Published online Oct 14, 2009. doi: 10.3748/wjg.15.4816
Revised: September 7, 2009
Accepted: September 14, 2009
Published online: October 14, 2009
AIM: To evaluate the hepatoprotective activity of a hydroalcoholic extract of the bark of Anogeissus latifolia; in vitro in primary rat hepatocyte monolayer culture and in vivo in the liver of Wistar rats intoxicated by carbon tetrachloride (CCl4).
METHODS: In the in vitro study, a primary hepatocyte monolayer culture was treated with CCl4 and extract of Anogeissus latifolia. Hepatoprotective activity was demonstrated in the CCl4 damaged primary monolayer culture. In the in vivo study, the hepatoprotective activity of a hydroalcoholic extract of Anogeissus latifolia was analyzed in liver injured CCl4-treated rats. Biochemical parameters including serum transaminases [aspartate aminotransferase (AST) and alanine aminotransferase (ALT)] and alkaline phosphatase (ALP) in serum were analyzed. The biochemical findings were supplemented with histopathological examination of rat liver sections.
RESULTS: In vitro: primary hepatocyte monolayer cultures were treated with CCl4 and extract of Anogeissus latifolia. A protective activity could be demonstrated in the CCl4 damaged primary monolayer culture. In vivo: Hydroalcoholic extract of Anogeissus latifolia (300 mg/kg) was found to have protective activity in rats with CCl4-induced liver damage as judged from serum marker enzyme activity.
CONCLUSION: The above findings lead to the conclusion that the hydroalcoholic extract of Anogeissus latifolia is hepatoprotective. Hence, we suggest that the inclusion of this plant in the management of liver disorders is justified.
-
Citation: Pradeep HA, Khan S, Ravikumar K, Ahmed MF, Rao MS, Kiranmai M, Reddy DS, Ahamed SR, Ibrahim M. Hepatoprotective evaluation of
Anogeissus latifolia :In vitro andin vivo studies. World J Gastroenterol 2009; 15(38): 4816-4822 - URL: https://www.wjgnet.com/1007-9327/full/v15/i38/4816.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.4816
| Treatment | Viable cells (%) | ALT (IU/L) | AST (IU/L) |
| Cell control | 93.88 ± 0.81 | 18.70 ± 0.46 | 21.53 ± 0.63 |
| Toxicant (1 mol/L CCl4) | 33.06 ± 114 | 50.20 ± 0.61 | 57.50 ± 0.94 |
| Std Liv52 (250 μL/mL) | 85.84 ± 1.19 (86.7)a | 19.46 ± 0.35 (97.59)a | 24.10 ± 0.37 (92.85)a |
| ALE (250 μg/mL) | 57.63 ± 1.58 (40.39)a | 41.33 ± 0.62 (28.16)a | 44.53 ± 1.25 (36.05)a |
| ALE (500 μg/mL) | 77.60 ± 0.57 (73.72)a | 38.50 ± 0.45 (37.14)a | 32.80 ± 0.65 (68.66)a |
| ALE (1000 μg/mL) | 81.24 ± 1.67 (79.20)a | 22.23 ± 0.50 (88.48)a | 27.33 ± 0.69 (83.87)a |
| Treatment (n = 6) | AST (IU/L) | ALT (IU/L) | ALP (IU/L) | Lipid peroxidation nmol of MDA/mg protein |
| Normal | 88.17 ± 5.47 | 54 ± 2.7 | 249.5 ± 18.2 | 4.1 ± 0.5 |
| CCl4 (500 μL/kg) | 254.9 ± 19.3c | 222.8 ± 10.14c | 328.5 ± 25.36a | 14.8 ± 1.3c |
| Liv52 (2 mL/kg) | 111.7 ± 8.7b | 71.4 ± 5.6b | 255 ± 24.1b | 5.4 ± 0.6b |
| ALE (300 mg/kg) | 117 ± 6.7b | 66.2 ± 6.1b | 258 ± 15.54b | 6.1 ± 0.5b |
Oxidative stress has been implicated in the pathogenesis of acute and chronic liver injury in a variety of pathophysiological conditions such as hepatotoxin exposure, intrahepatic cholestasis, alcoholic liver injury, liver ischemia and viral hepatitis[1-4]. Over-production of reactive oxygen species (ROS) and nitrogen species (RNS), along with significant decrease of antioxidant defense in these pathological conditions, impairs various cellular functions through the processes of lipid peroxidation, protein oxidation and nucleic base oxidation. Lipid peroxidation causes changes in the physical and chemical properties of cellular membranes, thus altering their fluidity and permeability, leading to impairment in membrane signal transduction and ion exchange, resulting in swelling, cytolysis, and finally, cell death. The oxidation of proteins and DNA also relates directly to cellular dysfunction and death[5]. Accordingly, effects of antioxidants or free radical scavengers have been widely tested for the prevention and treatment of acute and chronic liver injuries. In some of the studies, antioxidants have shown beneficial effects, specifically for prevention and treatment of chronic liver injury[6-8].
