Published online Nov 7, 2009. doi: 10.3748/wjg.15.5165
Revised: September 18, 2009
Accepted: September 25, 2009
Published online: November 7, 2009
AIM: To develop a protocol for direct hepatic lineage differentiation from early developmental progenitors to a population of mature hepatocytes.
METHODS: Hepatic progenitor cells and then mature hepatocytes from mouse embryonic stem (ES) cells were obtained in a sequential manner, induced by valproic acid (VPA) and cytokines (hepatocyte growth factor, epidermal growth factor and insulin). Morphological changes of the differentiated cells were examined by phase-contrast microscopy and electron microscopy. Reverse transcription polymerase chain reaction and immunocytochemical analyses were used to evaluate the gene expression profiles of the VPA-induced hepatic progenitors and the hepatic progenitor-derived hepatocytes. Glycogen storage, cytochrome P450 activity, transplantation assay, differentiation of bile duct-like structures and tumorigenic analyses were performed for the functional identification of the differentiated cells. Furthermore, FACS and electron microscopy were used for the analyses of cell cycle profile and apoptosis in VPA-induced hepatic differentiated cells.
RESULTS: Based on the combination of VPA and cytokines, mouse ES cells differentiated into a uniform and homogeneous cell population of hepatic progenitor cells and then matured into functional hepatocytes. The progenitor population shared several characteristics with ES cells and hepatic stem/progenitor cells, and represented a novel progenitor cell between ES and hepatic oval cells in embryonic development. The differentiated hepatocytes from progenitor cells shared typical characteristics with mature hepatocytes, including the patterns of gene expression, immunological markers, in vitro hepatocyte functions and in vivo capacity to restore acute-damaged liver function. In addition, the differentiation of hepatic progenitor cells from ES cells was accompanied by significant cell cycle arrest and selective survival of differentiating cells towards hepatic lineages.
CONCLUSION: Hepatic cells of different developmental stages from early progenitors to matured hepatocytes can be acquired in the appropriate order based on sequential induction with VPA and cytokines.
- Citation: Dong XJ, Zhang GR, Zhou QJ, Pan RL, Chen Y, Xiang LX, Shao JZ. Direct hepatic differentiation of mouse embryonic stem cells induced by valproic acid and cytokines. World J Gastroenterol 2009; 15(41): 5165-5175
- URL: https://www.wjgnet.com/1007-9327/full/v15/i41/5165.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.5165
| Gene | Sequence (5'-3') | Product size (bp) | Annealing temperature (°C) |
| β-actin-F | TTCCTTCTTGGGTATGGAAT | 200 | 55 |
| β-actin-R | GAGCAATGATCTTGATCTTC | ||
| AFP-F | CACTGCTGCAACTCTTCGTA | 300 | 52 |
| AFP-R | CTTTGGACCCTCTTCTGTGA | ||
| HNF3β-F | GACCTCTTCCCTTTCTACCG | 551 | 51 |
| HNF3β-R | TTGAAGGCGTAATGGTGC | ||
| TTR-F | TGCCTCGCTGGACTGGTAT | 334 | 52 |
| TTR-R | CAGAGTCGTTGGCTGTGAA | ||
| DPPIV-F | GATTCCATACCCAAAGGC | 587 | 55 |
| DPPIV-R | GGTCACAACTAAGGCACT | ||
| ALB-F | TCTTCGTCTCCGGCTCTG | 475 | 55 |
| ALB-R | CTGGCAACTTCATGCAAA | ||
| OCT4-F | GGCGTTCTCTTTGGAAAGGTGTTC | 313 | 57 |
| OCT4-R | CTCGAACCACATCCTTCTCT | ||
| AAT-F | AGAACCATTATCAGGCAGAA | 675 | 55 |
| AAT-R | AATAAGGAACGGCTAGTAAGA | ||
| DLK-F | GGGGTGACTTCCGTTGC | 510 | 52 |
| DLK-R | GCTCCTCGCCGCTGTTAT | ||
| HNF4-F | CTTCCAAGAGCTGCAGATTG | 517 | 55 |
| HNF4-R | CTTGTAGGATTCAGATCCCG | ||
| G6P-F | TCAATCTCCTCTGGGTGGC | 602 | 52 |
| G6P-R | GGCAAAGGGTGTAGTGTCAAG | ||
| TAT-F | CTTCAGTGCTGGATGTTCGC | 619 | 55 |
| TAT-R | CAGGGATTGGACGGGTTGTT | ||
| TDO-F | TAAACAGAGCCAGCAAAG | 868 | 56 |
| TDO-R | ATGAGCGTGTCAATGTCC | ||
| BG-F | CGTGAAGGATACGGGAGT | 581 | 55 |
| BG-R | CAGAGTTATTGACGAGGC | ||
| GGT-F | TGTCCCTGGTGAAATCCG | 577 | 55 |
| GGT-R | GGCATAGGCAAACCGAAA |
Hepatocyte transplantation is an alternative therapy strategy for liver failure or end-stage liver diseases[1,2]. However, the shortage of donor hepatocytes and the difficulties for large scale hepatocyte amplification and function maintenance limit the clinical application of this cell-based therapy[3]. Embryonic stem (ES) cells, known for their capacity to proliferate indefinitely and differentiate into almost all types of cells including hepatocytes, have raised the hope of cellular replacement therapy for liver failure. The essential prerequisite for this purpose is to develop well-defined protocols for directing cellular differentiation into hepatic lineage, followed by selective isolation and proliferation in vitro. There have been several protocols available up till now for hepatic fate specification from ES cells, for example, the protocols based on spontaneous differentiation, combination of growth factors, co-culture with non-parenchymal liver cells and gene modifications[4-8]. However, most of the protocols currently used were devised to induce mature hepatocytes by using embryoid bodies that may result in low yield or purity of functional hepatocytes. Little documentation exists about a strategy for acquiring different developmental hepatic cells, for example, guiding differentiation of ES cells to hepatic progenitors and then to an entire population of mature hepatocytes to meet the requirements of precise study regarding the mechanisms of hepatic differentiation and potential clinical applications. Furthermore, more concise and reproducible methods to acquire abundant hepatic cells still remain to be developed, among which the direct hepatic differentiation from an ES monolayer without using embryoid bodies is most promising[9].
Valproic acid (VPA), a histone deacetylase (HDAC) inhibitor, has been used as a new class of chemotherapeutic drug for cancer clinical purposes and as an inducer for stem cell differentiation[10-13]. It has exhibited profound therapeutic activity against hepatocellular carcinoma by inducing apoptosis and cell-cycle arrest[14-16], and dramatic effects on cellular differentiation from stem cells, including cardiomyocyte differentiation from ES cells, neuronal differentiation from neural stem cells, and osteogenic and hepatic differentiation from bone marrow-derived mesenchymal stem cells (MSC)[17-20]. In a previous study, we established a method for hepatic differentiation from MSC using VPA, which provided preliminary evidence that VPA could facilitate hepatic differentiation[21]. However, little is know about whether VPA can induce the hepatic differentiation of ES cells. Here, we report such research and the development of a protocol for direct hepatic lineage differentiation, from early developmental progenitors to a population of mature hepatocytes, based on sequential induction with VPA and cytokines. Results show that VPA can direct the hepatic specification of ES cells and largely participates in the differentiation of ES cells into hepatic progenitors. Further differentiation of hepatic progenitors into mature hepatocytes requires supplementation with cytokines. The present study may not only be helpful for the clinical application of hepatocyte transplantation, but also provide an in vitro research model for the better investigation and understanding of the entire developmental process of hepatocytes, from ES cells to hepatic progenitors, and then to mature hepatocytes. Furthermore, as VPA is an epigenetic modulator, so our results may also be of benefit to the research of mechanisms of epigenetic modifications during liver development.
