Published online Dec 14, 2009. doi: 10.3748/wjg.15.5813
Revised: November 5, 2009
Accepted: November 12, 2009
Published online: December 14, 2009
AIM: To construct the recombinant lentivirus expression plasmid, pLenti6/V5-NT4 p53(N15)-antennapedia (Ant), and study its effect on HepG2 cells.
METHODS: Plasmid pLenti6/V5-NT4 p53(N15)-Ant was constructed incorporating the following functional regions, including signal peptide sequence and pro-region of neurotrophin 4, N-terminal residues 12-26 of p53 and 17 amino acid drosophila carrier protein, Ant. Hepatocellular carcinoma (HepG2) cells were used for transfection. 3-[4,5-dimethyl-thiazol-2yl]-2,5 diphenyl tetrazolium bromide (MTT) assay, lactate dehydrogenase (LDH) release assay, transmission electron microscopy (TEM) and flow cytometric analysis (FCM) were employed to investigate the effects of LV-NT4(Si)-p53(N15)-Ant in vitro on HepG2 cells. In vivo experiment was also performed to investigate the inhibitory effect of LV-NT4(Si)-p53(N15)-Ant on tumor growth in nude mice.
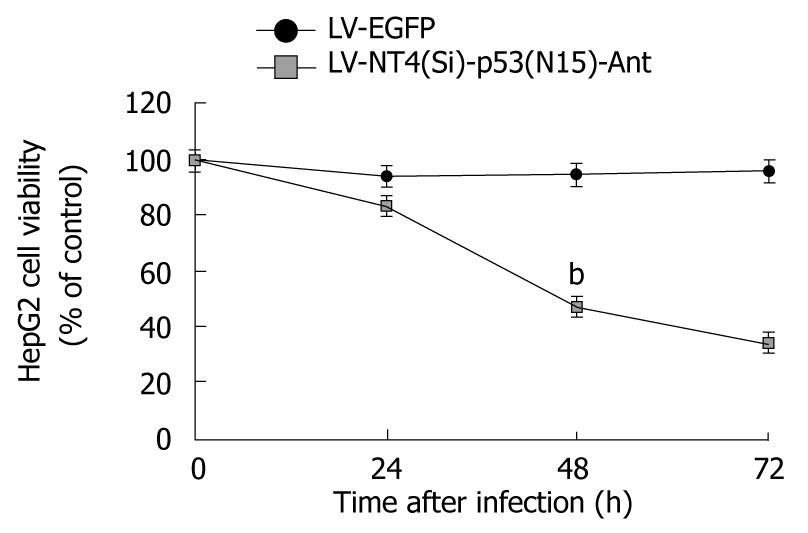
RESULTS: LV-NT4(Si)-p53(N15)-Ant significantly suppressed the growth of HepG2 cells. MTT assay showed that the growth of HepG2 cells was mucj more significantly inhibited by LV-NT4(Si)-p53(N15)-Ant than by LV-EGFP. The inhibition rate for HepG2 cell growth in the two groups was 46.9% and 94.5%, respectively, 48 h after infection with LV-NT4(Si)-p53(N15)-Ant, and was 33.9% and 95.8%, respectively, 72 h after infection with LV-NT4(Si)-p53(N15)-Ant (P < 0.01). Light microscopy and TEM showed morphological changes in HepG2 cells infected with LV-NT4(Si)-p53(N15)-Ant, but no significant changes in HepG2 cells infected with LV-EGFP. Changes were observed in ultra-structure of HepG2 cells infected with LV-NT4(Si)-p53(N15)-Ant, with degraded membranes, resulting in necrosis. LDH release from HepG2 cells was analyzed at 24, 48, 72 and 96 h after infection with LV-NT4(Si)-p53(N15)-Ant and LV-EGFP, which showed that LDH release was significantly higher in LV-NT4(Si)-p53(N15)-Ant treatment group (682 IU/L) than in control group (45 IU/L, P < 0.01). The longer the time was after infection, the bigger the difference was in LDH release. FCM analysis showed that LV-NT4(Si)-p53(N15)-Ant could induce two different kinds of cell death: necrosis and apoptosis, with apoptosis being the minor type and necrosis being the main type, suggesting that LV-NT4(Si)-p53(N15)-Ant exerts its anticancer effect on HepG2 cells by inducing necrosis. The in vivo study showed that LV-NT4(Si)-p53(N15)-Ant significantly inhibited tumor growth with an inhibition rate of 66.14% in terms of tumor size and weight.
CONCLUSION: LV-NT4(Si)-p53(N15)-Ant is a novel recombinant lentivirus expression plasmid and can be used in gene therapy for cancer.
- Citation: Song LP, Li YP, Wang N, Li WW, Ren J, Qiu SD, Wang QY, Yang GX. NT4(Si)-p53(N15)-antennapedia induces cell death in a human hepatocellular carcinoma cell line. World J Gastroenterol 2009; 15(46): 5813-5820
- URL: https://www.wjgnet.com/1007-9327/full/v15/i46/5813.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.5813
Hepatocellular carcinoma (HCC) is one of the most common causes of death worldwide, especially in Asian countries[1]. To date, none of the conventional treatment modalities can completely eradicate HCC cells. In recent years, gene therapy has been evaluated as a novel treatment modality for HCC[2-5], while conventional treatment modalities, including surgery, chemotherapy and liver transplantation, are still used[6-8].
p53 is an important tumor suppressor gene which regulates many important cellular activities, including apoptosis. Mutations and deletions of the p53 gene are common in HCC in a number of geographic regions. Since the mutation incidence ranges 5%-50%, p53 has become an ideal target for gene therapy. Kanovsky et al[9] and Do et al[10] reported that a p53 peptide, synthesized from residues 12-26 and fused with the Drosophila carrier protein antennapedia (Ant), can induce rapid tumor cell necrosis in all breast and pancreatic cancer cell lines irrespective of its status and exhibits a low cytotoxicity to normal cells, which is uncommonly observed in traditional cancer therapy. Human HCC cell line, HepG2, contains the wild-type p53 gene and can thus be used in research of the relation between HCC and p53 peptide gene therapy.