Anogeissus latifolia Wall (Combretaceae) is a large or moderate-sized tree characteristic of dry deciduous forests and available throughout India. The plant is traditionally used for the treatment of dysentery, snakebite, leprosy, diabetes, wounds and ulcers and skin diseases, in addition to hepatopathy[9]. The hydroalcoholic extract is reported to have antioxidant activity. It has been studied for total antioxidant activity, hydrogen-donating ability, nitric oxide, superoxide scavenging activity and hydrogen peroxide decomposition activity. Integral antioxidative capacity has been determined by chemiluminescence assay. It has also been studied in a lipid peroxidation assay with a thiobarbituric acid-reactive substances (TBARS) method using rat liver homogenate[10]. A variety of substances which might contribute to hepatoprotective activity have been identified in extracts of Anogeissus latifolia including tannins, gallic acid, ellagic acid and flavonoids such as lutin and quercetin, which are potential antioxidants[11-16]. The bark of the plant is also reported to possess several biological activities such as antiulcer, antimicrobial and wound healing activities. Gastroprotective potential of Anogeissus latifolia extract has been studied in aspirin-, cold-resistant stress (CRS)-, pylorus ligated- and ethanol-induced ulcers. The status of the antioxidant enzymes, superoxide dismutase and catalase, has also been studied in CRS-induced ulcers[17,18]. The bark of the plant was standardized for the presence of chemical constituents such as gallic acid and ellagic acid (0.95% w/w and 0.25% w/w, respectively) using High Performance Thin Layer Chromatography (HPTLC) by Govindrajan et al[17]. Further, we identified and quantified the other constituents of the bark, quercetin and rutin (1.875% w/w, 0.1617% w/w, respectively) using HPTLC; these are reported as potent antioxidants[15,16] and hepatoprotective agents[19,20]. Antioxidant action has been reported to play a crucial role in hepatoprotection[6-8]. The hydroalcoholic extract of Anogeissus latifolia is reported to have chemoprotective activity in paracetamol-induced toxicity in a rat model[21]. Thus, the present study was therefore undertaken to investigate the hepatoprotective activity of hydroalcoholic extract of Anogeissus latifolia in vitro and in vivo against CCl4 intoxicated rats.
Plant material and extraction: Bark of Anogeissus latifolia was collected from Chikmagalure, Karnataka, South India during the month of May. It was authenticated by Botanical survey of India, Coimbatore, Tamilnadu, India (No. BSI/SC/5/23/06-07/Tech.880).
The bark was shade-dried and powdered coarsely. The coarse powder (250 g) obtained was treated with n-hexane to remove the fatty substances; the bark was further submitted to exhaustive lipid extraction with 70% ethanol in Soxhlet apparatus and filtered. The extract was concentrated under reduced temperature and pressure to obtain dry residue (26.8 g)[10].