VPA was purchased from Sigma (St Louis, MO); fetal bovine serum (FBS) was purchased from Hyclone (Rockville, MD); murine leukemia inhibitory factor (LIF) was purchased from Chemicon (Temecula, CA); mouse hepatocyte growth factor (mHGF), mouse epidermal growth factor (mEGF), oncostatin M (OSM), Insulin-Transferrin-Selenium (ITS), and collagen I were all from R&D systems (Minneapolis, MN); Matrigel was purchased from BD Biosciences (Palo Alto, CA); sheep anti-ALB antibodies were purchased from Biodesign (Saco, ME); rabbit anti-AFP, mouse anti-CK19 were from Dako (Copenhagen, Denmark); rat anti-OCT-4 was from R&D; mouse anti-SSEA-1 was from Developmental Hybridoma Bank of Iowa University; goat anti-mouse DLK was from Santa Cruz Biotechnology (Santa Cruz, CA, USA); rat anti-A6 was presented by Dr. Valentina Factor of NIH; FITC-conjugated bovine anti-sheep IgG, FITC-conjugated rabbit anti-goat IgG, FITC-conjugated goat anti-rabbit IgG, TRITC-conjugated goat anti-mouse IgG, TRITC-conjugated goat anti-rat IgG were all purchased from Dako; FITC-conjugated goat anti-mouse IgM was from Jackson Immunoresearch Laboratories Inc. All other reagents were from Sigma (St.Louis, MO).
Mouse ES D3 cells, provided by the Cell Biology Institute of the Chinese Academy of Sciences, were cultured on mitomycin C inactivated MEF feeder layers in high-glucose DMEM supplemented with 15% FBS, 2 mmol/L L-Glu, 0.1 mmol/L N-ME, 1% NEAA and 10 ng/mL murine LIF as described previously[22]. Briefly, MEF feeder cells were isolated from ICR mice at embryo day 13.5 and cultured at 37°C and 5% CO2. At approximately 80% confluence, the feeder cells were incubated with 10 μg/mL mitomycin C for 4 h and washed three times with PBS. Then the cells were replated at 8 × 104 cells/cm2 on to tissue culture flasks. After allowed for attachment overnight, the ES cells were seeded.
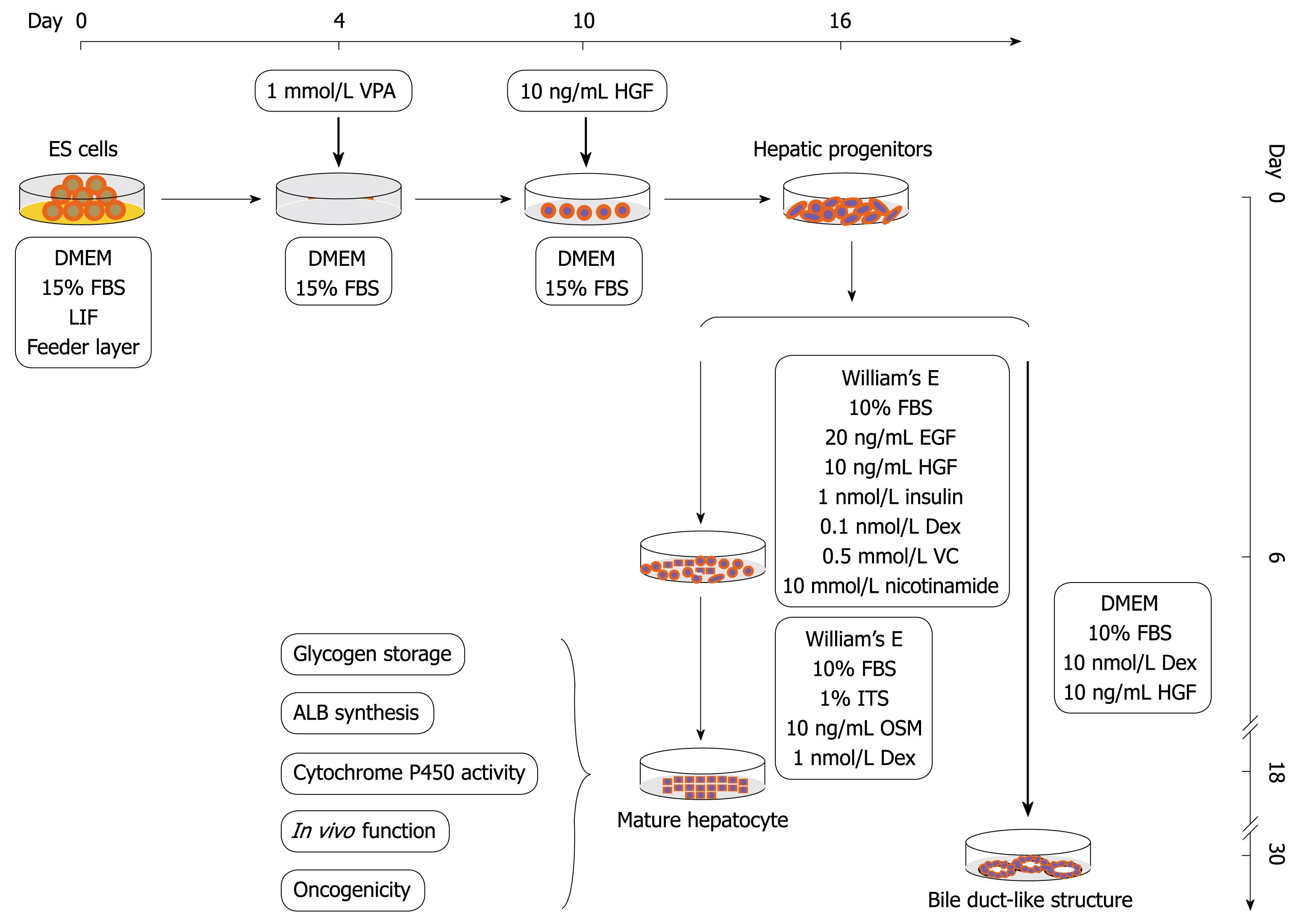
A protocol was designed to obtain the hepatic progenitor cells and then mature hepatocytes from mouse ES cells in a sequential manner (Figure 1). For differentiation of hepatic progenitor cells, ES cells were cultured in DME medium as described above with the exception of the withdrawal of feeder layer and LIF, and treated with 1 mmol/L VPA for 4-6 d, then the VPA was removed and recombinant mouse HGF 10 ng/mL was added for another 6-12 d until the hepatic progenitor cells became confluent. For differentiation of hepatocytes from hepatic progenitor cells, the progenitor cells were cultured in a William’s E medium supplemented with 20 ng/mL mEGF, 10 ng/mL mHGF, 10-6 mol/L insulin, 10-7 mol/L Dex, 0.5 mmol/L ascorbic acid diphosphate, 10 mmol/L nicotinamide and 10% FBS (maturation medium I) for 6 d, and then replaced with another William’s E medium containing 1% ITS, 10 ng/mL OSM and 10-6 mol/L Dex (maturation medium II) for another 6-12 d.
