Published online Dec 14, 2009. doi: 10.3748/wjg.15.5770
Revised: November 6, 2009
Accepted: November 13, 2009
Published online: December 14, 2009
Recent technological advances in miniaturization have allowed for a confocal scanning microscope to be integrated into a conventional flexible endoscope, or into trans-endoscopic probes, a technique now known as confocal endomicroscopy or confocal laser endomicroscopy. This newly-developed technology has enabled endoscopists to collect real-time in vivo histological images or “virtual biopsies” of the gastrointestinal mucosa during endoscopy, and has stimulated significant interest in the application of this technique in clinical gastroenterology. This review aims to evaluate the current data on the technical aspects and the utility of this new technology in clinical gastroenterology and its potential impact in the future, particularly in the screening or surveillance of gastrointestinal neoplasia.
-
Citation: Palma GDD. Confocal laser endomicroscopy in the “
in vivo ” histological diagnosis of the gastrointestinal tract. World J Gastroenterol 2009; 15(46): 5770-5775 - URL: https://www.wjgnet.com/1007-9327/full/v15/i46/5770.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.5770
In recent years, endoscopic image quality has improved as the devices have advanced technologically. Although techniques such as chromoendoscopy, high resolution and magnification endoscopy, narrow band imaging, and auto-fluorescence imaging improve the visualization and detection of mucosal lesions, biopsy of the targeted lesion must still be performed for a formal histological diagnosis of cellular and architectural atypia.
Suspicious areas identified during endoscopy are targeted and biopsied or removed endoscopically. However, there are several disadvantages that may be associated with biopsies or endoscopic resection, including bleeding or perforation. Non-representative biopsies may miss relevant portions of tissue, leading to underestimation of the diagnosis. Random biopsy sampling or endoscopic resection for non-neoplastic lesions can also be time-consuming. It would be ideal if a definite diagnosis could be made during endoscopy without a biopsy.
Recent technological advances in miniaturization have allowed for a confocal scanning microscope to be integrated into a conventional flexible endoscope, or into trans-endoscopic probes, a technique now known as confocal endomicroscopy (CEM) or confocal laser endomicroscopy (CLE). This newly-developed technology has enabled endoscopists to collect real-time in vivo histological images or “virtual biopsies” of the gastrointestinal (GI) mucosa during endoscopy, and has stimulated significant interest in the application of this technique in clinical gastroenterology[1-8].
This report aims to evaluate the current data on the utility of this new technology in clinical gastroenterology and its potential impact in the future, particularly in the screening or surveillance of GI neoplasia.
Confocal microscopy has been used in the biological sciences since 1961 when the concept of optical sectioning of a biological specimen was introduced.
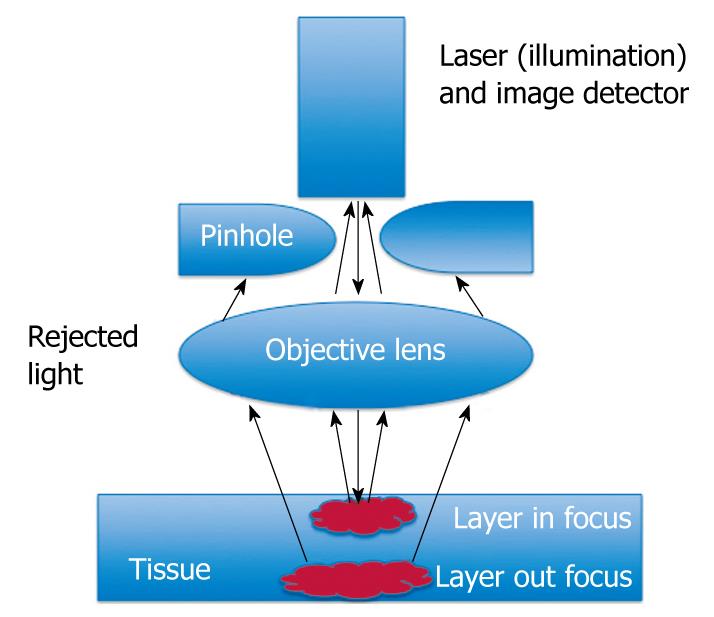
To create confocal images, a low-powered laser (an argon-ion laser that generates an excitation wavelength of 488 nm, blue laser light) is focused by an objective lens into a single point, within a fluorescent specimen. The same lens is used as both the condenser and objective folding. The point of illumination thus coincides with the point of detection within the specimen. Light emanating from that point is focused through a pinhole to a detector, and light emanating from outside the illuminated spot is rejected. The illumination and detection systems are in the same focal plane and are termed “confocal” (Figure 1).
After passing the pinhole, the fluorescent light is detected by a photodetection device (a photomultiplier tube or avalanche photodiode), transforming the light signal into an electrical one that is recorded by a computer. All detected signals from the illuminated spot are captured and measured.