Chemicals: All chemicals and solvents used were obtained from S.D. Fine Chemicals, Mumbai, Loba Chemie Indo Austranel Co., Mumbai, Ranbaxy laboratories Ltd., Punjab, Sigma Fine Chemicals, Mumbai and Hi media Laboratories, Mumbai, India. For various biochemical estimations, kits were procured from Ecoline, E. Merck Ltd., M.I.D.C., Taloja. Liv-52 syrup was procured from Market, manufactured by Himalaya Drug Company, Bangalore.
Animals: Healthy, adult female albino rats of Wistar strain, weighing 180-220 g were obtained from the animal house of J.S.S College of Pharmacy, Ooty, India. The animal house was well ventilated and the animals were exposed to 12 h day and night cycles at a temperature of 20 ± 2°C. The animals were housed in large spacious, hygienic polypropylene cages during the course of the experimental period. The animals were fed with water and standard rat pellet obtained from M/s Hindustan Lever Ltd., Bangalore, India (CPCSEA-JSSCP/IAEM/PHY.PHARM/2006-07).
Microlab 100, manufactured by M/s Vital Scientific N.V., The Netherlands, was used to estimate biochemical parameters for aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP). UV-160 Spectrophotometer, manufactured by Shimadzu Corporation, Japan, was used to estimate total phenol content, total flavonol content and lipid peroxidation.
Estimation of total phenolic content: Phenolic compounds are commonly found in both edible and inedible plants and they have been reported to have multiple biological effects, including antioxidant activity[22]. Total phenol was determined using the Folin-Ciocalteu method. This test is based on the oxidation of phenolic groups with phosphomolybdic and phosphotungstic acids. After oxidation a green blue complex is measured at 750 nm. The total phenol content of a tested material is related to its antioxidant activity[23].
Estimation of total flavonol content: Total flavonol content was determined by the method of Woisky[24]. This involved an aluminum chloride colorimetric method. The principle of this method is that aluminum chloride forms an acid stable complex with the C-3 or C-5 hydroxyl group of flavones and flavonols. In addition, aluminum chloride forms acid labile complexes with the ortho-dihydroxyl groups in the A or B ring of flavonoids[25].
Preparation of drug solution: The 70% ethanolic extract of Anogeissus latifolia was suspended in 1% carboxy methyl cellulose (CMC), for oral administration. The concentrations of extract selected were 100 mg and 300 mg/kg body weight. Liv-52 syrup was administered orally at 2 mL/kg body weight.
Carbon tetrachloride-induced hepatotoxicity: It is emphasized that hepatotoxins which cause acute hepatitis should have close resemblance with viral hepatitis, clinically, biochemically and histologically. Certain drugs are responsible for chronic hepatic disease. Chemically-induced hepatic injury for experimental studies should be severe enough to cause cell death or to modify hepatic functions. The mechanism of acute hepatic injury depends upon the chemical compounds used to induce toxicity. Carbon tetrachloride (CCl4) is one of the most powerful hepatotoxins in terms of severity of injury. It causes toxic necrosis leading to biochemical changes, having clinical features similar to those of acute viral hepatitis[26,27]. CCl4 at a dose of 0.5 mL/kg was dissolved in olive oil (1:1) and 0.1 mL was administered for each 100 g of rat body weight intraperitoneally.
Standard Liv-52 Syrup: Liv-52 is a poly herbal formulation introduced in 1954 as a specially formulated ayurvedic herbal remedy for the treatment of viral hepatitis and has been widely prescribed for infective hepatitis ever since[28]. It is an ayurvedic formulation available as tablets and syrup containing the following herbs: Capparis spinosa, Cichorium intybus, Solanum nigrum, Terminalia arjuna, Cassia occidentalis, Achillea millefolium, Tamarix galica and Phyllanthus amarus.