Gene expression analyses: Total RNA was extracted from the ES cells, hepatic progenitor cells, mature hepatocytes and adult mouse liver using Nucleospin RNA Kits (BD Biosciences, Palo Alto, CA). RNA samples were digested with DNase I for 15 min at room temperature. cDNAs were synthesized from 1 μg total RNA with a superscript III first-strand synthesis kit (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions, and amplified with the following program: initial denaturation at 95°C for 5 min followed by 30-40 cycles of 94°C for 30 s, 50-56°C for 30 s, 72°C for 30 s and final extension at 72°C for 10 min. The amplified products were analyzed by electrophoresis on 1.5% agarose gel and stained with ethidium bromide. All the primers used are listed in Table 1.
Immunocytochemical analyses: Cells were fixed using 4% paraformaldehyde (PFA) followed by ice-cooled methanol, and incubated with primary antibodies overnight at 4°C after blocking with 5% normal goat serum. For the application of SSEA-1 antigen, the cells were fixed with PFA only. Following washing with PBS three times, the cells were incubated with fluorescence-conjugated secondary antibodies, and examined under a Carl Zeiss confocol laser-scanning microscope.
Electron microscopy: Cells were fixed in 2.5% glutaraldehyde in 0.1 mmol/L phosphate buffer saline (pH 7.4) at 4°C for 24 h, post-fixed in 1% OsO4 for 1 h, and embedded in Epon 812. They were cut into 30-40 nm sections for ultrastructural evaluation using a Philips TECNAL-10 transmission electron microscope.
Glycogen storage: At the final stage of differentiation, the cells were assessed by periodic acid-Schiff reaction for glycogen storage as previously described[23]. Briefly, cells were fixed in Carnoy’s solution, oxidized in 1% periodic acid, treated with Schiff’s reagent and examined under a Nikon microscope.
Cytochrome P450 activity: Cytochrome P450 activity was examined by ethoxyresorufin O-dealkylase assay. Briefly, cells were maintained under the same conditions in the presence or absence of 5 μmol/L phenobarbital for 24 h followed by treatment with 5 μmol/L ethoxyresorufin for 2-3 h, and then observed under Carl Zeiss confocol laser-scanning microscope at 355 nm excitation and 581 nm emission.
Transplantation assay: To evaluate the in vivo function of differentiated hepatocytes, a transplantation assay was performed in CCl4-intoxicated mice as previously described[24]. Five ICR mice were injected intraperitoneally with 10% CCl4 in olive oil (1 mL/kg body weight). After 6 h, mice underwent intrasplenic transplantation of differentiated hepatocytes at 1 × 106 cells (0.1 mL of 1 × 107 cells/mL) per mouse. The injection site was ligated to prevent cell leakage and bleeding. Mice were then sacrificed 24 h after transplantation, and sera were collected separately. Liver function was assessed by measuring the total bilirubin (T-Bil), ALT, AST and urea levels. Ten CCl4-intoxicated and untreated mice were used as controls.
Differentiation of bile duct-like structures: To evaluate the differentiation potential of progenitors into cholangiocytes, a spontaneous and directed bile duct-like structural differentiation assay was designed. For spontaneous differentiation, the progenitors were inoculated into the collagen I coated 96-well plate, and cultured with William’s E medium supplemented with 10-6 mol/L Dex, 10 ng/mL mHGF for 20-40 d until the special bile duct-like structures appeared. For directed differentiation, the progenitors were seeded on a layer of Matrigel basement membrane matrix and cultured in the medium supplemented with 100 ng/mL mHGF and 50 ng/mL mEGF until the bile duct-like structures formed. Media were changed every 3 d.
Tumorigenic analyses: Undifferentiated ES cells and the hepatic progenitor cells were collected and suspended in DMEM medium (1 × 107 cells/mL). A 0.2 mL aliquot was injected subcutaneously into the backs of Balb/C nude mice. Each experimental group contained three animals. The animals were kept under a controlled lighting schedule with a 12-h dark period. Food and water were available ad libitum. All animals received humane care in compliance with institutional guidelines. Mice were sacrificed 6-8 wk after transplantation, the tumors and tumor-like tissues were fixed with formalin. After additional fixation in 4% paraformaldehyde for 2 h at 4°C, tissues were embedded in paraffin. Sections were stained with hematoxylin and eosin, and then microscopically observed.
Flow cytometry was performed to measure cell cycle distribution and apoptosis of VPA-treated cells. The measurements were made with a Becton Dickinson FACS Calibur machine, adapted for excitation with a 488 nm argon laser, and 582/42 nm band-pass filter for detecting propidium iodide emission.
The data are presented as mean ± SD. Statistical tests for the significance of differences between control and transplanted mice were performed by way of Student’s t test. P < 0.05 was considered as statistically significant.
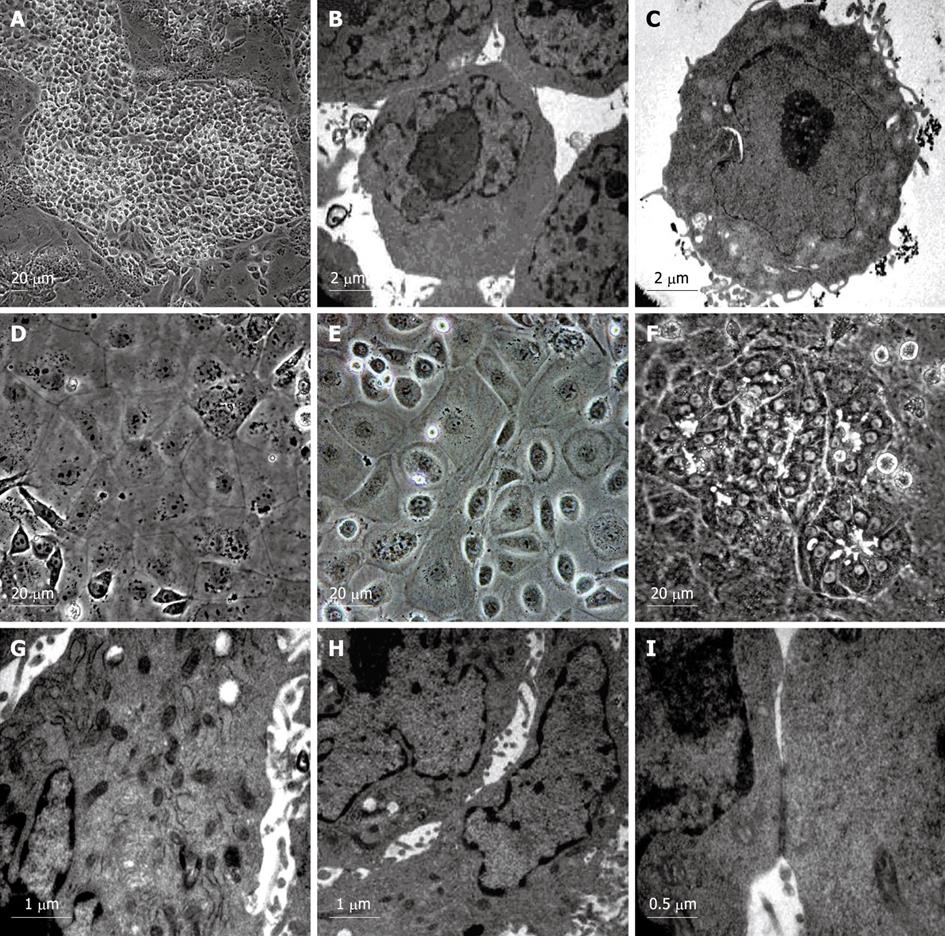
To examine whether VPA has an effect on hepatic fate differentiation from mouse ES cells, the ES cultures were subjected to VPA (1 mmol/L) after withdrawing feeder layers and LIF. After treatment for 4-6 d, a small round-shaped progenitor-like population appeared. These cells were then treated with recombinant mouse HGF (10 ng/mL) which allowed the promotion of cellular proliferation. After another 6-12 d, this progenitor-like population underwent rapid growth, and finally proliferated into confluence. The acquired progenitor-like cells exhibited 8-10 μm in diameter, scant cytoplasm and a high nuclear to cytoplasmic ratio (Figure 2A), and resembled the blast-like oval cells proliferating during severe liver injury or the hepatoblasts found in fetal liver. Electron microscopic observation showed that the progenitor-like cells had abundant cell surface microvilli and shared scanty organelles except for a few mitochondria as in ES cells (Figure 2B and C). Noticeably, it was found that few mature functional hepatocytes could be acquired by treating the ES cells with VPA independently, and further differentiation into mature hepatocytes needed the combination of various cytokines, which indicated that VPA probably participates mainly in the regulation of early hepatic differentiation.