It is important to study the transfer of fusion gene and the secretion of expressed protein for the assessment of enhanced cancer-killing effects of a protein. In this study, NT4 signal peptide and its pro-region were used as the regions responsible for protein and peptide secretion from cells. Lentivirus gene expression plasmids were constructed for NT4(Si)-ADNF-9 and NT4(Si)-NAP fusion proteins containing the NT4 signal peptide and pro-region to enhance their expression. The restriction enzyme site NaeI at the NT4 signal peptidase fissure site and two restriction enzyme sites, BamHI and XhoI, in NT4(Si)-p53(N15)-Ant could ensure the correct construct. A novel recombinant lentivirus expression plasmid, pLenti6/V5-NT4 p53(N15)-Ant, was constructed, containing a signal peptide sequence and pro-region of neurotrophin 4 (NT4) fused to p53(N15)-Ant peptide. Its effect on HepG2 cells, both in vitro and in vivo, was investigated.
Human hepatoma HepG2 cells containing wild-type p53 were cultured in RPMI 1640 containing 10% fetal bovine serum (FBS). 293T human kidney cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% FBS, 100 U/mL penicillin and 100 μg/mL streptomycin at 37°C in a humidified atmosphere containing 50 mL/L CO2. All cell lines were supplied by Xi’an Huaguang Bioengineering Company (Xi’an, China).
p53(N15)-Ant was synthesized by Beijing Sun Biotechnology Co, according to Kanovsky et al[9] and Genbank. Two restriction enzyme sites (NaeI and XhoI) were introduced upstream and downstream of this sequence, respectively. p53(N15)-Ant was subcloned into pGEM-T-easy for sequencing, then into pBV220/NT4. Signal peptide sequence and pro-region of NT4 were cloned from human genomic DNA and subcloned into the BamHI/NaeIsites of pBV220. After digestion with restriction enzymes, the resulting NT4 p53(N15)-Ant gene with BamHIand XhoIsites from the pBV220/NT4-p53-(N15)-Ant plasmid was inserted into the multiple cloning site of the expression plasmid, pLenti6/V5-D-TOPO (Invitrogen) containing a cytomegalovirus (CMV) promoter, upstream of the inserted gene. The resulting plasmid was named pLenti6/V5-NT4 p53(N15)-Ant.
To generate a control plasmid containing green fluorescent protein, EGFP was amplified from EGFP-C2 by PCR. The sequences of sense and anti-sense primers are 5'CGGGATCCATGGTGAGCAAGGGCGAGG-3' and 5'CGCTCGAGTCAAGTCCGGCCGGACTTGTAC-3', respectively. The resultant PCR fragments were digested with BamHI and XhoI (underlined), subcloned into pLenti6/V5-D-TOPO, and verified by DNA sequencing.
The four-plasmid-based lentiviral expression system was purchased from Invitrogen. Briefly, four kinds of plasmid including 4.2 μg of pLP1, 2 μg of pLP2, 2.8 μg of pLP/VSVG and 3 μg of lentivector pLenti6/V5-NT4 p53(N15)-Ant or pLenti6/EGFP, were cotransfected into 293T cells using the calcium phosphate coprecipitation method. The conditioned medium was harvested 72 h after transfection and filtered through a 0.45 μm filter. Concentrated viral stocks were prepared by ultra-centrifugation of 3 mL conditioned medium at 50 000 g for 1.5 h at 4°C in a SW41 rotor (Beckman). The pellet was resuspended in 30 μL complete medium and stored at -80°C. The resulting recombinant lentivirus was named LV-NT4 p53(N15)-Ant and LV-EGFP, respectively.
Viral titer was measured by assessing the viral p24 antigen concentration using ELISA (Beckman Coulter, Fullerton, California, USA), showing that one microgram per milliliter of p24 corresponds to approximately 2 × 106 transducing units per milliliter of EGFP virus, as assessed by titration in 293 T cells.
HepG2 cells were seeded into 6-well plates at a density of 1 × 106 cells/well, allowed to adhere for 24 h, and then infected with EGFP lentivirus at multiplicities of infection (MOI) of 4 transducing units (TU)/cell. EGFP-positive cells were observed and counted under a fluorescence microscope 48 h after infection.
Expression of the NT4 p53(N15)-Ant gene was detected by reverse transcription-polymerase chain reaction (RT-PCR). HepG2 cells were seeded in 100 mL culture flasks at a density of 1 × 106 cells/flask and infected with NT4-p53(N15)-Ant lentivirus at a MOI of 4 TU/cell. Forty-eight hours after infection, HepG2 cells were scraped from the flask surface with a brush (Paro-Isola, Thalwil, Switzerland). Total RNA was extracted using Trizol reagent (Gibco). A reverse transcription (RT) step was carried out for 60 min at 37°C using 1 μg total RNA treated with DNase and M-MLV reverse transcriptase (Invitrogen, USA) in the presence of random primers. PCR was performed in 50 μL reaction volume containing 10 μL (about 2.5 ng) cDNA, 1 μL of 10 mmol/L dNTP, 1 U of Taq DNA polymerase, 5 μL of 10 × Taq buffer, and 1 μL 50 pmol of forward primer (5'-CGGATCCATGCTCCCTCTCCCCTCATGC-3') and reverse primer (5'-CCTCGAGTCATCCGCGCTGTACCTTTACC-3') of the NT4 p53(N15)-Ant gene. Samples were subjected to RT-PCR at the following conditions: pre-denaturation for 5 min at 94°C, followed by 30 cycles of denaturation for 60 s at 94°C, annealing for 60 s at 60°C, extension for 90 s at 72°C, and a final extension for 5 min at 72°C. The RT-PCR products were separated by electrophoresis on 2% agarose gels (Qiagen, USA).