Hepatotoxin and test substances: For in vitro studies, CCl4 (0.1 mol/L), was used to produce submaximal toxicity in isolated rat hepatocytes. The test solutions were administered at dose levels of 125, 250 and 500 μg/mL. Liv-52 was used as a positive control at a dose level of 250 μL/mL. All the substances were dissolved in DMSO[29].
Isolation of rat hepatocytes: The rat hepatocytes were isolated according to the method of Seglen et al[30]. The livers were isolated under aseptic conditions and placed in HEPES (N-2-hydroxyethylpiperazine-N-2-ethanesulphonic acid) buffer I containing HEPES (0.01 mol/L), NaCl (0.142 mol/L) and KCl (0.0067 mol/L), pH 7.4. The livers were cut into small pieces and then incubated with a second buffer containing HEPES (0.1 mol/L), NaCl (0.0667 mol/L), KCl (0.0067 mol/L) and 0.5% Collagenase type IV, pH 7.6, for about 45 min at 37°C in an incubator with constant shaking. Hepatocytes were obtained after filtration and cold centrifugation (4°C, 200 rpm for 2 min, three times) and suspended in HEPES buffer I. The viability of the hepatocytes was assessed by trypan blue (0.2%) exclusion method[30].
Primary cultures of rat hepatocytes: The method of Tingstrom and Obrink[31] with slight modifications was used for the culturing of rat hepatocytes. The freshly isolated viable hepatocytes were suspended in culture medium RPMI-1640 supplemented with calf serum (10%), HEPES and gentamycin (1 μg/mL). These cells (approximately 1-1.2 × 106/mL) were then seeded into culture bottles and incubated at 37°C in an atmosphere of 5% CO2 in a carbon dioxide incubator. Upon incubation for 24 h the hepatocytes formed a monolayer. The newly formed cells were round and most appeared as individual cells. These cells were 95%-96% viable as confirmed by trypan blue exclusion test.
Hepatic cytotoxicity testing: The hydroalcoholic extract of Anogeissus latifolia was tested for hepatic cytotoxicity at 250, 500 and 1000 μg/mL on isolated rat hepatocytes. After 24 h of incubation at 37°C in a CO2 incubator, the percentage viability of hepatocytes was tested using trypan blue exclusion[32].
Hepatoprotective activity: Twenty-four hours after the establishment of the monolayer of hepatocytes, the medium was decanted and the culture was washed with HEPES buffer-I and finally the hepatocytes were suspended in Buffer-I. The hepatic cytotoxicity was induced with CCl4 (0.1 mol/L). Triplicate hepatocyte suspensions (0.1 mL) from different cultures were distributed into various culture tubes labeled as control, toxicant, standard (Liv-52 + toxicant) and test (test sample + toxicant). The control group received 0.1 mL of vehicle (30% DMSO) and toxicant groups received 0.1 mL of CCl4, while the test groups received 0.1 mL of respective test solutions (250, 500 and 1000 μg/mL) followed by 0.1 mL (0.1 mol/L) of hepatotoxin. The standard groups received 0.1 mL of Liv-52 (250 μL/mL) followed by hepatotoxin. The contents of all culture tubes were made up to 1 mL with HEPES buffer I. The contents of all the tubes were mixed well and incubated in a CO2 incubator for 24 h at 37°C. In test and standard groups the hepatocytes were preincubated with respective solutions for 30 min and then exposed to hepatotoxin. After incubation, hepatocyte suspensions were collected to assess cell damage. Cell viability was evaluated by trypan blue exclusion method. Hepatocyte suspensions were centrifuged at 200 rpm. The leakage of the enzymes ALT and AST secreted outside the cells was determined from the supernatant.