As the laser scans over the plane of interest, a whole image is obtained pixel-by-pixel and line-by-line, whereas the brightness of a resulting image pixel corresponds to the relative intensity of detected fluorescent light.
The gray-scale image created is an optical section representing one focal plane within the examined specimen.
Confocal microscopy provides the capacity for direct, non-invasive, serial optical sectioning of intact, thick, living specimens with a minimum of sample preparation as well as a marginal improvement in lateral resolution. Because confocal images depend on fluorescence, a fluorescent dye (contrast agent) is required to make objects visible.
A fluorescent contrast agent is used and is needed to achieve high contrast images using CEM. Potentially suitable agents in humans are fluorescein, acriflavine, tetracycline or cresyl violet. The contrast agents can be applied systemically (fluorescein, tetracycline) or topically (acriflavine, cresyl violet) by using a spraying catheter. Of these, intravenous fluorescein sodium (10%) and topically applied acriflavine (0.2%) have been most commonly used in humans. No data is so far available on the use of tetracycline and cresyl violet.
Fluorescein is an agent used for diagnostic fluorescein angiography or angioscopy of the retina and iris vasculature.
After intravenous injection, fluorescein binds extensively to serum albumin in the bloodstream. The unbound contrast diffuses across capillaries, entering the tissue and staining the extracellular matrix of the surface epithelium and the lamina propria for up to 30 min[9]. Cell nuclei and mucin are not stained by fluorescein and therefore appear dark. The mucosal structures that can be identified after fluorescein administration include enterocytes, cellular infiltrate, surface epithelial cells, blood vessels, and red blood cells. Fluorescein is a highly safe agent whose major side effects are short term (1-2 h) and include yellowish skin discoloration and 1-2 d of bright yellow-colored urine. Nausea and vomiting were reported during angiography and were transient and minor. Serious side effects, such as anaphylaxis or cardiac or respiratory effects, are extremely rare, and to date, have not been recorded in CEM.
Topical acriflavine is highly specific for labeling acidic constituents, and stains the nuclei of superficial layers of the mucosa. The staining provides clear visualization of cell nuclei in the uppermost mucosa and may allow better differentiation between intra-epithelial neoplasia and cancer of the GI tract. Although there has been a hypothetical concern about the risk of mutagenesis, no severe adverse reactions have been reported after the topical use of acriflavine. However, concerns about DNA damage by acriflavine stain have reduced its use in humans.
Fluorescein and acriflavine can also be used simultaneously which adds to the labeling properties.
CLE can be performed currently with 2 devices: (1) integrated into an endoscope (Pentax, Tokio, Japan, herein termed eCLE); and (2) as a stand-alone probe (herein termed pCLE) capable of passage through the accessory channel of most endoscopes (Cellvizio, Mauna Kea Technologies, Paris, France)[10-14].
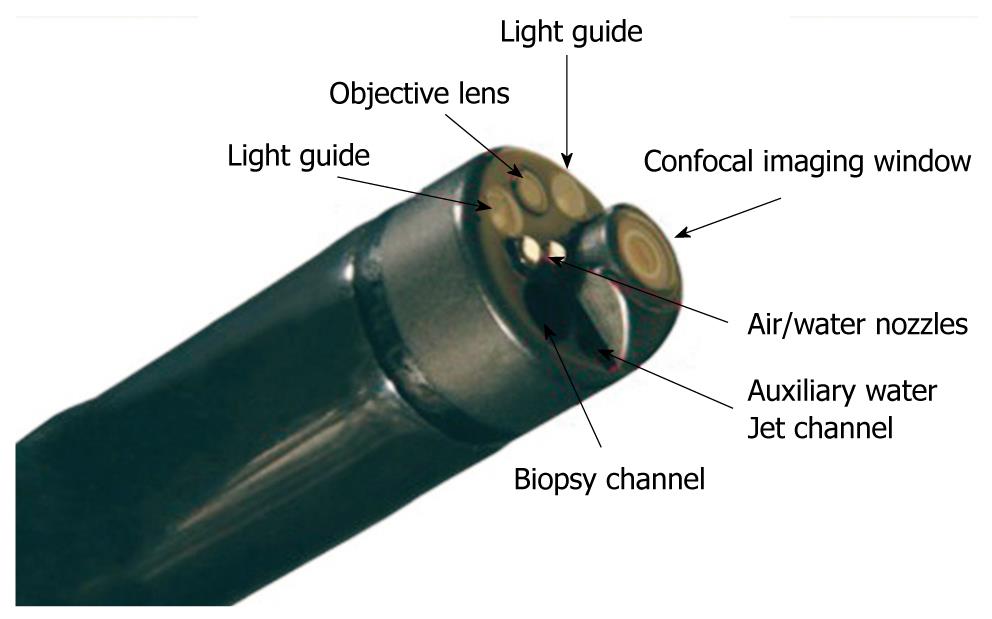
The components of the confocal laser endoscope are based on the integration of a confocal laser microscope in the distal tip of a conventional video endoscope, which enables confocal microscopy in addition to standard video endoscopy (Figure 2). The diameter of both the distal tip and the insertion tube is 12.8 mm. The distal tip contains an air and water jet nozzle, 2 light guides, an auxiliary water jet channel (used for topical application of the contrast agent) and a 2.8 mm working channel. This imaging system provides confocal imaging using an incident 488 nm wavelength laser, and enables the detection of fluorescence of 505-585 nm wavelength, with reduced image noise compared with the reflectance confocal systems. CLE imaging data are collected at a scan rate of rate of 1.6 frames per second (1024 × 512 pixels) or 0.8 frames per second (1024 × 1024 pixels) with an adjustable depth of scanning ranging from 0 to 250 μm, a field of view of 475 μm × 475 μm, a lateral resolution of 0.7 μm, and an axial resolution of 7 μm. Confocal images are generated simultaneously with the endoscopic images and the endoscope working channel can still be used.