H1299 cells (null for p53) were seeded onto cover slips and infected with LV-NT4(Si)-p53(N15)-Ant at a MOI of 4 TU/well. Polybrene was added to a final concentration of 8 μg/mL. Twelve hours after infection, 2 mL of a new DMEM medium was added. Forty-eight hours after infection, H1299 cells were washed and fixed with cold acetone. Protein expression of p53(N15)-Ant in H1299 cells was determined by immunocytochemical staining with anti-p53 antibody (DO-1) (Santa Cruz Biotechnology, Santa Cruz, CA, USA) using the Vectastain Elite ABC kit following its manufacturer’s instructions (Vector Laboratories, Burlingame, CA, USA).
To determine the effect of lentivirus-NT4(Si)-p53(N15)-Ant on HCC, HepG2 cells were seeded in 96-well plates at a concentration of 5 × 103 cells, grown for 24 h, infected with lentivirus-NT4(Si)-p53(N15)-Ant or lentivirus-EGFP at a MOI of 4 TU/cell with polybrene (8 μg/mL) for 12 h. The culture medium was then replaced with a fresh medium. MTT assay was performed at different time points (24, 48 and 72 h) after infection with lentivirus according to its manufacturer’s instructions (Xi’an, China).
After infection with NT4(Si)-p53(N15)-Ant, morphology of HepG2 cells was observed under an inverted light microscope and recorded.
Forty-eight hours after infection with NT4(Si)-p53(N15)-Ant, HepG2 cells were trypsinized, fixed with 2.5% glutaraldehyde for 2 h at 4°C, washed with 0.1 mol/L dimethyl arsenic trioxide buffer, fixed with 1% osmium acid for 2 h, dehydrated, and then embedded in 618 domestic epoxy resin, followed by polymerization for 24 h. The embedded block was cut into ultra-thin sections. The sections were double stained with uranyl acetate and lead citrate, and observed under a transmission electron microscope.
To determine LDH leakage into the extra-cellular fluid, supernatant was collected at different time points (24, 48, 72, and 96 h) after infection with NT4(Si)-p53(N15)-Ant. LDH in the supernatant was detected with a RA-100 automatic biochemical analyzer.
When HepG2 cells were grown to 2 × 105/mL, they were seeded into 9-well plates at a volume of 3 mL/well, and left to adhere for 24 h. The cells were then divided into control group and lentivirus treatment group. The cells in control group were grown in a serum-free medium. Forty-eight hours after infection with lentivirus, the cells were washed twice with PBS at 4°C, re-suspended in 250 μL of a combination buffer solution, and the cell concentration was adjusted to 1 × 105/mL. Five microliter of Annexin V/FITC and 5 μL of 20 μg/mL propidium iodide were added to 100 μL of cell suspension. After incubation in the dark for 15 min, the cells were analyzed by flow cytometry (FCM).
Twenty 6-8 wk old male and female BALB/c (nu/nu) mice, weighing 20.3 ± 2 g, were purchased from Laboratory Animal Center, Fourth Military Medical University (Xi’an, China).
HepG2 cells (2 × 105) were injected subcutaneously on the right side of each mouse’s back to induce tumor formation. The animals were divided into three groups when the tumor volume reached 30 mm3 with 0.1 mL LV-NT4(Si)-p53(N15)-Ant (2 × 106 TU/mL) injected into their tumor. Mice treated with PBS and LV-EGFP served as controls with 0.1 mL LV-EGFP (2 × 106 TU/mL) injected into their tumor (injection at different directions). Infection efficiency of lentivirus was observed under a confocal laser scanning microscope one week after infection.
The animals were sacrificed 3 wk after injection of lentivirus. Tumors were removed and weighed with their size measured using a vernier caliper and their volume calculated according to the following formula: (3.14 × L × W × H)/6. Tumors were fixed in 4% formaldehyde and embedded in paraffin. Tumor tissue was cut into 5-μm thick sections which were stained with HE.
Tumor growth inhibition rate = (A-B)/A * K, where A is the average tumor weight of control group, B is the average tumor weight of treatment group, K represents 100%.
The data were expressed as mean ± SD. LDH release and MTT were analyzed by t-test and χ2 test, respectively. The data for animal experiment were processed by analysis of variance. Data analysis was performed using the SPSS 15.0. P < 0.05 was considered statistically significant.
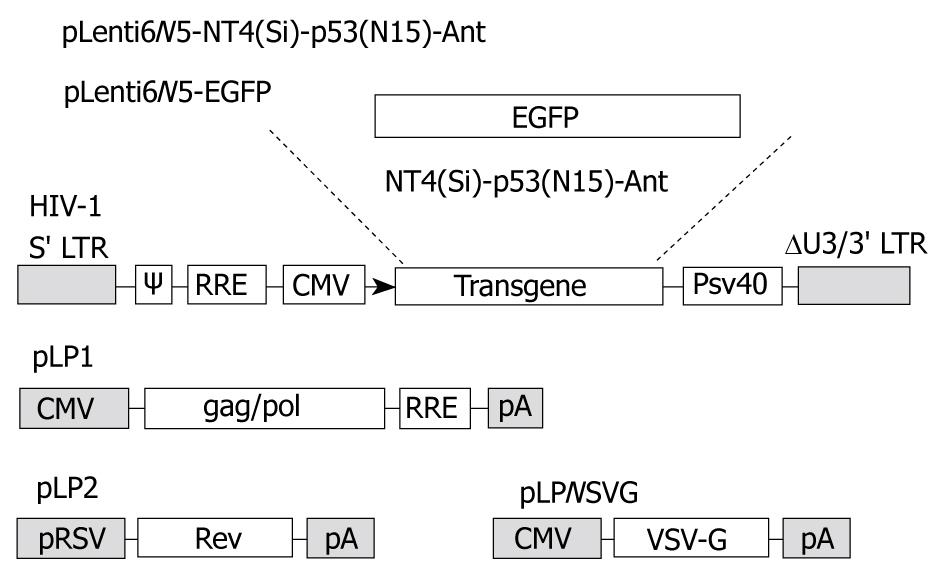
The lentivirus expression vector, pLenti6 V5-D-TOPO we constructed, contains the elements required for virion packaging, such as 5’ and 3’ long terminal repeats and ψ packaging signal. The packaging system of LV-NT4(Si)-p53(N15)-Ant and LV-EGFP is shown in Figure 1.