Assessment of hepatoprotective activity: The effect of different extracts on liver protection was determined by measuring an increase in the percentage of viable cells in that group of cells incubated with extracts, compared with the control and toxicant-alone groups. Reversal of toxin-induced elevations in the level of enzymes was also considered to assess hepatoprotective activity. Kits procured from Ecoline, E. Merck Ltd., using an autoanalyser, carried out the biochemical estimations (Table 1).
In vivo acute toxicity studies: Acute oral toxicity was induced according to the Organization for Economic Co-Operation and Development (OECD) 423 guidelines procedure[33]. Healthy, young adult Wistar albino rats of weight variation not exceeding ± 20% of the mean weight were selected. The animals were fasted for 4 h with free access to water only. Anogeissus latifolia was administered orally at a dose of 5 mg/kg initially. Mortality, if any, was observed for 3 d. If mortality was observed in two out of three animals, then the dose administered was considered as toxic dose. However, if the mortality was observed in only one animal out of three animals then the same dose was repeated again to confirm the toxic effect. If no mortality was observed, then higher doses (50, 300, 2000 mg/kg) of Anogeissus latifolia were employed for further toxicity studies. Anogeissus latifolia did not produce any behavioral changes and mortality up to the dose of 3000 mg/kg body weight. Hence, 1/10th of this dose, i.e. 300 mg/kg (high dose) was used for the study.
Experimental design: Twenty four albino Wistar rats, weighing about 180-220 g were divided into 4 groups of six animals each. Group I served as solvent control (normal animals). Group II served as CCl4 toxicant control and received 1% CMC, 2 mL/kg b.w. Group III served as positive control and received Liv-52, 2 mL/kg b.w., while Group IV received the freshly prepared Anogeissus latifolia suspended in 1% CMC at a dose level of 300 mg/kg b.w. The animals were treated for 7 d and on the 7th d after one hour of dosing, the toxicant CCl4 (500 μL/kg i.p.) was administered to all the groups except Group I. After 24 h, the animals were anesthetized and blood was collected by sino-orbital puncture for the assessment of various enzyme activities. The blood was centrifuged at 2000 rpm for 10 min. The serum was separated and was used for various biochemical estimations such as AST, ALT, and ALP. The animals were sacrificed later and the liver was perfused and excised. Part of the liver was stored in 10% formalin saline for histopathological studies. The remaining was frozen at -70°C and was used for the estimation of lipid peroxidation.
Estimation of lipid peroxidation by thiobarbituric acid reactive substances (TBARS): The level of lipid peroxidation in liver homogenate was determined by the method of Niehaus and Samuelson[34]. Malondialdehyde and other thiobarbituric acid reactive substances were quantified by their reactivity with thiobarbituric acid in acidic conditions. The reaction generates a pink colored chromophore, which can be read in a colorimeter at 535 nm.
The statistical analysis was carried out by one-way analysis of variance (ANOVA). The values are represented as mean ± SE. Comparison of mean values of different groups treated with different dose levels of extracts and positive controls were estimated by Tukey’s Multiple Comparison Test. P < 0.05 was considered significant.
When normal hepatocytes were treated with the extracts under test conditions, there were no alterations in the values of percentage viable cells as compared to the control at the dose level up to 1000 μg/mL, indicating that the extracts were not toxic to the cells.
Incubation of hepatocytes with CCl4 (0.1 mol/L) resulted in 65% depletion in viability of hepatocytes. Similarly an elevation of about 268.45% and 267.06% of ALT and AST levels were observed, respectively, upon intoxication with CCl4. Hepatocytes treated with Anogeissus latifolia showed a concentration-dependant (100-1000 μg/mL) protective effect by restoring the viability of hepatocytes (40.36%-79.20%), AST (28.16%-88.48%) and ALT (36.05%-83.87%) levels, while the positive control Liv-52 showed good protective effect by restoring viability (86.7%), AST (97.5%) and ALT (92.8%). The maximum protection was seen with 1000 μg/mL of Anogeissus latifolia. Results are represented in Table 1.