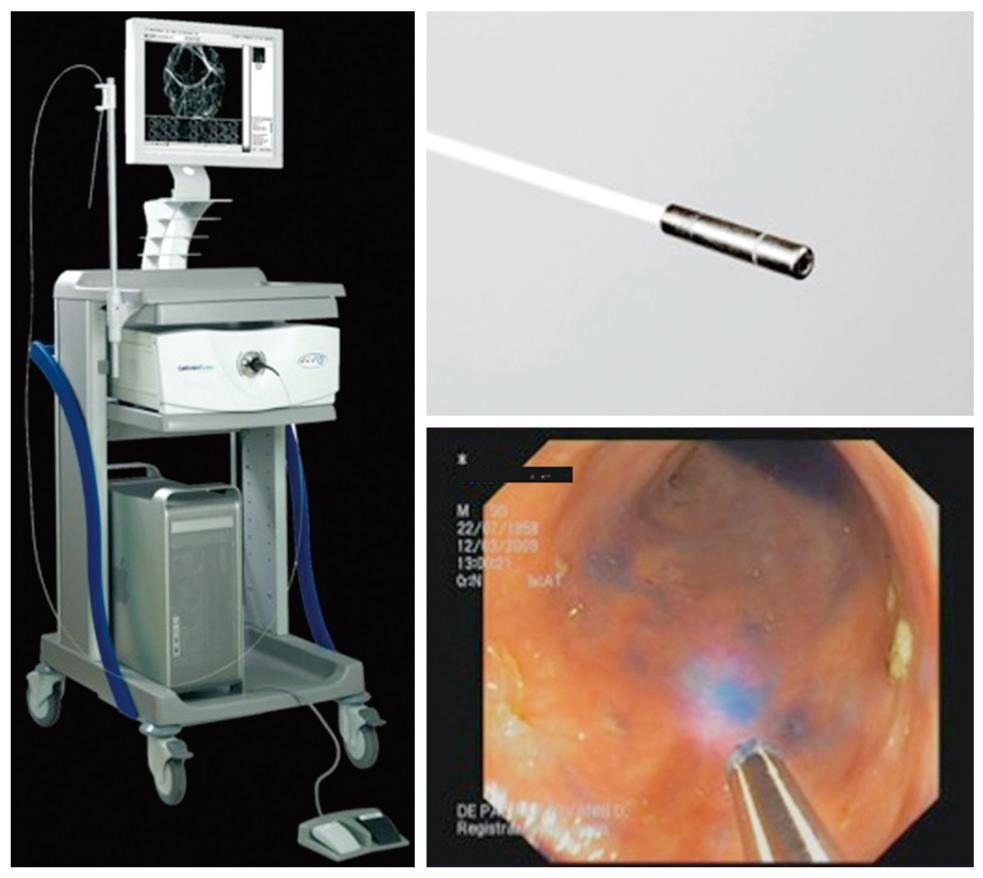
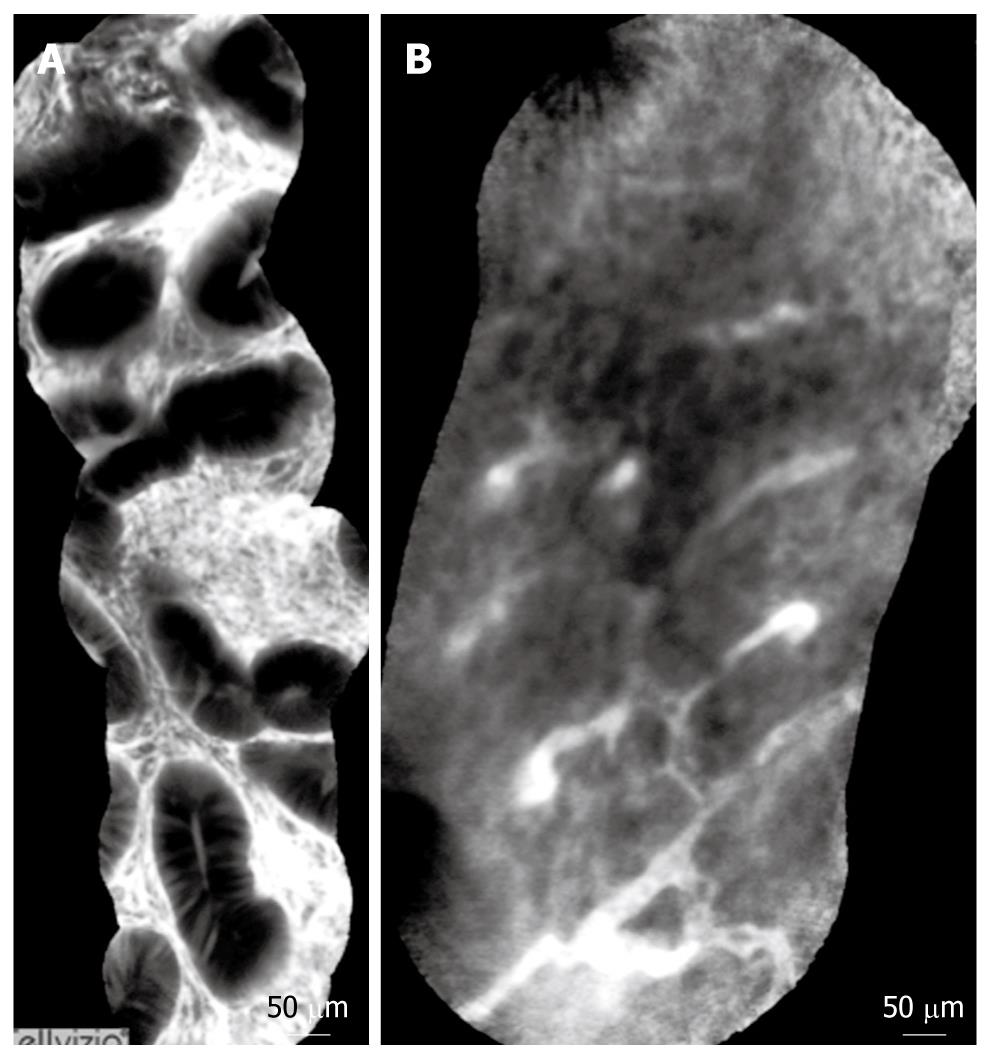
The Cellvizio® Endomicroscopy System (Figure 3) is based on a different catheter probe with a semiconductor laser that oscillates at 488 nm. The latest model of Cellvizio confocal miniprobes created for GI tract applications include CholangioFlex, GastroFlex, ColoFlex, GastroFlex-UHD, and ColoFlex-UHD. CholangioFlex probes designed for use during endoscopic retrograde cholangiopancreatography require an endoscope accessory channel of at least 1.2 mm, whereas the other probes, which are designed for use in esophagogastroduodenoscopy and colonoscopy, require a channel larger than 2.8 mm. All probes generate dynamic (12 frames per second) images with a scanning field of 30 000 pixels.
This system has a field of view of 240-600 μm (CholangioFlex probes: 325 μm; GastroFlex and ColoFlex: 600 μm; GastroFlex-UHD and ColoFlex-UHD: 240 μm) with a lateral resolution of 1-3.5 μm (the lateral resolution for CholangioFlex and for GastroFlex and ColoFlex probes is 3.5 μm; the lateral resolution for GastroFlex-UHD and ColoFlex-UHD is 1 μm). This system has a fixed imaging plane depth, and different confocal miniprobes are required to vary the depth of imaging. The depth of imaging for CholangioFlex probes is 40-70 μm, 70-130 μm for GastroFlex and ColoFlex), and 55-65 μm for GastroFlex-UHD and ColoFlex-UHD.
Single video frames are reconstructed by a special computer algorithm (‘‘mosaicing’’) in an image with an enlarged field of view (4 mm × 2 mm).
Once a suspicious area of the mucosa is identified, contrast agents (fluorescein and/or acriflavine) can be given, and a CEM examination of the targeted area is performed by placing the distal tip of the endoscope or the distal tip of the catheter probe against the targeted mucosa. Gentle suction and/or an endoscopic cap can be used to stabilize the equipment and minimize excessive movement, which is important to reduce movement artifacts during imaging. At the area of interest, images can be obtained using an image-capture foot pedal and stored digitally.
The orientation of the “cut” plane of confocal in vivo histological images is horizontal whereas in conventional histological specimens it is longitudinal. CLE images cannot display a simultaneous overview of the mucosal and submucosal structures, unlike conventional histology. CLE images provide a thorough view of the mucosal architecture and allow rapid differentiation between normal, regenerative, and neoplastic mucosa of the GI tract. Differentiation between grades of intra-epithelial neoplasm by CEM, however, is not yet possible with the currently available staining techniques.
Endoscopists who perform CEM require a fundamental knowledge of the normal and disease micro-architecture of the GI tract. An onsite pathologist or at least a review of the stored images by a pathologist and correlation with biopsy histology is recommended during the learning process.