The DNA sequence of the p53(N15)-Ant gene was identical to those of p53(N15) and 17 amino acid Ant [9]. The length of NT4-p53(N15)-Ant was 349 bp, including a 247 bp fragment of the signal peptide sequence and pro-region of neurotrophin 4 with the two restriction sites (BamHI and NaeI). The DNA fragment of p53(N15)-Ant was 108 bp with NaeI and XhoI. Digestion of pLenti6/V5-NT4-p53(N15)-Ant with BamHI and NaeI followed by electrophoresis verified that the NT4-p53(N15)-Ant secretory expression cassette was correctly inserted into the multiple cloning site (MCS) of pLenti6/V5-D-TOPO, and pLenti6/V5-EGFP was confirmed in the same way as pLenti6/V5-NT4-p53(N15)-Ant.
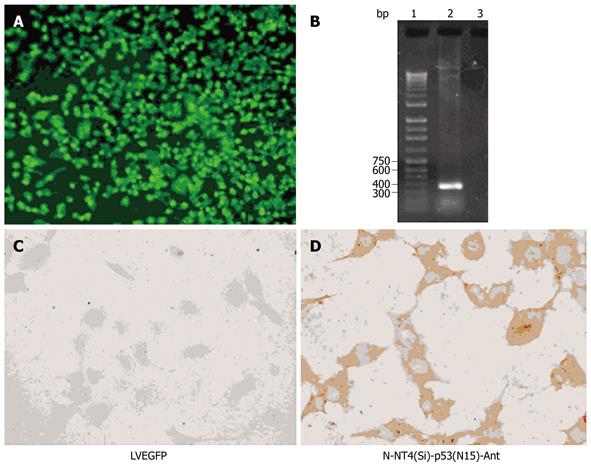
Fluorescent microscopic analysis showed that EGFP was expressed in over 60% of HepG2 cells 48 h after infection with pLenti6/V5-EGFP at a MOI of 4 TU/cell (Figure 2A and B).
Since H1299 cells lack of the p53 gene (null-p53), H1299 lung cancer cells were used as target cells for the expression of p53(N15)-Ant. p53 protein could not be detected with immunohistochemical staining before or after infection with LV-EGFP (Figure 2C). However, 48 h after infection with LV-NT4(Si)-p53(N15)-Ant, p53 protein was mainly expressed in cytoplasm of H1299 lung cancer cells (Figure 2D). Anti-DO-1 could identify and bind to amino acids 11-25 of p53, suggesting that LV-NT4(Si)-p53(N15)-Ant can effectively infect the target cells and express the p53 fusion peptide.
HepG2 cells, infected with pLenti6/V5-NT4-p53(N15)-Ant in our study, were significantly killed, whereas pLenti6/V5–EGFP had no effect on cell viability, suggesting that growth of HepG2 cells can be inhibited by the transferred exogenous gene rather by viral toxicity (Figure 3).
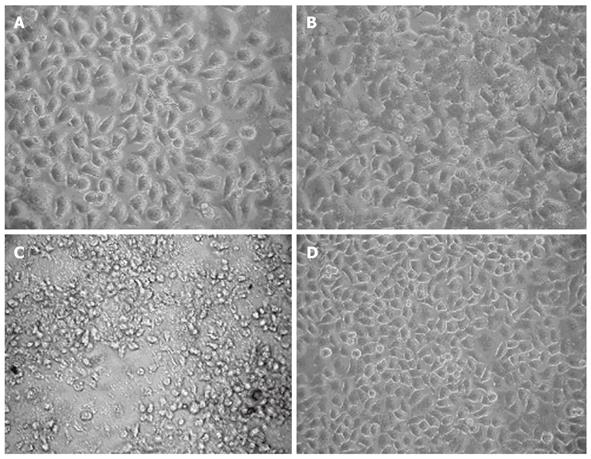
Significant morphological changes were observed in HepG2 cells at different time points after infection with LV-NT4(Si)-p53(N15)-Ant but not after infection with LV-EGFP and LV-NT4(Si)-p53(N15)-Ant (Figure 4). However, the cells became swollen and their boundary was blurred 48 h after infection with LV-NT4(Si)-p53(N15)-Ant, while their viability was significantly declined with noticeable cell shrinkage, fragments and detachment 72 h after infection with LV-NT4(Si)-p53(N15)-Ant, but without significant morphological changes 72 h after infection with LV-EGFP infection.