For maturation of functional hepatocytes from hepatic progenitors, the progenitor cells were further cultured in William’s E medium (containing 10% FBS) supplemented with cytokines (EGF, HGF and insulin) and chemical inducers (Dex, ascorbic acid diphosphate and nicotinamide) for 6 d. Then, the cells were cultured in another medium containing ITS, OSM and Dex. After 6-12 d, two kinds of hepatocyte-like cells appeared. One exhibited typical hepatocyte features with a diameter of 20-40 μm, flattened and cuboidal morphology, and had acquired abundant granules in the cytoplasm (Figure 2D and E). The other displayed a rising/piled morphology with dark cytoplasm and light nuclei, and bile canaliculi-like structures were often found between these cells (Figure 2F). Ultrastructural analyses revealed that both kinds of hepatocyte-like cells contained numerous mitochondria and endoplasmic reticulum (Figure 2G). Bile canaliculi were also observed between adjacent cells and sealed with tight junctions (Figure 2H and I). These results showed that functional hepatocytes could be acquired from VPA-induced progenitors under sequential induction combinations.
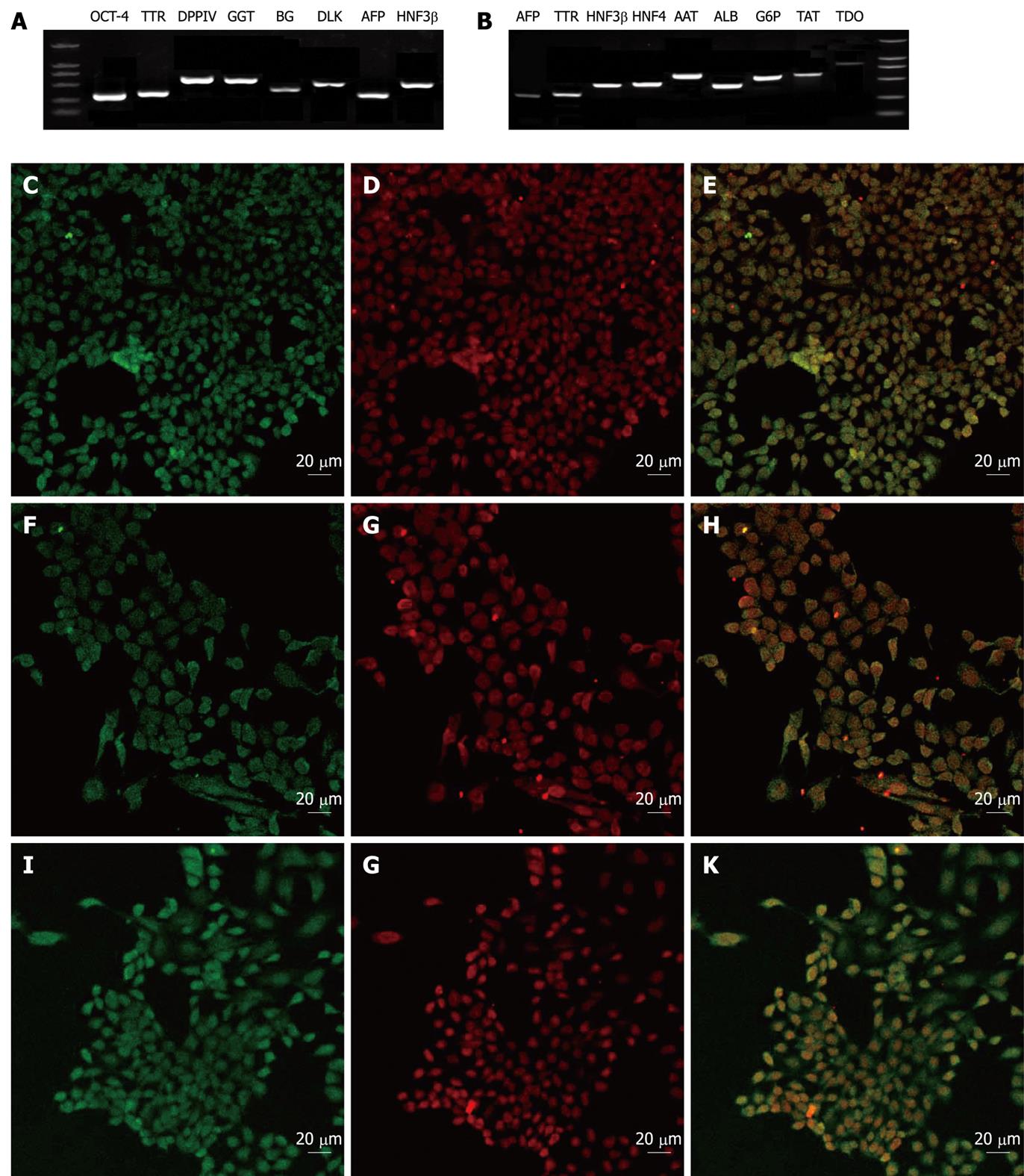
To evaluate the characterization of the VPA-induced hepatic progenitors and the hepatic progenitor-derived hepatocytes, a number of gene expression profiles were examined at mRNA and/or protein levels. The results showed that the undifferentiated ES cells expressed SSEA-1 and OCT-4, but no hepatic markers (data not shown). However, the progenitor cells expressed most typical markers of hepatic/hepatic stem cells, such as AFP, TTR, DPPIV, GGT, BG, HNF3β, CK19 and Dlk (Figure 3), but did not express the ES marker SSEA-1 or mature hepatocyte marker ALB (data not shown). Interestingly, the progenitor cells expressed the ES cell marker OCT-4, which suggested that the VPA-induced hepatic progenitor cells may share partial properties of ES cells (Figure 3A and D). Accordingly, the hepatic progenitor-derived hepatocytes expressed typical markers of mature liver cells, including ALB, AAT, HNF4, G6p, TAT, AFP, TTR, HNF3β and TDO (Figure 3B).
