Published online Oct 14, 2009. doi: 10.3748/wjg.15.4833
Revised: August 22, 2009
Accepted: August 29, 2009
Published online: October 14, 2009
AIM: To investigate the expression of leukemia related protein 16 (LRP16), and the possible relationship between LRP16 expression and clinicopathological indices in 336 gastric carcinoma patients.
METHODS: Immunohistochemistry was used to detect LRP16 expression in 336 cases of paraffin-embedded gastric carcinoma tissues and 60 cases of distal normal mucosa. The relationships between LRP16 expression and patients’ age, tumor size, histological grade, clinical stage, metastatic status and prognosis were analysed.
RESULTS: The expression of LRP16 was 58.6% (197/336) in gastric carcinoma and 31.7% (19/60) in distal normal gastric mucosa. The expression of LRP16 in carcinoma was significantly higher than that in normal mucosa tissues (χ2 = 14.929, P = 0.001). LRP16 protein expression was found in 44.1% (63/143) carcinomas at stage I and II, and 69.4% (134/193) carcinomas at stage III and IV (χ2 = 21.804, P = 0.001), and in 56.9% (182/320) of cancers without metastasis but 93.8% (15/16) of those with metastasis (χ2 = 8.543, P = 0.003). The expression of LRP16 was correlated with tumor size, infiltrative depth, clinical stage, lymphatic invasion and distant metastasis (all P < 0.05). Follow-up data showed that there was a significant difference in median survival time between cancer patients with expression of LRP16 (27.0 mo) and those without (48.0 mo, Log rank =31.644, P = 0.001).
CONCLUSION: The expression of LRP16 may be associated with invasion, metastasis and prognosis of gastric cancer.
- Citation: Li YZ, Zhao P, Han WD. Clinicopathological significance of LRP16 protein in 336 gastric carcinoma patients. World J Gastroenterol 2009; 15(38): 4833-4837
- URL: https://www.wjgnet.com/1007-9327/full/v15/i38/4833.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.4833
| Variable | LRP16 | Statistics value | |
| Positive group (n = 197) | Negative group (n = 139) | ||
| Gender | |||
| Male | 165 (60.2) | 109 (39.8) | χ2 = 1.544 |
| Female | 32 (51.6) | 30 (48.4) | P = 0.214 |
| Age (yr) | |||
| ≤ 40 | 19 (55.9) | 15 (44.1) | χ2 = 1.544 |
| 41-65 | 114 (57.6) | 84 (42.4) | P = 0.756 |
| > 65 | 64 (61.5) | 40 (38.5) | |
| Tumor size (cm) | |||
| < 4 | 36 (46.8) | 41 (53.2) | χ2 = 11.593 |
| 4-7 | 117 (58.2) | 84 (41.8) | P = 0.003a |
| ≥ 8 | 44 (75.9) | 14 (24.1) | |
| Histologic differentiation | |||
| Well differentiated | 8 (47.1) | 9 (52.9) | χ2 = 1.165 |
| Moderately differentiated | 40 (61.5) | 25 (38.5) | P = 0.558 |
| Poorly differentiated | 149 (58.7) | 105 (41.3) | |
| Depth of invasion, T stage | |||
| T1 | 10 (43.5) | 13 (56.5) | χ2 = 9.041 |
| T2 | 55 (53.4) | 48 (46.6) | P = 0.029a |
| T3 | 113 (60.4) | 74 (39.6) | |
| T4 | 19 (82.6) | 4 (17.4) | |
| Lymph node metastasis | |||
| 0 | 49 (44.1) | 62 (55.9) | χ2 = 18.946 |
| 1-6 | 77 (60.2) | 51 (39.8) | P = 0.000a |
| 7-15 | 49 (68.1) | 23 (31.9) | |
| > 15 | 21 (84.0) | 4 (16.0) | |
| Distant metastasis | |||
| Negative | 182 (56.9) | 138 (43.1) | χ2 = 8.543 |
| Positive | 15 (93.8) | 1 (6.2) | P = 0.003a |
| TNM stage | |||
| I-II stage | 63 (44.1) | 80 (55.9) | χ2 = 21.804 |
| III-IV stage | 134 (69.4) | 59 (30.6) | P = 0.000a |
| Prognostic variables | B | SE | Wald value | P value | RR |
| Gender | 0.041 | 0.112 | 0.134 | 0.714 | 1.042 |
| Age | 0.523 | 0.078 | 45.169 | 0.000a | 1.687 |
| Histologic differentiation | 0.143 | 0.105 | 1.830 | 0.176 | 1.153 |
| Histologic type | 0.192 | 0.051 | 14.344 | 0.000a | 1.212 |