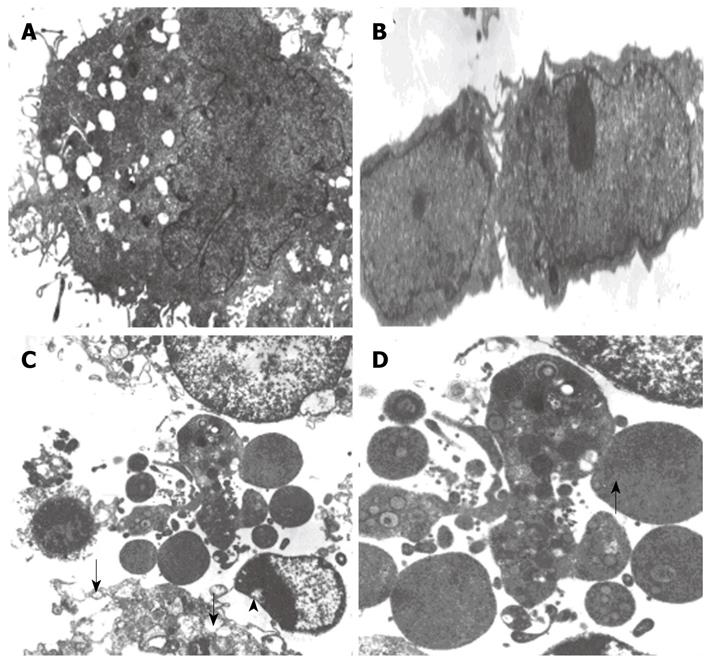
Forty-eight hours after infection with LV-EGFP, the membranes and nuclear membranes of HepG2 cells were intact with some crimples in the nuclear membranes, some microvilli on the surface of HepG2 cells, normal structure of mitochondria and ER (Figure 5A), and 2 completely divided HepG2 cells (Figure 5B), suggesting that LV-EGFP has no significant effect on cell growth and division. Forty-eight hours after infection with LV-NT4(Si)-p53(N15)-Ant, significant changes in ultra-structure of HepG2 cells, as well as in cell membranes and mitochondria, were observed under electron microscope. Cell membranes were incomplete with several fractures and the cells appeared to leak contents. Mitochondria were swollen with vacuolization. Vacuoles were also seen under the nuclear membrane with bare nuclei (Figure 5C and D). These findings suggest that NT4(Si)-p53(N15)-Ant may exert its anticancer effect mainly by inducing necrosis of cell membranes.
LDH was significantly increased in LV-NT4(Si)-p53(N15)-Ant treatment group, but not in LV-EGFP treatment group, with no significant difference between the two groups. The longer the time was after the infection, the greater the difference was between the groups, suggesting that cell membranes are damaged.
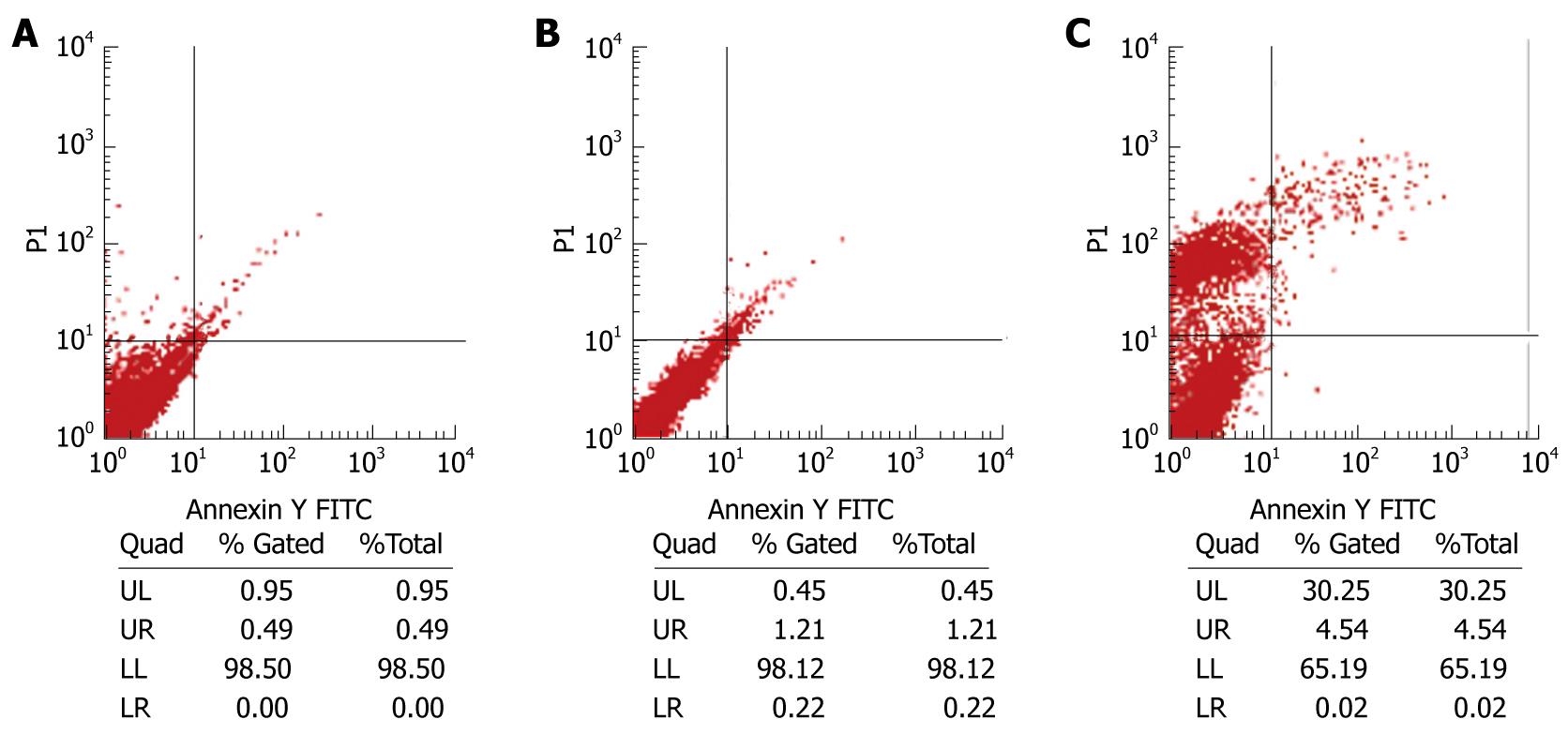
Flow cytometry (FCM) was employed to detect the fluorescence intensity after HepG2 cells were double-stained with Annexin V and PI. A plot composed of 4 quadrants was detected by flow cytometry, which showed that the number of necrotic cells was significantly greater in LV-NT4(Si)-p53(N15)-Ant treatment group than in LV-EGFP treatment group 48 h after infection with lentivirus. The apoptosis level was also higher in cells expressing LV-p53(N15) than in controls. However, the number of necrotic cells was still much greater than that of apoptotic cells (Figure 6), suggesting that the NT4(Si)-p53(N15)-Ant gene kills tumor cells mainly by inducing necrosis of cell membrane.