Single administration of CCl4 (500 μL/kg, i.p) to vehicle control rats showed significant increases in ALT (222.8 ± 10.14 IU/mL, P < 0.001), AST (254.9 ± 19.3 IU/mL, P < 0.001) and ALP (328.5 ± 25.36 IU/mL, P < 0.01) levels when compared to normal control rats (54 ± 2.7 IU/mL, 88.17 ± 5.47 IU/mL and 249.5 ± 18.2 IU/mL, respectively) (Figure 1). Anogeissus latifolia administered at 300 mg/kg produced significant reductions in ALT 66.2 ± 6.1 IU/mL (P < 0.01), AST 117 ± 6.7 IU/mL (P < 0.01) and ALP 258 ± 15.54 IU/mL (P < 0.01) levels when compared to CCl4-administered rats. Liv-52 also showed significant reductions in ALT 71.4 ± 5.6 IU/mL (P < 0.01), AST 111.7 ± 8.7 IU/mL (P < 0.01) and ALP 258 ± 15.54 IU/mL (P < 0.01) levels when compared to CCl4-administered rats (Table 2).
Lipid peroxidation was significantly elevated following CCl4 administration (14.8 ± 1.3 nmol, P < 0.001) when compared to normal control (4.1 ± 0.5 nmol) (Figure 1). Anogeissus latifolia at a dose of 300 mg/kg, b.w., resulted in significant (P < 0.01) reductions in lipid peroxidation (6.1 ± 0.5 nmol) when compared to toxicant control. Liv-52 at 2 mL/kg b.w., showed significant (P < 0.01) reductions in lipid peroxidation (5.4 ± 0.6 nmol) when compared to CCl4-administered rats (Table 2).
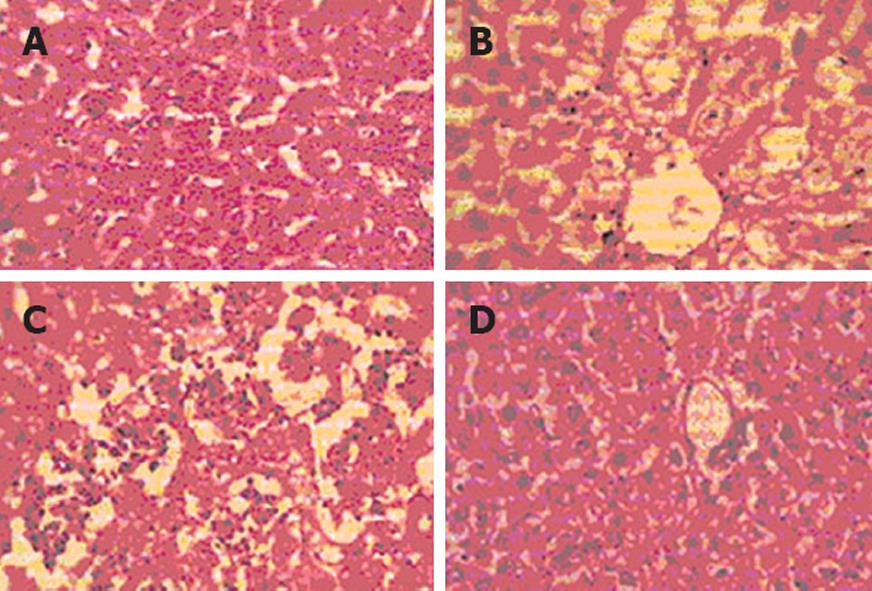
The protective effect of the hydroalcoholic extract of Anogeissus latifolia was further confirmed by histopathological examination of the control (Figure 1A), CCl4-treated and extract-treated groups. The liver of CCl4-treated rats (Figure 1B) shows damaged liver cells and ballooning changes of the hepatocytes. The histopathological pattern of the livers treated with extracts at 100 mg and 300 mg/kg (Figure 1C) shows mild feathery changes, little ballooning degeneration of hepatocytes along with normal hepatocytes. Positive control liver treated with Liv 52 (Figure 1D) shows a normal lobular pattern with minimal pooling of blood in the sinusoidal spaces. The present study reveals the hepatoprotective activity of the hydroalcoholic extract of Anogeissus latifolia against well-known hepatotoxin CCl4.