Some studies of the learning curve for CLE performed by endoscopists demonstrates that highly accurate, efficient in vivo prediction of Barrett’s esophagus can be achieved after approximately 20-30 independently performed CLE procedures.
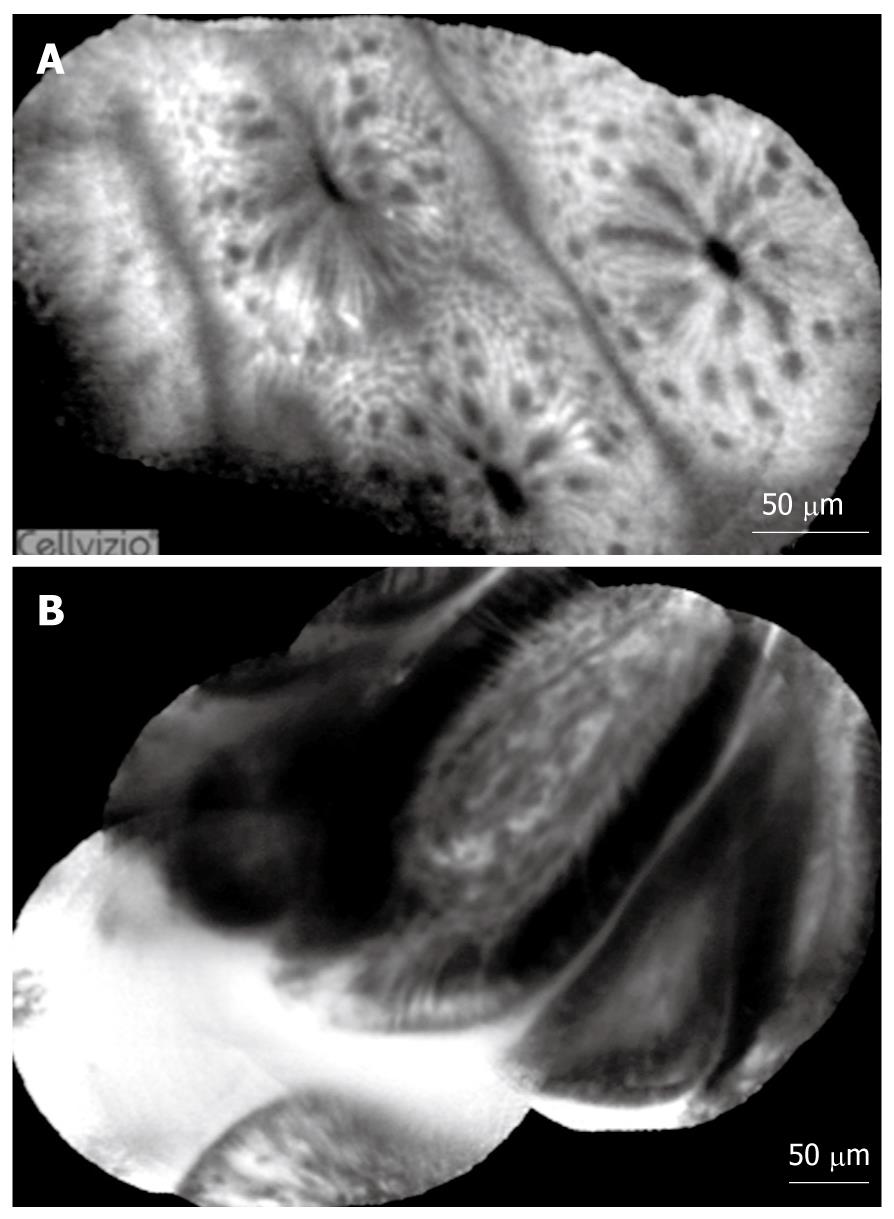
Normal and pathological aspects of CLE imaging of the upper and lower GI tract are shown in Tables 1, 2, 3, and Figures 3, 4, 5.