| Depth of invasion | 0.240 | 0.119 | 4.063 | 0.044a | 1.271 |
| Lymph node metastasis | 0.278 | 0.100 | 7.746 | 0.005a | 1.321 |
| Distant metastasis | 1.058 | 0.214 | 24.495 | 0.000a | 2.881 |
| TNM stage | -0.016 | 0.131 | 0.015 | 0.901 | 0.984 |
| Tumor size | 0.686 | 0.082 | 70.279 | 0.000a | 1.986 |
| LRP16 expression | 0.174 | 0.094 | 3.466 | 0.063 | 1.190 |
Leukemia related protein 16 gene (LRP16), localized on chromosome 11q12.1, is an important estrogen-responsive gene[1-5]. It has been found expressed at a high level in testicles, ovaries and mucosa of colon, at a moderate level in prostate, small intestine, spleen and thymus, and at a low level in peripheral blood leucocytes[2,3]. LRP16 is also an estrogen receptor alpha (ERα), coactivator[6], and it may play an important role in ER signaling pathways. Since gastric cancer is the second leading cause of cancer-related death worldwide[7], it is important for us to assess its prognosis according to the expression of some markers. It has been reported that ER is expressed in gastric cancer[8], but no reports have ever evaluated the expression of LRP16 gene in gastric carcinoma so far. In this study we retrospectively analyzed the relationships between the expression of LRP16 and clinicopathological factors in 336 Chinese patients with gastric cancer.
Paraffin embedded sections of 336 gastric carcinomas and 60 distal normal gastric tissues were obtained from the Department of Pathology, Chinese People’s Liberation Army (PLA) General Hospital (Beijing, China) from 1998 to 2001. Of these patients, 17 were grade I, 65 grade II and 254 grade III, according to histological grading; 66 were stage I, 77 stage II, 147 stage III and 46 stage IV, according to clinical TNM stage revised by UIAC in 2003; and 75 were tubular adenocarcinoma (well-moderately differentiated adenocarcinoma), 32 were mucinous adenocarcinoma, 183 were poorly differentiated adenocarcinoma, 35 were signet-ring cell carcinoma, 11 were other gastric carcinoma according to histological type, respectively. By March, 2008 (the time of data analysis), 251 patients were dead, and 85 patients were alive. The median survival time was 36 mo (range, 0.17-120 mo).
All samples were fixed in 10% buffered formalin and embedded in paraffin. Sections were cut 4 μmol/L thick from wax blocks, mounted on to APES-coated glass slides. Slides were deparaffinized in xylene twice for 10 min, rehydrated through graded ethanols to distilled water before incubation for 10 min with 3% hydrogen peroxidase-methanol to inhibit endogenous peroxidase activity, and heated in 0.01 mol/L citrate buffer (pH 6.0) in a microwave oven for 5 min at 100°C after reaching boiling point for antigen retrieval. Then the slides were taken out of microwave oven to be cooled at room temperature for 15 min. After incubating for 20 min in a blocking solution containing 10% normal goat serum in PBS, sections were incubated at 4°C overnight in a humidified chamber with rabbit polyclonal antibody to human LRP16 (recognized and isolated in 1999 by Department of Molecular Biology of our hospital) diluted 1:400 in blocking solution. The sections were rinsed in PBS and incubated for 30 min with biotinylated secondary antibody (Poly peroxidase anti-mouse/rabbit IgG, Zymed). After washing in PBS, the sections were then incubated for 30 min at 37°C. 3,3’-Diaminobenzidine was used as the chromogen. Slides were counterstained for 3 min with hematoxylin solution. Normal ovarian tissue was used as a positive control for every lesion, whereas the primary antibody was replaced by PBS as a negative control.