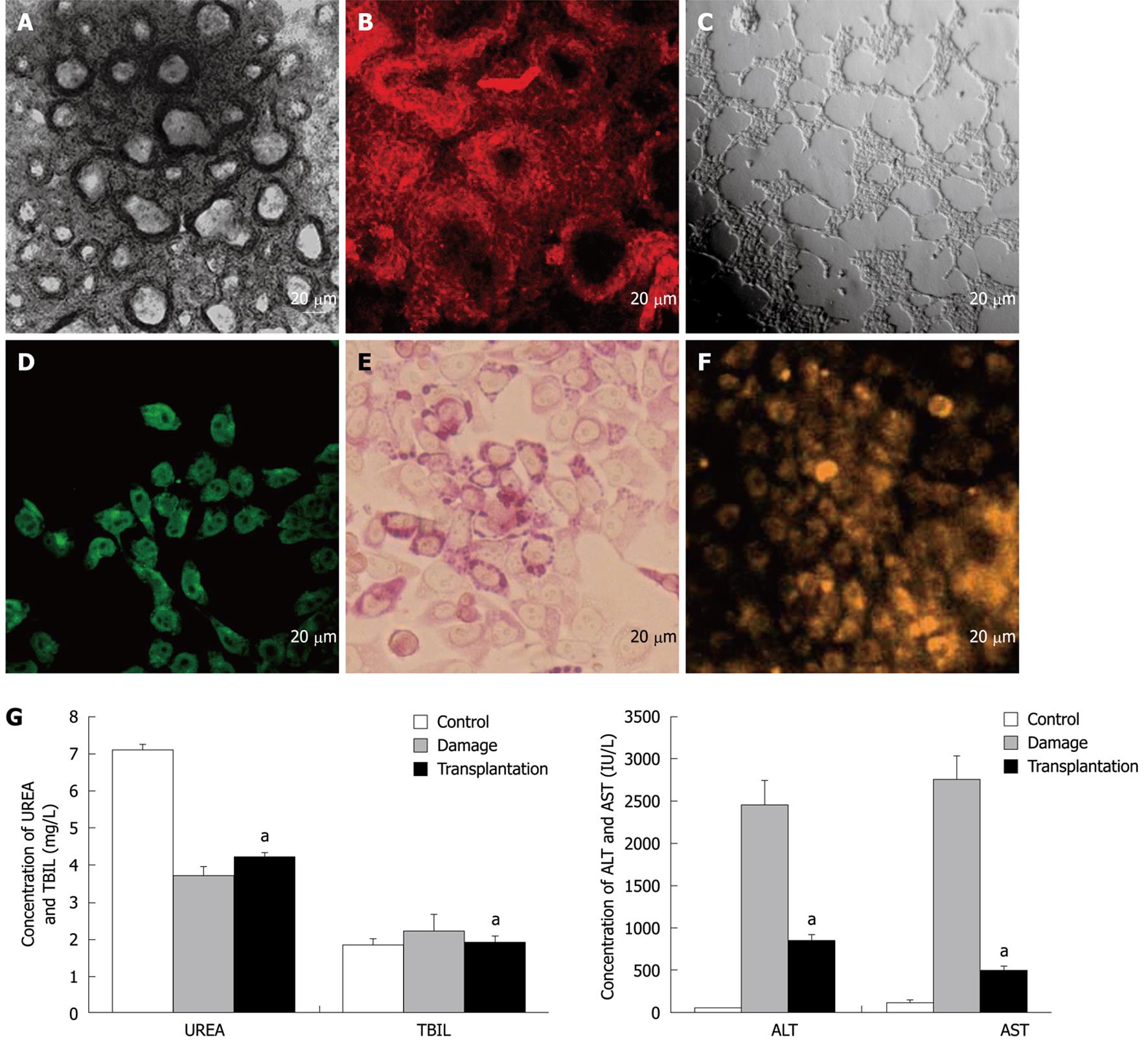
For functional evaluation of the progenitor cells, the in vitro bi-differentiation potential of the progenitors into bile duct-like structures and mature hepatocytes was determined. The results showed that bile duct-like structures could be formed from the progenitor cells in two ways. In the first way, the progenitor cells were cultured on collagen I coated dishes in the medium supplemented with 10-6 mol/L Dex and 10 ng/mL mHGF for about 30 d until the foci of small dark cells appeared, and these foci became organized and developed as doughnut-like structures identical to bile duct units (Figure 4A). In the second way, the progenitor cells were seeded on a layer of Matrigel basement membrane matrix and cultured in the medium supplemented with 100 ng/mL mHGF and 50 ng/mL mEGF as previously described. Many well-defined duct-like structures comprised of neatly aligned cells were found throughout the dish 1-2 d after inoculation, which developed into spherical 3-dimensional structures consisting of tightly packed columnar epithelium along with a central lumen when further maintained for another 15 d (Figure 4C). Immunofluorescence analysis demonstrated that the structures were CK19-positive (Figure 4B) and AFP-negative (data not shown), which are typical hallmarks seen in the bile duct.
Accordingly, typical hepatocytes could also be attained from the progenitors as mentioned above. Functional characterization analyses showed that the differentiated hepatocytes could synthesize albumin (Figure 4D), store glycogen (Figure 4E) and possessed cytochrome P450 activity (Figure 4F) after differentiation for 2-3 wk. Furthermore, an in vivo transplantation assay of hepatocytes in acute-injured liver was performed. The results showed that when the differentiated hepatocytes were transplanted 6 h after liver intoxication, serum T-Bil, serum ammonia, AST and ALT levels were significantly (P < 0.05) improved towards normal levels compared to control mice without hepatocyte transplantation (Figure 4G), suggesting that the hepatocytes functioned normally in vivo and their transplantation improved the liver function of CCl4-treated mice.