| Squamous cell epithelium | Squamous cell neoplasia | |
| Cellular criteria | Dark, homogeneous epithelial cells; regular architecture and clearly visible borders | Dark cells with different sizes; no clearly visible borders; irregular architecture |
| Vascular criteria | Capillaries directed to luminal epithelium without leakage of fluorescein | Twisted and irregular vessels; elongated capillaries; capillary leakage |
| Confocal diagnosis | Vessel architecture | Crypt architecture |
| Gastric-type epithelium | Capillaries with a regular shape only visible in the deeper parts of the mucosal layer | Regular columnar-lined epithelium with round glandular openings and typical cobblestone appearance |
| Barrett’s epithelium | Subepithelial capillaries with a regular shape underneath columnar-lined epithelium visible in the upper and deeper parts of the mucosal layer | Columnar-lined epithelium with intermittent dark mucin in goblet cells in the upper parts of the mucosal layer. In the deeper parts, villous, dark, regular cylindrical Barrett’s epithelial cells are present |
| Neoplasia | Irregular capillaries visible in the upper and deeper parts of the mucosal layer. Leakage of vessels leads to a heterogeneous and brighter signal intensity within the lamina propria | Black cells with irregular apical and distal borders and shapes, with strong dark contrast against the surrounding tissue |
| Grading | Vessel architecture | Crypt architecture |
| Normal | Hexagonal, honeycomb appearance that presents a network of capillaries outlining the stroma surrounding the luminal openings of the crypts | Regular luminal openings and distribution of crypts covered by a homogeneous layer of epithelial cells, including goblet cells |
| Regeneration | Hexagonal, honeycomb appearance with no increase or only a slight increase in the number of capillaries | Star-shaped luminal crypt openings or focal aggregation of regular-shaped crypts with a regular or reduced amount of goblet cells |
| Neoplasia | Dilated and distorted vessels with increased leakage; irregular architecture, with little or no orientation to the adjoining tissue | Ridge-lined irregular epithelial layer with loss of crypts and goblet cells; irregular cell architecture, with little or no mucin |
The current potential indications for CLE imaging are broad and include almost all current applications of endoscopic biopsy.
Unequivocally, this technology is best used in conjunction with other “red-flag” techniques because of its minute scanning area, and thus is only appropriate for classification of tissue at a site already detected by standard or optically enhanced endoscopy. Ideally, the no-dye “red-flag” techniques such as narrow band imaging or auto-fluorescence imaging, should be used to screen the mucosa for “areas of interest”, which can then be interrogated by CEM for a “histological” diagnosis. An example would be use of narrow-band imaging to detect regions of suspicion in Barrett’s esophagus, followed by CLE to confirm intraepithelial neoplasia, and guide immediate therapy.
The confocal laser endoscope can be used routinely for screening and surveillance. Suspicious lesions can be examined in a targeted fashion by placing the endomicroscopy window onto the lesion. Confocal images can be graduated according to cellular and vascular changes. The images correlate well with conventional histology after targeted biopsies.
Numerous studies have addressed the clinical applications of CLE, in particular in the study of precancerous lesions of the upper and lower GI tract[15,16].
In patients with Barrett’s esophagus, CLE can diagnose Barrett’s epithelium and Barrett’s-associated neoplastic changes with an accuracy > 90% for both e-CLE or p-CLE[17-23].
CLE in the stomach allows good visualization of normal and pathologic gastric pit patterns, making it a potentially useful tool for diagnosis of gastric cancer and precancerous conditions. Direct in vivo identification of Helicobacter pylori infection can be obtained[24-27].
In patients with suspected celiac disease, confocal endomicroscopy can demonstrate villous atrophy and an increased number of intraepithelial lymphocytes, enabling immediate in vivo diagnosis of celiac disease[28].
The presence of neoplastic changes in a colonic mucosa can be predict with high accuracy (> 95%). CLE has several potential roles in polyp management. The best-studied application is to distinguish between hyperplastic and adenomatous polyps, thus negating the need to remove hyperplastic polyps. In patients with long-term ulcerative colitis, chromoscopy with supplemental CEM, has recently been shown to further increase the yield for intraepithelial neoplasia above and beyond methylene blue. pCLE also has the capacity to differentiate normal from inflamed tissue, and thus target biopsies for the purpose of grading and mapping the extent of colitis[29-34].
CEM can also be helpful for the diagnosis of microscopic colitis in patients with chronic diarrhea. In patients with collagenous colitis, it allows direct in vivo visualization of collagenous bands under the epithelial layer of the colon, and in patients with lymphocytic colitis, it can demonstrate crypt distortion and an increased distance between the colonic crypt. Thus, CLE has the potential to replace or direct a large number of random biopsies in patients with chronic diarrhea, where the confocal image is normal[35-37].
Pancreato-biliary applications are under way using the probe-based CLE. The major role of pCLE in the bile duct is likely to detect cancer in indeterminate bile duct and pancreatic strictures[38,39].
CLE is a rapidly emerging field of gastroenterology that bridges the interface between endoscopy and histology. It further expands our ability to image living tissue in real time and to provide therapy in the same setting. The immediate impact will be the ability to target biopsies much more precisely, and eliminate a large number of random biopsies. Currently available devices for CEM have a very narrow field of view and allow only visualization of the superficial mucosal layer of the GI tract. Further technological developments are needed to enlarge the field of view, which will facilitate the use of CEM for cancer screening and surveillance. Increased depth of penetration is also needed to assess depth of invasion during cancer staging.