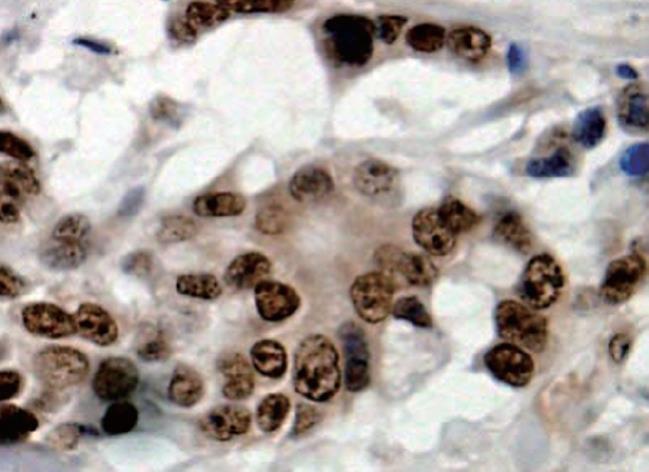
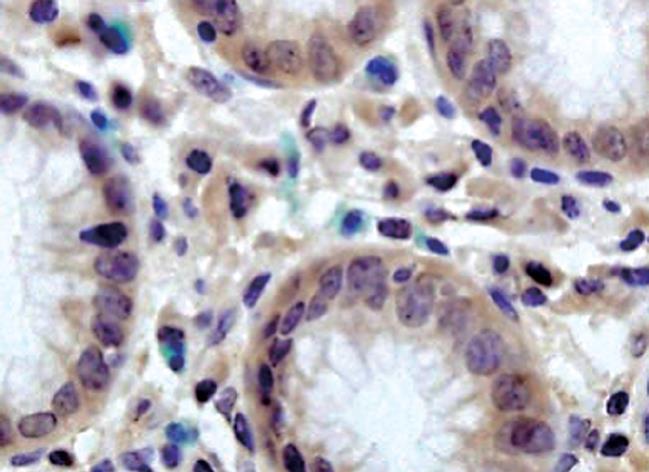
In scoring LRP16 protein expression, both the extent and intensity of immunopositivity in the cell nucleus were considered. The intensity of staining was scored as follows: 0, negative; 1, weak; 2, moderate; 3, strong. The extent of staining was scored as follows: 0, < 5%; 1, > 5%-25%; 2, > 25%-50%; 3, > 50%-75%; 4, > 75% of the cells in the respective lesions. The final score was determined by multiplying the intensity of staining and the extent of staining scores, yielding a range from 0 to 12. Scores 9-12 were defined as preserved or strong staining pattern (++), 5-8 were defined as weak staining pattern (+) and 0-4 were defined as markedly reduced or negative expression (-).
For the statistical analysis, SPSS 13.0 for Windows (SPSS Inc, Chicago, Illinois) was used. The clinical variables were analyzed with the χ2 test. The survival rates were calculated by the Kaplan-Meier method, and the outcomes of treatment were evaluated with the log-rank test. Finally, multivariate analysis was performed to determine the independent prognostic factors by Cox proportional hazards regression model. P < 0.05 was considered statistically significant.
LRP16 was assessed by IHC in 336 gastric cancer cases, with the following results: negative expression (-) in 41.4% (139/336) cases, weak staining (+) in 37.2% (125/336) cases, strong staining (++) in 21.4% (72/336) cases. In normal cases, LRP16 showed a negative expression in 68.3% (41/60) cases, a weak expression in 21.7% (13/60) cases, and a strong expression in 10.0% (6/60) cases. In total, LRP16 protein was expressed (+ or ++) in 58.6% (197/336) gastric carcinoma, but was expressed (+ or ++) only in 31.7% (19/60) of distal normal stomach mucosa (Figures 1 and 2). LRP16 protein was localized mainly in the nucleus of cancer cells or normal epithelial cells. A greatly significant difference was found in the expression of LRP16 protein between gastric carcinoma and normal gastric mucosa tissues (χ2 = 14.929, P = 0.001).