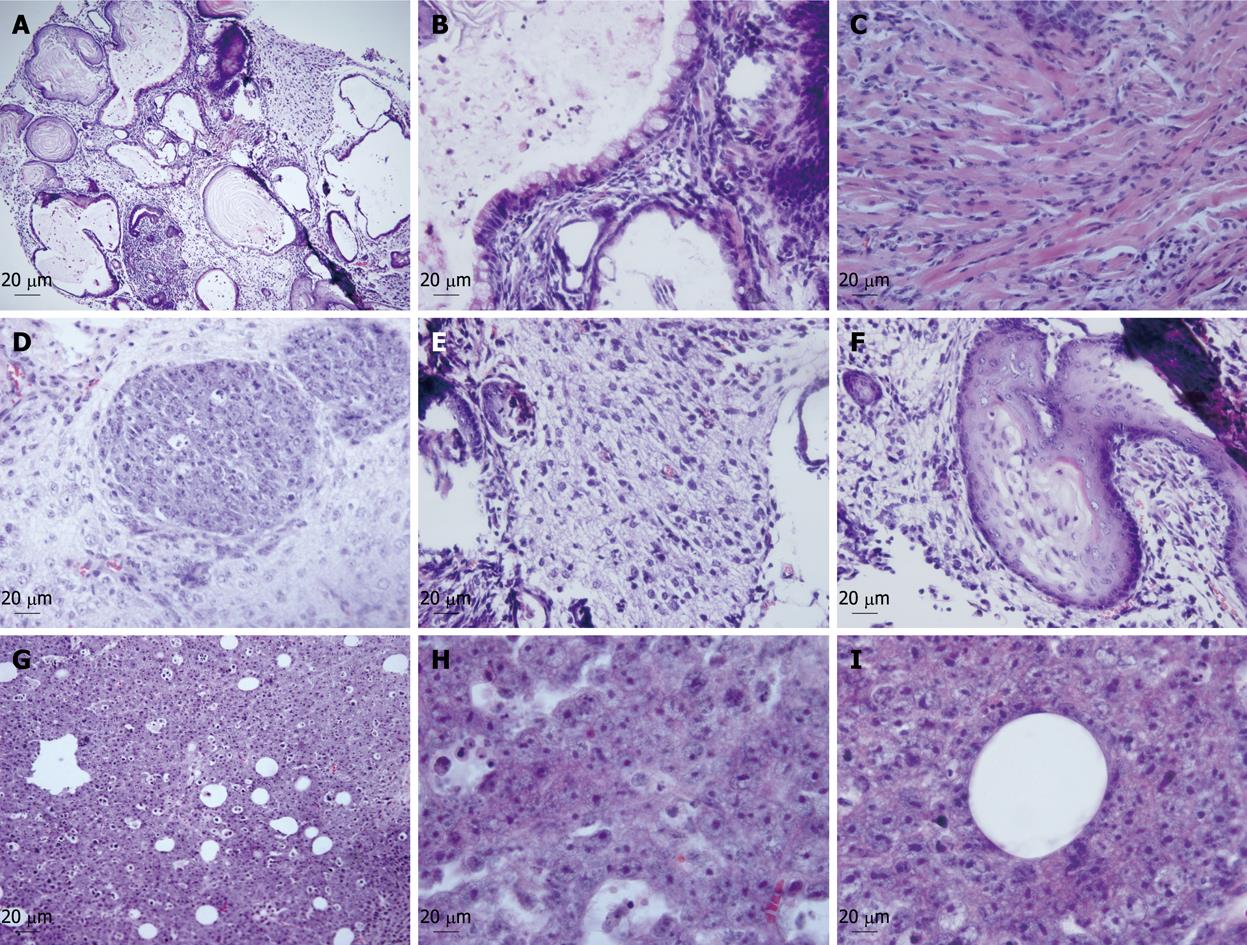
To evaluate the in vivo oncogenicity and differentiation potential of the progenitor cells, we introduced these cells and undifferentiated mES cells (as control) into Balb/c nude mice. Two weeks post-inoculation, the mice implanted with ES cells developed apparent teratomas at the injection site, while the mice with progenitor cells developed tumor-like bumps about 3-4 wk later. After the development of 6-8 wk, the teratomas and tumor-like bumps were fixed and examined with HE staining. The teratomas derived from ES cells were composed of a variety of types of differentiated cells from all three primary germ layers including neuroectodermal cells, adipocytes, muscle and epithelium (Figure 5A-F). However, the tumor-like bumps derived from the progenitor cells contained only epithelial and mesenchymal-like cells, and duct-like structures surrounded by epithelial cells were also observed (Figure 5G-I). The results further confirmed that the progenitor cells have in vivo differentiation potential into hepatic lineages, and indicated the difference in in vivo potential between the undifferentiated ES cells and hepatic progenitor cells.
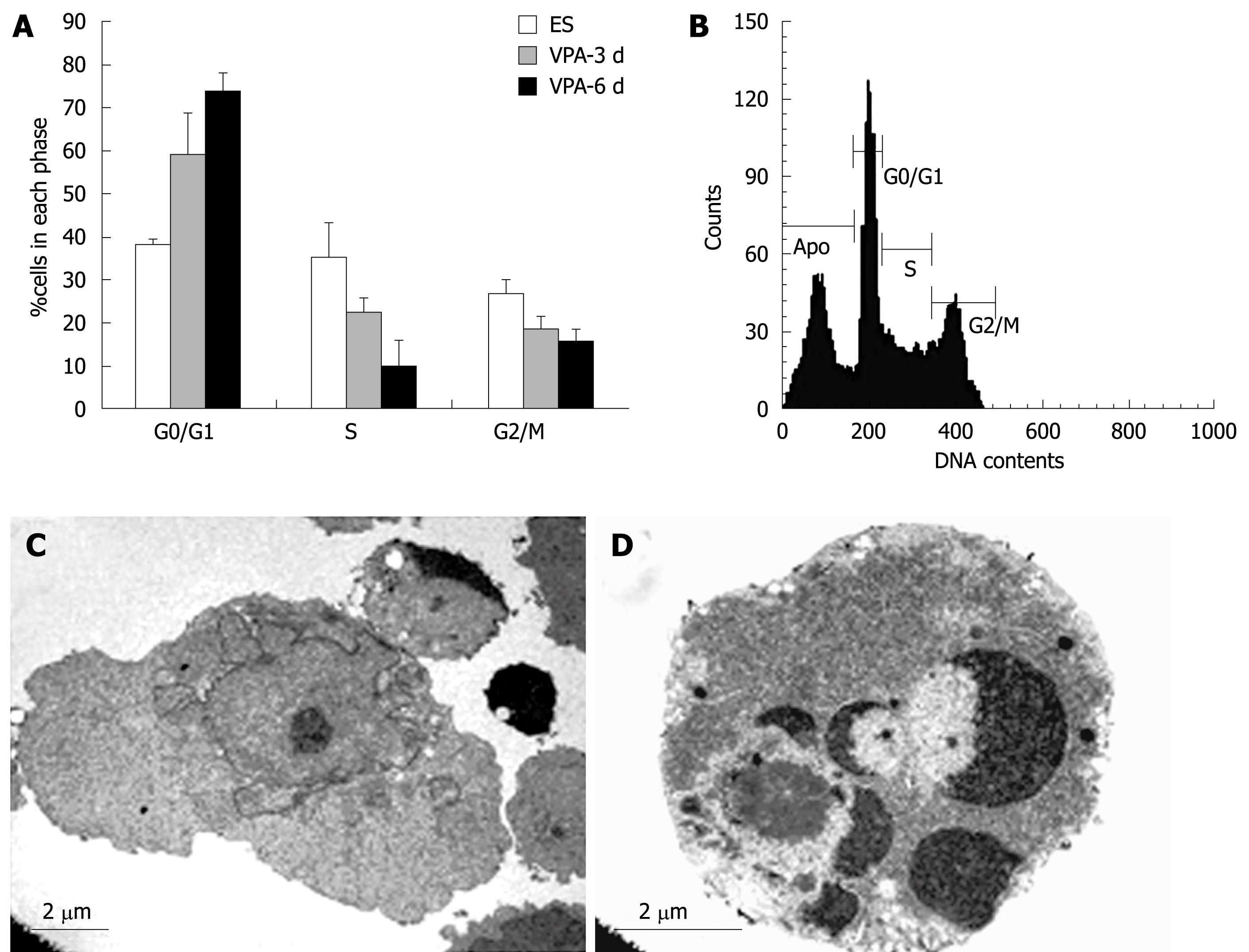
To evaluate whether VPA had an effect on cell cycle profile, ES cells were exposed to 1 mmol/L VPA for 0, 3, 6 d and cell cycle analyses were performed with FACS. Results indicated that exposure to VPA decreased the proportion of cells in S phase and increased the G0/G1 phase proportion. Approximately 75% of the cells were arrested in the G0/G1 phase and only 10% of the cells were in the S phase after 6 d of treatment with VPA, whereas over 37% of control ES cells were in the S phase (Figure 6A). These data indicated that VPA could reduce the proliferation of ES cells and cause the inhibition of G1-S transition. In addition, DNA content analysis of the cells treated with VPA for 3 d showed that about 28% of the cells adopted apoptotic features (Figure 6B). Furthermore, the ultrastructural observations also showed that a considerable number of cells presented typical apoptotic morphology after being treated with VPA for more than 5 d (Figure 6C and D).