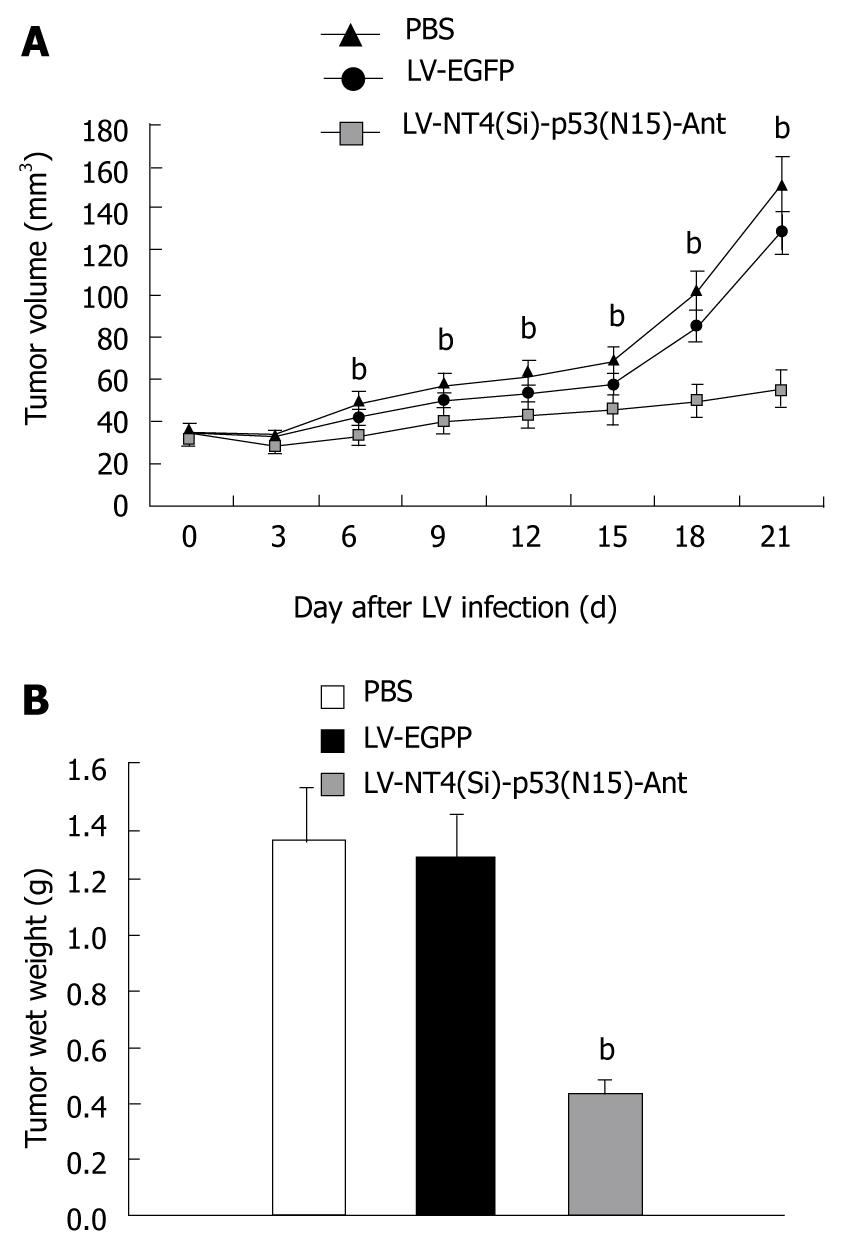
Nude mice developed tumor mass within 10 d after inoculation with HepG2 cells. Three weeks after infection with lentivirus, the size and weight of tumor mass were measured (Figure 7A and B). The inhibition rate of tumor growth for LV-NT4(Si)-p53(N15)-Ant was 66.14%.