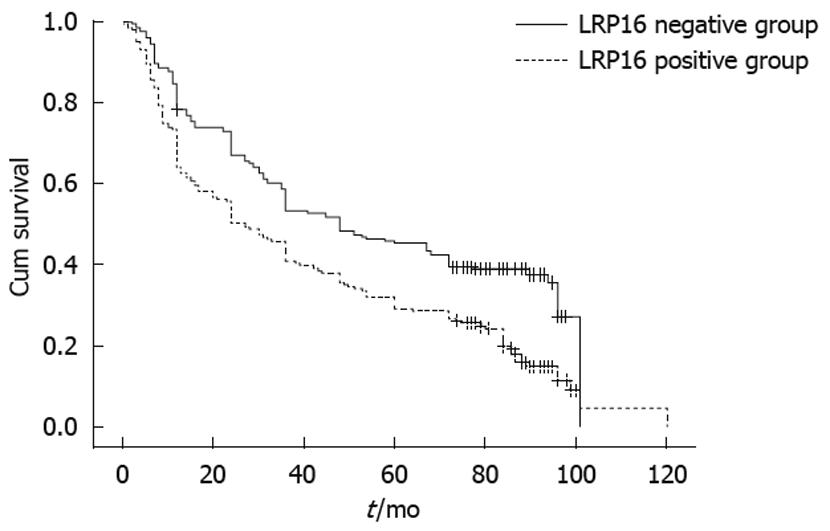
The proportion of LRP16 expression showed an increasing trend from smaller tumor to bigger tumor. Significant positive correlations were found between LRP16 expression and depth of invasion, lymph node metastasis, and distant metastasis (all P < 0.05). Meanwhile, an increasing trend in LRP16 expression was also observed in clinical stage, from 44.1% (63/143) at stage I and II to 69.4% (134/193) at stage III and IV carcinomas (χ2 =21.804, P = 0.001) (Table 1). Moreover, follow-up data showed that there was a significant difference in median survival time between the carcinomas with LRP16 expression (27 mo) and those without (48 mo), and the overall 5-year survival rate (45.8%) of the LRP16 positive group was better than that of the LRP16 positive group (39.1%) (Log rank = 31.64, P = 0.001) (Figure 3).
By Cox proportional hazards model, age, tumor size, histological type, depth of invasion, lymph node metastasis, and distant metastasis were proved to be statistically significant, but LRP16 was not an independent prognostic indicator (Table 2).
LRP16 was originally isolated from lymphocytes in order to identify a leukemia relapse-related gene, but there was no difference between patients primarily diagnosed with acute myeloid leukemia (AML)[9]. Later some studies demonstrated that LRP16 gene plays an important role in the carcinogenesis and progression of hormone-dependent breast cancer[5,6]. LRP16 overexpression markedly promoted the proliferation of MCF-7 human breast cancer cells by promoting G1/S transition through increasing the cyclin E and cyclin D1 protein level[4,6]. On the contrary, suppression of LRP16 gene expression inhibited MCF-7 cell growth and sensitized tumor cells to radiation[10]. It has been proposed that estrogen affects the expression of LRP16 gene, and its expression was strongly dependent on the estrogen activities[9,11]. However, ectopic expression of LRP16 in ERα-negative cells has no effect on proliferation[6]. Among breast cancer patients, LRP16 expression was significantly correlated with tumor size, lymph node metastasis, and clinical stage[12]. Clinical data has shown that LRP16 is overexpressed in primary breast cancer samples compared with their matched normal tissues[12]. In our study, LRP16 expression is in relation to tumor size, depth of invasion, lymph node metastasis, distant metastasis and TNM stage. The expression of LRP16 in carcinoma is significantly higher than that in normal mucosa. Two types of ERs, ERα and ERβ, have both been identified in non-cancerous and cancerous gastric tissue[13,14]. Therefore, we propose that LRP16, a coactivator of ERα, may have a similar function on gastric cancer as on breast cancer. Activation of the ER signaling pathway plays important roles in multi-tissue development[15-18], which implies that LRP16 may display an important function in ERα target tissue development.