The bark of Anogeissus latifolia was selected to evaluate its antihepatotoxic effect in preclinical models on the basis of its utility profile in the traditional system of medicine. Subsequently, a survey of literature suggested that it has been used for different diseases including inflammation, diabetes, diarrhoea and skin diseases, as well as hepatopathy. The bark has been evaluated scientifically for its antioxidant and wound healing activity. There was, however, no evidence of any scientific studies on its hepatoprotective action. A qualitative chemical examination showed the presence of carbohydrates, glycosides, phenolic compounds, flavonoids and tannins. The presence of polyphenols and flavonoids supports its antioxidant potential. Total phenol content and total flavonol content was estimated in the extract, and found to be 64.43% and 43.9 mg/g of extract respectively. Since the bark has been reported to contain quercetin and rutin[9], we estimated the quantity of these substances in the extract by HPTLC and this was found to be 1.875% w/w, and 0.1617% w/w respectively. The drug also contains gallic acid. The high percentage of quercetin, rutin and gallic acid in the extract justifies the potent antioxidant activity[13,15,16] which results in the hepatoprotective potential of the extract. Quercetin and rutin are reported to be potential therapeutic agents as they reduce oxidative DNA damage, lipid peroxidation and quench free radicals[35,36]. The drug, thus, is a rich source of various antioxidant chemicals which may exert a cumulative antioxidant effect producing favourable actions in various disease conditions such as hepatopathy, diabetes, inflammation and wound healing.
The hepatotoxicity induced by CCl4 is due to its metabolite CCl3*, a free radical that binds to lipoprotein and leads to peroxidation of lipids of the endoplasmic reticulum[37]. The ability of a hepatoprotective drug to reduce the injurious effects, or to preserve the normal hepatic physiological mechanisms which have been disturbed by a hepatotoxin, is an index of its protective effects. Although serum enzyme levels are not a direct measure of hepatic injury, they show the status of the liver. The lowering of enzyme levels is a definite indication of hepatoprotective action of the drug. The serum ALT, AST, and ALP levels are reliable markers of liver function[38]. In our study, an increase in LPO level in liver suggests enhanced lipid peroxidation leading to tissue damage and failure of antioxidant defense mechanisms resulting in excessive free radical. In CCl4-induced hepatitis, administration of Anogeissus latifolia at 300 mg/kg b.w. produced significant reductions in ALT, AST, ALP levels and lipid peroxidation. Thus it could be suggested that Anogeissus latifolia has hepatoprotective activity in this model, a concept which was further supported by the histopathological results. The reactive species-mediated hepatotoxicity can be effectively managed upon administration of agents possessing antioxidant[39], free radical scavenger[40] and anti-lipid peroxidant[41] activities. The inhibitors of cytochrome P450 isoenzymes (CYPs) are known to reduce the toxicity of CCl4[19]. Rutin and quercetin, which are constituents of Anogeissus latifolia extract, have been reported to inhibit CYPs[42] and might have contributed favorably toward the observed hepatoprotection. Anogeissus latifolia, being a potent antioxidant, free radical scavenger, contributed favourably in this regard towards the observed hepatoprotection. The in vitro and histopathological studies are direct evidence of efficacy of this drug as a hepatoprotectant. Thus, the presence of rutin, quercetin and other antioxidants in Anogeissus latifolia may be the contributing factor towards its hepatoprotective activity and justifies the folkloric use of the plant in liver diseases.
To evaluate the hepatoprotective activity of a hydroalcoholic extract of the bark of Anogeissus latifolia; in vitro in primary rat hepatocyte monolayer culture and in vivo in the liver of Wistar rats intoxicated by CCl4.