p53 protein plays an essential role in cell activities[11,12]. Under various stress conditions, such as genotoxic damage, hypoxia, ribonucleotide depletion or oncogene activation, p53 molecule accumulates and is activated. Activated p53 acts as a transcription factor and activates or represses a variety of genes involved in cell-cycle regulation, induction of apoptosis or senescence. Therefore, the p53 gene represents an ideal target for cancer therapy. In non-stressed cells, p53 protein is present at a very low cellular concentration, because it interacts with the hdm2 protein, the human analogue of murine mdm2, which acts as a ligase of ubiquitin[13,14] and promotes p53 degradation[9,10]. Recent advances in regulation of p53 by hdm2 offer the possibility of generating new anticancer agents that activate wild-type p53 in tumors. Since hdm2 down-regulates p53, inhibition of this interaction would lead to an accumulation of p53 in tumor cells, eventually inducing their death[10,15]. p53(N15)-Ant has been considered a novel cancer therapeutic peptide because it induces cancer cell death and does not seem to be cytotoxic to normal cells and may, therefore, prove useful as a general anticancer agent. In this study, we focused on how to improve the anticancer effect of peptide therapy by employing two strategies. The first was to enable the expressed therapeutic peptide to be secreted, and the second was to use the lentivirus gene transfer system. It is well-known that the signal peptide is located at the terminal of amino acid sequence of a secreted protein and plays an important role in protein targeting and translocation in both prokaryotic and eukaryotic cells. NT4 is a member of the neurotrophin family, involved in controlling survival and differentiation of vertebrate neurons[16]. Besides the common features of neurotrophins, NT4 has many unusual features. Unlike other neurotrophins, the expression of NT4 is ubiquitous and less influenced by environmental signals[17]. In the present study, NT4 structure analysis showed that the human NT4 initiation codon was followed by a signal peptide sequence, a pro-region and an apparent dibasic cleavage site, which was followed by the sequence of the mature NT4. The signal peptide sequence and pro-region of human NT4 are notably shorter (by = 243 bp) than those of other neurotrophins[18]. In our previous study, the pre-protein NT4 could be cleaved into the signal peptide and the mature protein by Escherichia coli (E. coli) endopeptidase. There is a native NaeI enzyme site in front of the site in the NT4 signal peptide, thus ensuring the correct cleavage of therapeutic peptide, and making this signal peptide sequence highly convenient for the expression of other exogenous peptides in a secretory manner. It has been shown that it is not only feasible, but also improves the therapeutic effect[19]. In this study, we successfully constructed a LV containing the NT4(Si)-p53(N15) fusion gene with in vitro recombinant DNA technology.
To transfer the NT4(Si)-p53(N15)-Ant gene into the target cells and to achieve a consistent expression, selection of the gene transfer vector is a critical step. Lentiviruses, such as HIV and SIV, are a new generation of vectors and have many advantages over the traditional virus vectors, such as retroviral or adenovirus vector. These LVs can infect both dividing and non-dividing cells and efficiently integrate into the host DNA, thus providing stable transgene expression over several months[20,21]. At the same time, LV is very safe because all lentivirus accessory genes have been removed, virus production components have been split into 3 or 4 separate parts, and self-inactivating deletions have been introduced into the vector[21,22]. Since the aim of cancer gene therapy is to remove all cancer cells including those in a precancerous stage, only extensive gene transfer and long-term gene expression in cancer cells are appropriate. Thus, LV has a greater promise to become an ideal vector for gene delivery.
In this study, MTT assay showed that LV-NT4(Si)-p53(N15)-Ant could significantly induce HepG2 cell death 48 h after infection, LDH was significantly increased in the LV-NT4(Si)-p53(N15)-Ant treatment group but not in the LV-EGFP treatment group, suggesting that the cell membranes are damaged. Moreover, cytotoxicity occurred in a time-dependent manner. The ultra-structure of HepG2 cells, especially cell membranes and mitochondria, was significantly changed after infection with LV-NT4(Si)-p53(N15)-Ant, which was different from that during apoptosis and necrosis. In addition, FCM revealed that two cell death modes (apoptosis and necrosis) occurred 48 h after infection with LV-NT4(Si)-p53(N15)-Ant with necrosis being common and apoptosis being rare, revealing that LV-NT4(Si)-p53(N15)-Ant exerts its effect on HCC mainly by inducing necrosis of cell membranes. This is probably related to the process of LV infection, integration of the therapeutic gene into the host genome and gene expression.
The in vivo study also showed that LV-NT4(Si)-p53(N15)-Ant significantly inhibited tumor growth. Since p53(N15) contains overlapping sequences from the p53 mdm-2 binding domain, p53(N15)-Ant peptide may block the interaction of mdm-2 with other proteins. Recently, a study investigating the secondary structure of the p53 fusion peptide has revealed that p53(N15)-Ant peptide plays an important role in membrane interaction[15], showing that p53(N15)-Ant peptide forms distinctive S-shape helix-loop-helix structures, which can rapidly disrupt cancer cell membranes by forming a toroidal-like pore, resulting in necrosis. The two mechanisms exist simultaneously.
In conclusion, we have established a way to express lentivirus-introduced p53(N15)-Ant 32-peptide. The growth of HepG2 cells can be significantly inhibited by LV-NT4(Si)-p53(N15)-Ant. LV-NT4(Si)-p53(N15)-Ant gene therapy may be used as a novel anticancer strategy. Further study on the biological characteristics of the p53 peptide is needed by transfecting LV-NT4(Si)-p53(N15)-Ant into other cancer cell lines.
Peptide has emerged as a new anticancer agent in recent years. Numerous reports suggest that many low molecular weight peptides possess an anticancer effect but have almost no cytotoxic effect on normal cells. p53(N15)-Ant has been considered a novel cancer therapeutic peptide because it induces cancer cell death and does not seem to be cytotoxic to normal cells.
It has been reported that p53 peptide, synthesized from residues 12-26 and fused with Drosophila carrier protein antennapedia (Ant), induces rapid tumor cell necrosis in all breast and pancreatic cancer cell lines tested, irrespective of the p53 status, whereas it shows a low cytotoxicity to normal cells. In this study, the authors constructed a novel recombinant lentivirus expression plasmid LV-NT4(Si)-p53(N15)-Ant and demonstrated its anticancer effect.
The signal peptide plays an important role in protein targeting and translocation in both prokaryotic and eukaryotic cells. In this experiment, the authors used the NT4 signal peptide to enable the therapeutic peptide to be secreted. Lentivirus vectors were also employed. LV-NT4(Si)-p53(N15)-Ant was constructed and successfully cultured at a high titer and verified to induce necrosis of cancer cells.
This work has established a way to express lentivirus-introduced p53(N15)-Ant 32-peptide. LV-NT4(Si)-p53(N15)-Ant dependent gene therapy may be a promising novel anticancer strategy. p53(N15)-Ant may represent a promising agent of gene therapy for hepatocellular carcinoma.