In conclusion, LRP16 protein may play an important role in the carcinogenesis, progress and prognosis of Chinese gastric carcinoma and LRP16 expression detected by immunohistochemistry may be a simple and useful molecular marker to predict the prognosis in gastric carcinoma patients. The association between LRP16 and ER in gastric cancer development needs to be further investigated, from which LRP16 targeting with anti-estrogen therapy may be applied in gastric cancer patients.
Gastric cancer is the second leading cause of cancer death worldwide and it is important for us to assess its prognosis according to the expressions of some markers. The expression of LRP16 may be associated with invasion, metastasis and prognosis of gastric cancer. Therefore, LRP16 may play a significant role in the evolution of gastric carcinoma.
LRP16 is an important estrogen-responsive gene. Some studies have demonstrated that LRP16 gene plays an important role in the carcinogenesis and progression of hormone-dependent breast cancer. It has also been reported that ER is expressed in gastric cancer, but no reports have evaluated the expression of LRP16 gene in gastric carcinoma so far. In this study the authors retrospectively analyzed the relationships between the expression of LRP16 and the clinical and pathological factors for Chinese patients with gastric cancer.
Recent reports have demonstrated the important roles of LRP16 in in vitro cell studies. Particularly in breast cancers, LRP16 is over-expressed. This is the first study to report that LRP16 is also over-expressed in gastric carcinoma. Furthermore, our study shows that LRP16 expression is in relation to tumor size, depth of invasion, lymph node metastasis, distant metastasis and TNM stage in gastric cancer.
The LRP16 expression status detected by immunohistochemistry may be a simple and useful molecular marker to predict the prognosis in gastric carcinoma patients.
LRP16, Leukemia related protein 16 gene, localized on chromosome 11q12.1, is an important estrogen-responsive gene. It has been proposed that the expression of LRP16 gene is strongly dependent on the estrogen activities. LRP16 gene plays an important role in the carcinogenesis and progression of hormone-dependent breast cancer. Estrogen receptors (ERs), including ERα, ERβ and the recently discovered ERβcx, have been identified in non-cancerous and cancerous gastric tissue. The biological mechanisms behind this are not yet clear.
The authors examined the expression of LRP16 protein, and the possible relationship between LRP16 expression and clinicopathological indices in gastric carcinoma patients. It revealed that the expression of LRP16 might be associated with invasion, metastasis and TNM stage of gastric cancer. The results are interesting and may provide us with a new molecular marker to assess the prognosis of gastric carcinoma.
Peer reviewer: Dr. Eithne Costello, Royal Liverpool University Hospital, School of Cancer Studies, Division of Surgery and Oncology, 5th Floor UCD Building, Daulby Street, Liverpool, L69 3GA, United Kingdom
S- Editor Li LF L- Editor O'Neill M E- Editor Zheng XM
| 1. | Yu L, Han WD, Lou FD, Wang QS, Zhao Y, Caligiuri MA. Cloning of leukemia associated gene LRP16 in acute myeloid leukemia. Junyi Jinxiu Xueyuan Xuebao. 2000;21:81-84. |