Peer reviewer: Dr. William Kemp, MB, BS (Hons), FRACP, Department of Gastroenterology, Alfred Hospital, PO Box 315 Prahran, 55 Commercial Road, Melbourne 3181, Australia
S- Editor Wang JL L- Editor Cant MR E- Editor Ma WH
| 1. | Wang TD. Confocal microscopy from the bench to the bedside. Gastrointest Endosc. 2005;62:696-697. |
| 2. | Polglase AL, McLaren WJ, Skinner SA, Kiesslich R, Neurath MF, Delaney PM. A fluorescence confocal endomicroscope for in vivo microscopy of the upper- and the lower-GI tract. Gastrointest Endosc. 2005;62:686-695. |
| 3. | Kiesslich R, Goetz M, Neurath MF. Confocal laser endomicroscopy for gastrointestinal diseases. Gastrointest Endosc Clin N Am. 2008;18:451-466, viii. |
| 4. | Kantsevoy SV, Adler DG, Conway JD, Diehl DL, Farraye FA, Kaul V, Kethu SR, Kwon RS, Mamula P, Rodriguez SA. Confocal laser endomicroscopy. Gastrointest Endosc. 2009;70:197-200. |
| 5. | Venkatesh K, Cohen M, Evans C, Delaney P, Thomas S, Taylor C, Abou-Taleb A, Kiesslich R, Thomson M. Feasibility of confocal endomicroscopy in the diagnosis of pediatric gastrointestinal disorders. World J Gastroenterol. 2009;15:2214-2219. |
| 6. | Dunbar K, Canto M. Confocal endomicroscopy. Curr Opin Gastroenterol. 2008;24:631-637. |
| 7. | Kiesslich R, Goetz M, Neurath MF. Virtual histology. Best Pract Res Clin Gastroenterol. 2008;22:883-897. |
| 8. | Nguyen NQ, Leong RW. Current application of confocal endomicroscopy in gastrointestinal disorders. J Gastroenterol Hepatol. 2008;23:1483-1491. |
| 9. | Becker V, von Delius S, Bajbouj M, Karagianni A, Schmid RM, Meining A. Intravenous application of fluorescein for confocal laser scanning microscopy: evaluation of contrast dynamics and image quality with increasing injection-to-imaging time. Gastrointest Endosc. 2008;68:319-323. |
| 10. | Polglase AL, McLaren WJ, Delaney PM. Pentax confocal endomicroscope: a novel imaging device for in vivo histology of the upper and lower gastrointestinal tract. Expert Rev Med Devices. 2006;3:549-556. |
| 11. | Becker V, Vercauteren T, von Weyhern CH, Prinz C, Schmid RM, Meining A. High-resolution miniprobe-based confocal microscopy in combination with video mosaicing (with video). Gastrointest Endosc. 2007;66:1001-1007. |
| 12. | Meining A, Saur D, Bajbouj M, Becker V, Peltier E, Hofler H, von Weyhern CH, Schmid RM, Prinz C. In vivo histopathology for detection of gastrointestinal neoplasia with a portable, confocal miniprobe: an examiner blinded analysis. Clin Gastroenterol Hepatol. 2007;5:1261-1267. |
| 13. | Leeuwenhoek meets Kussmaul: the evolution of endoscopist to endo-pathologist. Gastroenterology. 2006;131:347-349. |
| 14. | Wallace MB, Fockens P. Probe-based confocal laser endomicroscopy. Gastroenterology. 2009;136:1509-1513. |
| 15. | Hoffman A, Goetz M, Vieth M, Galle PR, Neurath MF, Kiesslich R. Confocal laser endomicroscopy: technical status and current indications. Endoscopy. 2006;38:1275-1283. |
| 16. | Liu H, Li YQ, Yu T, Zhao YA, Zhang JP, Zhang JN, Guo YT, Xie XJ, Zhang TG, Desmond PV. Confocal endomicroscopy for in vivo detection of microvascular architecture in normal and malignant lesions of upper gastrointestinal tract. J Gastroenterol Hepatol. 2008;23:56-61. |
| 17. | Dunbar KB, Okolo P 3rd, Montgomery E, Canto MI. Confocal laser endomicroscopy in Barrett’s esophagus and endoscopically inapparent Barrett’s neoplasia: a prospective, randomized, double-blind, controlled, crossover trial. Gastrointest Endosc. 2009;70:645-654. |
| 18. | Pohl H, Rosch T, Vieth M, Koch M, Becker V, Anders M, Khalifa AC, Meining A. Miniprobe confocal laser microscopy for the detection of invisible neoplasia in patients with Barrett's oesophagus. Gut. 2008;57:1648-1653. |
| 19. | Becker V, Vieth M, Bajbouj M, Schmid RM, Meining A. Confocal laser scanning fluorescence microscopy for in vivo determination of microvessel density in Barrett’s esophagus. Endoscopy. 2008;40:888-891. |
| 20. | Liu H, Li YQ, Yu T, Zhao YA, Zhang JP, Zuo XL, Li CQ, Zhang JN, Guo YT, Zhang TG. Confocal laser endomicroscopy for superficial esophageal squamous cell carcinoma. Endoscopy. 2009;41:99-106. |