NT4 signal peptide and its pro-region are protein sequences that can direct the secretion of proteins and peptides from cells. Such a mechanism is thought to be helpful in fusion peptide gene therapy as it can enhance the therapeutic effect.
In this study, Song et al showed that the constructed lentivirus vector expressed in cancer cells could inhibit the growth of HepG2 cells. This vector can induce necrosis and apoptosis, and inhibit tumor growth in vivo. The study in general is significant and interesting.
Peer reviewers: Lin Zhang, PhD, Associate Professor, Department of Pharmacology & Chemical Biology, University of Pittsburgh Cancer Institute, University of Pittsburgh School of Medicine, UPCI Research Pavilion, Room 2.42d, Hillman Cancer Center, 5117 Centre Ave., Pittsburgh, PA 15213-1863, United States; Sung-Gil Chi, Professor, School of Life Sciences and Biotechnology, Korea University, #301, Nok-Ji Building, Seoul 136-701, Korea; Jian-Zhong Zhang, Professor, Department of Pathology and Laboratory Medicine, Beijing 306 Hospital, 9 North Anxiang Road, PO Box 9720, Beijing 100101, China
S- Editor Wang JL L- Editor Wang XL E- Editor Tian L
| 1. | Di Bisceglie AM, Carithers RL Jr, Gores GJ. Hepatocellular carcinoma. Hepatology. 1998;28:1161-1165. |
| 2. | Ruiz J, Qian C, Drozdzik M, Prieto J. Gene therapy of viral hepatitis and hepatocellular carcinoma. J Viral Hepat. 1999;6:17-34. |
| 3. | Qian C, Drozdzik M, Caselmann WH, Prieto J. The potential of gene therapy in the treatment of hepatocellular carcinoma. J Hepatol. 2000;32:344-351. |
| 4. | Mitry RR, Mansour MR, Havlík R, Habib NA. Gene therapy for liver tumours. Adv Exp Med Biol. 2000;465:193-205. |
| 5. | Bálint EE, Vousden KH. Activation and activities of the p53 tumour suppressor protein. Br J Cancer. 2001;85:1813-1823. |
| 6. | Boyd SD, Tsai KY, Jacks T. An intact HDM2 RING-finger domain is required for nuclear exclusion of p53. Nat Cell Biol. 2000;2:563-568. |
| 7. | Courtois S, Verhaegh G, North S, Luciani MG, Lassus P, Hibner U, Oren M, Hainaut P. DeltaN-p53, a natural isoform of p53 lacking the first transactivation domain, counteracts growth suppression by wild-type p53. Oncogene. 2002;21:6722-6728. |
| 8. | Donehower LA. The p53-deficient mouse: a model for basic and applied cancer studies. Semin Cancer Biol. 1996;7:269-278. |
| 9. | Kanovsky M, Raffo A, Drew L, Rosal R, Do T, Friedman FK, Rubinstein P, Visser J, Robinson R, Brandt-Rauf PW. Peptides from the amino terminal mdm-2-binding domain of p53, designed from conformational analysis, are selectively cytotoxic to transformed cells. Proc Natl Acad Sci USA. 2001;98:12438-12443. |
| 10. | Do TN, Rosal RV, Drew L, Raffo AJ, Michl J, Pincus MR, Friedman FK, Petrylak DP, Cassai N, Szmulewicz J. Preferential induction of necrosis in human breast cancer cells by a p53 peptide derived from the MDM2 binding site. Oncogene. 2003;22:1431-1444. |
| 11. | Haupt Y, Maya R, Kazaz A, Oren M. Mdm2 promotes the rapid degradation of p53. Nature. 1997;387:296-299. |
| 12. | Kubbutat MH, Jones SN, Vousden KH. Regulation of p53 stability by Mdm2. Nature. 1997;387:299-303. |
| 13. | Laín S. Protecting p53 from degradation. Protecting p53 from degradation. Biochem Soc Trans. 2003;31:482-485. |
| 14. | Momand J, Jung D, Wilczynski S, Niland J. The MDM2 gene amplification database. Nucleic Acids Res. 1998;26:3453-3459. |
| 15. | Rosal R, Brandt-Rauf P, Pincus MR, Wang H, Mao Y, Li Y, Fine RL. The role of alpha-helical structure in p53 peptides as a determinant for their mechanism of cell death: necrosis versus apoptosis. Adv Drug Deliv Rev. 2005;57:653-660. |
| 16. | Smith DJ, Leil TA, Liu X. Neurotrophin-4 is required for tolerance to morphine in the mouse. Neurosci Lett. 2003;340:103-106. |
| 17. | Timmusk T, Belluardo N, Metsis M, Persson H. Widespread and developmentally regulated expression of neurotrophin-4 mRNA in rat brain and peripheral tissues. Eur J Neurosci. 1993;5:605-613. |
| 18. | Ip NY, Ibáñez CF, Nye SH, McClain J, Jones PF, Gies DR, Belluscio L, Le Beau MM, Espinosa R 3rd, Squinto SP. Mammalian neurotrophin-4: structure, chromosomal localization, tissue distribution, and receptor specificity. Proc Natl Acad Sci USA. 1992;89:3060-3064. |
| 19. | Fan G, Egles C, Sun Y, Minichiello L, Renger JJ, Klein R, Liu G, Jaenisch R. Knocking the NT4 gene into the BDNF locus rescues BDNF deficient mice and reveals distinct NT4 and BDNF activities. Nat Neurosci. 2000;3:350-357. |
| 20. | Lever AM, Strappe PM, Zhao J. Lentiviral vectors. J Biomed Sci. 2004;11:439-49. |
| 21. | Naldini L, Blömer U, Gallay P, Ory D, Mulligan R, Gage FH, Verma IM, Trono D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263-267. |
| 22. | Zufferey R, Nagy D, Mandel RJ, Naldini L, Trono D. Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat Biotechnol. 1997;15:871-875. |