To develop well-defined in vitro protocols for directing cellular differentiation into hepatic lineage has become critical for better investigating the mechanisms of hepatocyte differentiation, and for providing seed cells for hepatic tissue engineering as well as for clinical purposes. Most of the protocols currently developed to induce the hepatic differentiation from ES cells were devised by using embryoid bodies. However, several disadvantages may exist in this method. For example, it is time-consuming to prepare the embryoid bodies, and hepatic differentiation through embryoid bodies may result in low yield and purity of functional hepatocytes. Therefore, direct hepatic fate differentiation from an ES monolayer without using embryoid bodies is a most promising concept. A previous study has demonstrated that hepatocyte-like cells could be directly induced from human ES cells by using a combination of cytokines, providing preliminary evidence of the possibilities for this method[25]. However, the direct hepatic differentiation of ES cells still remains a challenge and needs to be further developed. In the present study, we report a novel strategy for the direct hepatic fate differentiation of ES cells, which allows hepatic differentiation from progenitor cells to functional hepatocytes, based on a combination of VPA and cytokines. The results show this strategy has obvious advantages, such as being easy to operate, well reproducible and capable of acquirement of abundant and uniform progenitor cells and mature hepatocytes, the latter of which are suitable for the large-scale requirements of cell replacement therapy. Noticeably, following this strategy, we can harvest progenitor cells and mature hepatocytes in a sequential order. Thus, it may provide an in vitro research model which could meet the requirement of precise study regarding the mechanisms of hepatic differentiation at different developmental stages.
In addition, histone acetylation is considered to be one of the most important epigenetic regulation processes involved in gene expression, which is largely controlled by HDAC inhibitors including VPA. The observation that VPA could induce hepatic progenitor differentiation from ES cells suggested that epigenetic regulation mediated by histone acetylation might play an important role in early hepatic development. Therefore, the VPA-induced hepatic differentiation may also provide a model for the study of early hepatic developmental events, such as the relationship between the initiation of hepatic differentiation and epigenetic modification.
By FACS and ultrastructural analysis, it was found that treatment of ES cells with VPA significantly reduced the proportion of the cells in the S phase and promoted the accumulation in the G0/G1 phase, which was accompanied by cellular apoptosis. This finding suggests that cell cycle arrest and apoptosis are involved in the VPA-induced hepatic specification. Further study is needed to elucidate the exact molecular and cellular mechanisms underlying the VPA-induced hepatic cell fate determination from ES cells.
Several lines demonstrated that the VPA-induced progenitor cells possessed some distinctive characteristics distinguished from ES cells or traditionally identified hepatoblasts or hepatic oval cells. For example, the progenitor cells exhibited typical epithelial morphology when cultured in a collagen coated dish, while ES cells formed multilayer compact colonies. There were many condensed-stained heterochromatin areas in the nucleus of ES cells, while the progenitor cells were almost all euchromatic. Also, the results of an in vivo differentiation assay revealed that ES cells formed typical teratomas containing the structures of three primary germ layers, while the progenitor cells formed tumor-like bumps containing only epithelial and mesenchymal cells. Moreover, the acquired progenitor cells expressed a number of typical markers of hepatoblasts or oval cells, such as AFP, TTR, Foxa2, DPPIV, GGT and BG. Importantly, Dlk, a marker of hepatic stem cells (hepatoblasts or hepatic oval cells) recently reported[26-28], was also expressed by the progenitor cells. However, investigation of the hepatic oval cell marker, A6, was negative[29,30]. In addition, the progenitor cells expressed OCT-4, a marker of multi-lineage stem/progenitor cells[31-33]. These data indicated that the acquired progenitor cells may represent a novel subset at a developmental stage between ES cells and the known hepatic stem/progenitor cells, suggesting that a novel progenitor population was involved in the VPA-induced hepatic differentiation. This finding will be of benefit for understanding more about the early differentiation of hepatocytes.
Importantly, mouse is an often-used model species for human disease, so the investigation of hepatic differentiation of mouse ES cells will be of benefit for the better understanding of mechanisms underlying human hepatic differentiation and the development involved. Our results also support the establishment of new strategies to acquire human hepatic progenitor cells as well as hepatocytes, which will be helpful for the solution of obtaining cell sources for clinical cytotherapy.
Embryonic stem (ES) cells, known for their capacity to proliferate indefinitely and differentiate into almost all types of cells including hepatocytes, have raised the hope of cellular replacement therapy for liver failure. There have been several protocols available for hepatic fate specification from ES cells, however, most of the protocols currently used result in low yield or purity of functional hepatocytes. Valproic acid (VPA), a histone deacetylase (HDAC) inhibitor, has been demonstrated to facilitate the hepatic differentiation of mesenchymal stem cells. However, little is know about whether VPA could induce the hepatic differentiation of ES cells.
The research hotspot is to develop well-defined protocols for directing cellular differentiation into hepatic lineage, followed by selective isolation and proliferation in vitro.
In the present study, the authors report a novel strategy for the direct hepatic fate differentiation of ES cells, which allows the hepatic differentiation from progenitor cells to functional hepatocytes, based on using a combination of VPA and cytokines. The results show that this strategy has obvious advantages, such as being easy to operate, well reproducible and capable of acquirement of abundant and uniform progenitor cells and mature hepatocytes, the latter of which are suitable for the large-scale requirements of cell replacement therapy. Noticeably, following this strategy, they can harvest progenitor cells and mature hepatocytes in a sequential order. Thus, it may provide an in vitro research model which could meet the requirements of precise study regarding the mechanisms of hepatic differentiation at different developmental stages. In addition, the observation that VPA could induce hepatic progenitor differentiation from ES cells suggested that epigenetic regulation mediated by histone acetylation might play an important role in early hepatic development. Therefore, the VPA-induced hepatic differentiation may also provide a model for the study of early hepatic developmental events, such as the relationship between the initiation of hepatic differentiation and epigenetic modification.
The present study may not only be helpful for the clinical application of hepatocyte transplantation, but also provide an in vitro research model for the better investigation and understanding of the entire developmental process of hepatocytes, from ES cells to hepatic progenitors, and then to a population of mature hepatocytes.
VPA, a HDAC inhibitor, has been used as a new class of chemotherapeutic drug for cancer clinical purposes and as an inducer for stem cell differentiation.
This is an important area for research to find new sources of hepatocytes for future clinical application. The authors have performed a good study.