| 2. | Han WD, Yu L, Lou FD, Wang QS, Zhao Y, Shi ZJ, Jiao HY, Zhou JJ. Cloning and expression characterization of the full length cDNA for a novel leukemia-associated gene LRP16. Zhongguo Shengwu Huaxue yu Fenzi Shengwu Xuebao. 2001;17:209-214. |
| 3. | Han WD, Lou FD, Yu L, Wang QS, Han XP, Li XJ. SAGE pattern of LRP16 gene and its expression in normal blood and leukemia cells. Junyi Jinxiu Xueyuan Xuebao. 2002;23:161-163. |
| 4. | Han WD, Mu YM, Lu XC, Xu ZM, Li XJ, Yu L, Song HJ, Li M, Lu JM, Pan CY. Estrogen stimulates human breast cancer MCF-7 cell proliferation by up-regulation of LRP16 mRNA via activation of estrogen receptor-alpha. Zhonghua Neifenmi Daixie Zazhi. 2004;20:165-168. |
| 5. | Han WD, Mu YM, Lu XC, Xu ZM, Li XJ, Yu L, Song HJ, Li M, Lu JM, Zhao YL. Up-regulation of LRP16 mRNA by 17beta-estradiol through activation of estrogen receptor alpha (ERalpha), but not ERbeta, and promotion of human breast cancer MCF-7 cell proliferation: a preliminary report. Endocr Relat Cancer. 2003;10:217-224. |
| 6. | Han WD, Zhao YL, Meng YG, Zang L, Wu ZQ, Li Q, Si YL, Huang K, Ba JM, Morinaga H. Estrogenically regulated LRP16 interacts with estrogen receptor alpha and enhances the receptor's transcriptional activity. Endocr Relat Cancer. 2007;14:741-753. |
| 7. | Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108. |
| 8. | Kiang DT. The presence of steroid receptors in "nontarget" tissues and its significance. Am J Clin Pathol. 1993;99:120-122. |
| 9. | Zhao YL, Han WD, Li Q, Mu YM, Lu XC, Yu L, Song HJ, Li X, Lu JM, Pan CY. Mechanism of transcriptional regulation of LRP16 gene expression by 17-beta estradiol in MCF-7 human breast cancer cells. J Mol Endocrinol. 2005;34:77-89. |
| 10. | Han WD, Yang D, Li Q, Zhang XL, Zhao YL, Ma L, Mu YM. Improvement of radiation sensitivity by inhibiting expression of the human LRP16 gene in tumor cells. Junyi Jinxiu Xueyuan Xuebao. 2005;26:183-185. |
| 11. | Lu XC, Lou FD, Han WD, Zhu XD, Mu YM, Xu ZM, Yu L. [Analysis of LRP16 gene promoter activity]. Zhongguo Shiyan Xueyexue Zazhi. 2006;14:146-149. |
| 12. | Liao DX, Han WD, Zhao YL, Pu YD, Mu YM, Luo CH, Li XH. [Expression and clinical significance of LRP16 gene in human breast cancer]. Aizheng. 2006;25:866-870. |
| 13. | Chandanos E, Lindblad M, Rubio CA, Jia C, Warner M, Gustafsson JA, Lagergren J. Tamoxifen exposure in relation to gastric adenocarcinoma development. Eur J Cancer. 2008;44:1007-1014. |
| 14. | Wang M, Pan JY, Song GR, Chen HB, An LJ, Qu SX. Altered expression of estrogen receptor alpha and beta in advanced gastric adenocarcinoma: correlation with prothymosin alpha and clinicopathological parameters. Eur J Surg Oncol. 2007;33:195-201. |
| 15. | Gerits N, Kostenko S, Moens U. In vivo functions of mitogen-activated protein kinases: conclusions from knock-in and knock-out mice. Transgenic Res. 2007;16:281-314. |
| 16. | Morissette M, Jourdain S, Al Sweidi S, Menniti FS, Ramirez AD, Di Paolo T. Role of estrogen receptors in neuroprotection by estradiol against MPTP toxicity. Neuropharmacology. 2007;52:1509-1520. |
| 17. | Zaitsu M, Narita S, Lambert KC, Grady JJ, Estes DM, Curran EM, Brooks EG, Watson CS, Goldblum RM, Midoro-Horiuti T. Estradiol activates mast cells via a non-genomic estrogen receptor-alpha and calcium influx. Mol Immunol. 2007;44:1977-1985. |
| 18. | Morales LB, Loo KK, Liu HB, Peterson C, Tiwari-Woodruff S, Voskuhl RR. Treatment with an estrogen receptor alpha ligand is neuroprotective in experimental autoimmune encephalomyelitis. J Neurosci. 2006;26:6823-6833. |