate PC secretion pathways in intestinal epithelial cells: a high capacity basolateral and a low capacity apical vesicular excretion route. This now needs confirmation by cellular imaging techniques and the use of native intestinal mucosal cells. The fact that 60%-90% of the phospholipids in mucus represent PC[8,9], indicates a specific PC secretion mechanism. The highly enriched PC arrangement within lamellar membranes establishes the protective hydrophobic surface layer of the mucus[8,9]. In the case of ulcerative colitis, where a decrease in the mucus PC levels was detected, this may be due to impairment of PC synthesis, PC loading of secretory vesicles, apical membrane fusion and PC release into the mucus or adherence to mucus proteins. After verification of the data in native mucosal cell models, the experimental system can be used to examine whether the PC secretion mechanism can be modified for therapeutic purposes.
Intestinal mucus and the mucosal barrier are very important aspects of intestinal disease.
Impaired mucosal barrier function, its pathophysiology and its impact on inflammatory bowel disease have become a very important focus of research.
The pathway by which phosphatidylcholine is secreted into the intestine to form mucus is not yet known. In this paper we provide evidence, that there are two separate secretion routes.
The used methodology and the acquired results might be the basis for future work, to confirm the data in vivo and to elucidate the role of phosphatidylcholine in pathophysiology.
Phospholipids, mainly phosphatidylcholine are the constituents of intestinal mucus. Due to their hydrophobicity they are part of the intestinal barrier.
The reviewers appreciated the innovative approach and techniques of this work. These data need to be confirmed in other experimental setups.
Peer reviewers: Akira Andoh, MD, Department of Internal Medicine, Shiga University of Medical Science, Seta Tukinowa, Otsu 520-2192, Japan; Elias A Kouroumalis, Professor, Department of Gastroenterology, University of Crete, Medical School, Department of Gastroenterology, University Hospital, PO Box 1352, Heraklion, Crete 71110, Greece
S- Editor Tian L L- Editor Webster JR E- Editor Ma WH
| 1. | Karner M, Ehehalt R, Stremmel W. Retarded release phosphatidylcholine: A new therapeutic option for ulcerative colitis. Immunoregulation in inflammatory bowel diseases current understanding and innovation. Heidelberg, Dordrecht, New York: Springer 2007; 161-170. |
| 2. | Ehehalt R, Wagenblast J, Erben G, Lehmann WD, Hinz U, Merle U, Stremmel W. Phosphatidylcholine and lysophosphatidylcholine in intestinal mucus of ulcerative colitis patients. A quantitative approach by nanoElectrospray-tandem mass spectrometry. Scand J Gastroenterol. 2004;39:737-742. |
| 3. | Braun A, Treede I, Gotthardt D, Tietje A, Zahn A, Ruhwald R, Schoenfeld U, Welsch T, Kienle P, Erben G. Alterations of phospholipid concentration and species composition of the intestinal mucus barrier in ulcerative colitis: a clue to pathogenesis. Inflamm Bowel Dis. 2009;15:1705-1720. |
| 4. | Stremmel W, Merle U, Zahn A, Autschbach F, Hinz U, Ehehalt R. Retarded release phosphatidylcholine benefits patients with chronic active ulcerative colitis. Gut. 2005;54:966-971. |
| 5. | Stremmel W, Ehehalt R, Autschbach F, Karner M. Phosphatidylcholine for steroid-refractory chronic ulcerative colitis: a randomized trial. Ann Intern Med. 2007;147:603-610. |
| 6. | Croft NM. Phospholipid in UC: novel, safe and works--is it too good to be true? Gastroenterology. 2006;130:1003-1004; discussion 1004-1005. |
| 7. | Ehehalt R, Jochims C, Lehmann WD, Erben G, Staffer S, Reininger C, Stremmel W. Evidence of luminal phosphatidylcholine secretion in rat ileum. Biochim Biophys Acta. 2004;1682:63-71. |
| 8. | Lichtenberger LM. The hydrophobic barrier properties of gastrointestinal mucus. Annu Rev Physiol. 1995;57:565-583. |
| 9. | Kao YC, Lichtenberger LM. A method to preserve extracellular surfactant-like phospholipids on the luminal surface of rodent gastric mucosa. J Histochem Cytochem. 1990;38:427-431. |
| 10. | Rooney SA, Young SL, Mendelson CR. Molecular and cellular processing of lung surfactant. FASEB J. 1994;8:957-967. |
| 11. | van Helvoort A, Smith AJ, Sprong H, Fritzsche I, Schinkel AH, Borst P, van Meer G. MDR1 P-glycoprotein is a lipid translocase of broad specificity, while MDR3 P-glycoprotein specifically translocates phosphatidylcholine. Cell. 1996;87:507-517. |
| 12. | Alpers DH, Zhang Y, Ahnen DJ. Synthesis and parallel secretion of rat intestinal alkaline phosphatase and a surfactant-like particle protein. Am J Physiol. 1995;268:E1205-E1214. |
| 13. | Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497-509. |
| 14. | De Matteis MA, Di Campli A, D’Angelo G. Lipid-transfer proteins in membrane trafficking at the Golgi complex. Biochim Biophys Acta. 2007;1771:761-768. |
| 15. | Stremmel W. Absorption of fat and fat-soluble vitamins. Structure and function of the small intestine. Amsterdam: Elsevier Science Publishers 1987; 175-184. |