Peer reviewer: Robin Hughes, Institute of Liver Studies, King’s College London School of Medicine, Bessemer Road, London, SE5 9PJ, United Kingdom
S- Editor Tian L L- Editor Logan S E- Editor Zheng XM
| 1. | Tanaka K, Soto-Gutierrez A, Navarro-Alvarez N, Rivas-Carrillo JD, Jun HS, Kobayashi N. Functional hepatocyte culture and its application to cell therapies. Cell Transplant. 2006;15:855-864. |
| 2. | Strom SC, Bruzzone P, Cai H, Ellis E, Lehmann T, Mitamura K, Miki T. Hepatocyte transplantation: clinical experience and potential for future use. Cell Transplant. 2006;15 Suppl 1:S105-S110. |
| 3. | Nussler A, Konig S, Ott M, Sokal E, Christ B, Thasler W, Brulport M, Gabelein G, Schormann W, Schulze M. Present status and perspectives of cell-based therapies for liver diseases. J Hepatol. 2006;45:144-159. |
| 4. | Zhou QJ, Huang YD, Xiang LX, Shao JZ, Zhou GS, Yao H, Dai LC, Lu YL. In vitro differentiation of embryonic stem cells into hepatocytes induced by fibroblast growth factors and bone morphological protein-4. Int J Biochem Cell Biol. 2007;39:1714-1721. |
| 5. | Yamamoto H, Quinn G, Asari A, Yamanokuchi H, Teratani T, Terada M, Ochiya T. Differentiation of embryonic stem cells into hepatocytes: biological functions and therapeutic application. Hepatology. 2003;37:983-993. |
| 6. | Chinzei R, Tanaka Y, Shimizu-Saito K, Hara Y, Kakinuma S, Watanabe M, Teramoto K, Arii S, Takase K, Sato C. Embryoid-body cells derived from a mouse embryonic stem cell line show differentiation into functional hepatocytes. Hepatology. 2002;36:22-29. |
| 7. | Yamada T, Yoshikawa M, Kanda S, Kato Y, Nakajima Y, Ishizaka S, Tsunoda Y. In vitro differentiation of embryonic stem cells into hepatocyte-like cells identified by cellular uptake of indocyanine green. Stem Cells. 2002;20:146-154. |
| 8. | Cai J, Zhao Y, Liu Y, Ye F, Song Z, Qin H, Meng S, Chen Y, Zhou R, Song X. Directed differentiation of human embryonic stem cells into functional hepatic cells. Hepatology. 2007;45:1229-1239. |
| 9. | Teratani T, Yamamoto H, Aoyagi K, Sasaki H, Asari A, Quinn G, Sasaki H, Terada M, Ochiya T. Direct hepatic fate specification from mouse embryonic stem cells. Hepatology. 2005;41:836-846. |
| 10. | Marks P, Rifkind RA, Richon VM, Breslow R, Miller T, Kelly WK. Histone deacetylases and cancer: causes and therapies. Nat Rev Cancer. 2001;1:194-202. |
| 11. | Cress WD, Seto E. Histone deacetylases, transcriptional control, and cancer. J Cell Physiol. 2000;184:1-16. |
| 13. | Huang L. Targeting histone deacetylases for the treatment of cancer and inflammatory diseases. J Cell Physiol. 2006;209:611-616. |
| 14. | Venturelli S, Armeanu S, Pathil A, Hsieh CJ, Weiss TS, Vonthein R, Wehrmann M, Gregor M, Lauer UM, Bitzer M. Epigenetic combination therapy as a tumor-selective treatment approach for hepatocellular carcinoma. Cancer. 2007;109:2132-2141. |
| 15. | Pathil A, Armeanu S, Venturelli S, Mascagni P, Weiss TS, Gregor M, Lauer UM, Bitzer M. HDAC inhibitor treatment of hepatoma cells induces both TRAIL-independent apoptosis and restoration of sensitivity to TRAIL. Hepatology. 2006;43:425-434. |
| 16. | Armeanu S, Pathil A, Venturelli S, Mascagni P, Weiss TS, Göttlicher M, Gregor M, Lauer UM, Bitzer M. Apoptosis on hepatoma cells but not on primary hepatocytes by histone deacetylase inhibitors valproate and ITF2357. J Hepatol. 2005;42:210-217. |
| 17. | Hsieh J, Nakashima K, Kuwabara T, Mejia E, Gage FH. Histone deacetylase inhibition-mediated neuronal differentiation of multipotent adult neural progenitor cells. Proc Natl Acad Sci USA. 2004;101:16659-16664. |
| 18. | Siebzehnrubl FA, Buslei R, Eyupoglu IY, Seufert S, Hahnen E, Blumcke I. Histone deacetylase inhibitors increase neuronal differentiation in adult forebrain precursor cells. Exp Brain Res. 2007;176:672-678. |
| 19. | Rambhatla L, Chiu CP, Kundu P, Peng Y, Carpenter MK. Generation of hepatocyte-like cells from human embryonic stem cells. Cell Transplant. 2003;12:1-11. |
| 20. | Cho HH, Park HT, Kim YJ, Bae YC, Suh KT, Jung JS. Induction of osteogenic differentiation of human mesenchymal stem cells by histone deacetylase inhibitors. J Cell Biochem. 2005;96:533-542. |
| 21. | Chen Y, Pan RL, Zhang XL, Shao JZ, Xiang LX, Dong XJ, Zhang GR. Induction of hepatic differentiation of mouse bone marrow stromal stem cells by the histone deacetylase inhibitor VPA. J Cell Mol Med. 2008;. |
| 22. | Zhou QJ, Shao JZ, Xiang LX, Hu RZ, Lu YL, Yao H, Dai LC. Generation of embryoid bodies from mouse embryonic stem cells cultured on STO feeder cells. Cell Biol Int. 2005;29:817-825. |
| 23. | Kania G, Blyszczuk P, Jochheim A, Ott M, Wobus AM. Generation of glycogen- and albumin-producing hepatocyte-like cells from embryonic stem cells. Biol Chem. 2004;385:943-953. |
| 24. | Kumashiro Y, Asahina K, Ozeki R, Shimizu-Saito K, Tanaka Y, Kida Y, Inoue K, Kaneko M, Sato T, Teramoto K. Enrichment of hepatocytes differentiated from mouse embryonic stem cells as a transplantable source. Transplantation. 2005;79:550-557. |
| 25. | Hay DC, Zhao D, Ross A, Mandalam R, Lebkowski J, Cui W. Direct differentiation of human embryonic stem cells to hepatocyte-like cells exhibiting functional activities. Cloning Stem Cells. 2007;9:51-62. |
| 26. | Tanimizu N, Tsujimura T, Takahide K, Kodama T, Nakamura K, Miyajima A. Expression of Dlk/Pref-1 defines a subpopulation in the oval cell compartment of rat liver. Gene Expr Patterns. 2004;5:209-218. |
| 27. | Tanimizu N, Saito H, Mostov K, Miyajima A. Long-term culture of hepatic progenitors derived from mouse Dlk+ hepatoblasts. J Cell Sci. 2004;117:6425-6434. |
| 28. | Tanimizu N, Nishikawa M, Saito H, Tsujimura T, Miyajima A. Isolation of hepatoblasts based on the expression of Dlk/Pref-1. J Cell Sci. 2003;116:1775-1786. |
| 29. | Engelhardt NV, Factor VM, Medvinsky AL, Baranov VN, Lazareva MN, Poltoranina VS. Common antigen of oval and biliary epithelial cells (A6) is a differentiation marker of epithelial and erythroid cell lineages in early development of the mouse. Differentiation. 1993;55:19-26. |
| 30. | Petersen BE, Grossbard B, Hatch H, Pi L, Deng J, Scott EW. Mouse A6-positive hepatic oval cells also express several hematopoietic stem cell markers. Hepatology. 2003;37:632-640. |
| 31. | Carlin R, Davis D, Weiss M, Schultz B, Troyer D. Expression of early transcription factors Oct-4, Sox-2 and Nanog by porcine umbilical cord (PUC) matrix cells. Reprod Biol Endocrinol. 2006;4:8. |
| 32. | Mossman AK, Sourris K, Ng E, Stanley EG, Elefanty AG. Mixl1 and oct4 proteins are transiently co-expressed in differentiating mouse and human embryonic stem cells. Stem Cells Dev. 2005;14:656-663. |
| 33. | Rathjen J, Lake JA, Bettess MD, Washington JM, Chapman G, Rathjen PD. Formation of a primitive ectoderm like cell population, EPL cells, from ES cells in response to biologically derived factors. J Cell Sci. 1999;112:601-612. |