| 21. | Pech O, Rabenstein T, Manner H, Petrone MC, Pohl J, Vieth M, Stolte M, Ell C. Confocal laser endomicroscopy for in vivo diagnosis of early squamous cell carcinoma in the esophagus. Clin Gastroenterol Hepatol. 2008;6:89-94. |
| 22. | Deinert K, Kiesslich R, Vieth M, Neurath MF, Neuhaus H. In-vivo microvascular imaging of early squamous-cell cancer of the esophagus by confocal laser endomicroscopy. Endoscopy. 2007;39:366-368. |
| 23. | Goetz M, Hoffman A, Galle PR, Neurath MF, Kiesslich R. Confocal laser endoscopy: new approach to the early diagnosis of tumors of the esophagus and stomach. Future Oncol. 2006;2:469-476. |
| 24. | Kakeji Y, Yamaguchi S, Yoshida D, Tanoue K, Ueda M, Masunari A, Utsunomiya T, Imamura M, Honda H, Maehara Y. Development and assessment of morphologic criteria for diagnosing gastric cancer using confocal endomicroscopy: an ex vivo and in vivo study. Endoscopy. 2006;38:886-890. |
| 25. | Guo YT, Li YQ, Yu T, Zhang TG, Zhang JN, Liu H, Liu FG, Xie XJ, Zhu Q, Zhao YA. Diagnosis of gastric intestinal metaplasia with confocal laser endomicroscopy in vivo: a prospective study. Endoscopy. 2008;40:547-553. |
| 26. | Li WB, Zuo XL, Zuo F, Gu XM, Yu T, Zhao YA, Zhang TG, Zhang JP, Li YQ. Characterization and identification of gastric hyperplastic polyps and adenomas by confocal laser endomicroscopy. Surg Endosc. 2009;Epub ahead of print. |
| 27. | Kiesslich R, Goetz M, Burg J, Stolte M, Siegel E, Maeurer MJ, Thomas S, Strand D, Galle PR, Neurath MF. Diagnosing Helicobacter pylori in vivo by confocal laser endoscopy. Gastroenterology. 2005;128:2119-2123. |
| 28. | Zambelli A, Villanacci V, Buscarini E, Lupinacci G, De Grazia F, Brambilla G, Menozzi F, La Mantia L, Bassotti G. Confocal laser endomicroscopy in celiac disease: description of findings in two cases. Endoscopy. 2007;39:1018-1020. |
| 29. | Kiesslich R, Goetz M, Vieth M, Galle PR, Neurath MF. Technology insight: confocal laser endoscopy for in vivo diagnosis of colorectal cancer. Nat Clin Pract Oncol. 2007;4:480-490. |
| 30. | Hurlstone DP, Tiffin N, Brown SR, Baraza W, Thomson M, Cross SS. In vivo confocal laser scanning chromo-endomicroscopy of colorectal neoplasia: changing the technological paradigm. Histopathology. 2008;52:417-426. |
| 31. | Hurlstone DP, Baraza W, Brown S, Thomson M, Tiffin N, Cross SS. In vivo real-time confocal laser scanning endomicroscopic colonoscopy for the detection and characterization of colorectal neoplasia. Br J Surg. 2008;95:636-645. |
| 32. | Goetz M, Toermer T, Vieth M, Dunbar K, Hoffman A, Galle PR, Neurath MF, Delaney P, Kiesslich R. Simultaneous confocal laser endomicroscopy and chromoendoscopy with topical cresyl violet. Gastrointest Endosc. 2009;70:959-968. |
| 33. | Hurlstone DP, Brown S. Techniques for targeting screening in ulcerative colitis. Postgrad Med J. 2007;83:451-460. |
| 34. | Hurlstone DP, Thomson M, Brown S, Tiffin N, Cross SS, Hunter MD. Confocal endomicroscopy in ulcerative colitis: differentiating dysplasia-associated lesional mass and adenoma-like mass. Clin Gastroenterol Hepatol. 2007;5:1235-1241. |
| 35. | Meining A, Schwendy S, Becker V, Schmid RM, Prinz C. In vivo histopathology of lymphocytic colitis. Gastrointest Endosc. 2007;66:398-399, discussion 400. |
| 36. | Kiesslich R, Hoffman A, Goetz M, Biesterfeld S, Vieth M, Galle PR, Neurath MF. In vivo diagnosis of collagenous colitis by confocal endomicroscopy. Gut. 2006;55:591-592. |
| 37. | Zambelli A, Villanacci V, Buscarini E, Bassotti G, Albarello L. Collagenous colitis: a case series with confocal laser microscopy and histology correlation. Endoscopy. 2008;40:606-608. |
| 38. | Meining A, Phillip V, Gaa J, Prinz C, Schmid RM. Pancreaticoscopy with miniprobe-based confocal laser-scanning microscopy of an intraductal papillary mucinous neoplasm (with video). Gastrointest Endosc. 2009;69:1178-1180. |
| 39. | Meining A, Frimberger E, Becker V, Von Delius S, Von Weyhern CH, Schmid RM, Prinz C. Detection of cholangiocarcinoma in vivo using miniprobe-based confocal fluorescence microscopy. Clin Gastroenterol Hepatol. 2008;. |