Great effort has been and is still being made to minimize costs and side effects of synthetic drugs, which are being used in the treatment of liver diseases.
This product is effective both in in vitro and in vivo studies with no side effects and is cost effective. The product was observed to have an excellent reparative effect on the CCl4-damaged hepatocytes.
This article helps to understand and implement the process of treatment with herbal medicines in liver diseases, which is safe in every aspect.
The author/authors of this manuscript have assessed the protective effect of Anogeissus latifolia in CCL4-induced hepatoxicity of rats. Their study has shown that Anogeissus latifolia contributes to increased viability of cultured hepatocytes in vitro and also causes reduced ALT and AST levels in intoxicated rats.
Peer reviewer: Dr. Sk Md Fazle Akbar, Assistant Professor, Third Department of Internal Medicine, Ehime University School of Medicine, Shigenobu-Cho, Ehime 791-0295, Japan
S- Editor Tian L L- Editor Logan S E- Editor Ma WH
| 1. | Stehbens WE. Oxidative stress, toxic hepatitis, and antioxidants with particular emphasis on zinc. Exp Mol Pathol. 2003;75:265-276. |
| 2. | Jaeschke H, Knight TR, Bajt ML. The role of oxidant stress and reactive nitrogen species in acetaminophen hepatotoxicity. Toxicol Lett. 2003;144:279-288. |
| 3. | McDonough KH. Antioxidant nutrients and alcohol. Toxicology. 2003;189:89-97. |
| 4. | Jaeschke H, Smith CV, Mitchell JR. Reactive oxygen species during ischemia-reflow injury in isolated perfused rat liver. J Clin Invest. 1988;81:1240-1246. |
| 6. | Gupta M, Mazumder UK, Sambath KR. Antioxidant and protective effect of Ervatamia coronaria Stapf leaves against carbon tetrachloride induced liver injury. European Bull Drug Res. 2004;12:13-22. |
| 7. | Gupta M, Mazumder UK, Siva KT. Antioxidant and hepatoprotective effect of Bauhinia racemosa against paracetamol and CCl4 induced liver damage in rats. Iranian J Pharmacol Therapeutics. 2004;3:12-20. |
| 8. | Kukongviriyapan V, Janyacharoen T, Kukongviriyapan U, Laupattarakasaem P, Kanokmedhakul S, Chantaranothai P. Hepatoprotective and antioxidant activities of Tetracera loureiri. Phytother Res. 2003;17:717-721. |
| 9. | Central council for Research in Ayurveda and Siddha, New Delhi. Anonymous, Pharmacognosy of Indigenous drugs, Vol-I. 1985;250-259. |
| 10. | Govindarajan R, Vijayakumar M, Rao CV, Shirwaikar A, Rawat AK, Mehrotra S, Pushpangadan P. Antioxidant potential of Anogeissus latifolia. Biol Pharm Bull. 2004;27:1266-1269. |
| 11. | Reddy KK, Rajadurai S, Nayudamma Y. Studies on Dhava (Anogeissus latifolia) Tannins: Part III- Polyphenols of bark, sapwood and heartwood of Dhava. Indian J Chem. 1965;27:308-310. |
| 12. | Deshpande VH, Patil AD, Ramarao AV, Venkataraman K. 3,3’-Di-O-methylellagic Acid-4’-β-D-xyloside and 3,4,3’-tri-O-methylflavellagic acid-4’-β-D-glucoside from Anogeissus latifolia bark. Indian J Chem. 1976;14B:641-643. |
| 13. | Aruoma OI, Murcia A, Butler J, Halliwell B. Evaluation of the antioxidant and prooxidant actions of gallic acid and its derivatives. J Agric Food Chem. 1993;41:1880-1885. |
| 14. | Festa F, Aglitti T, Duranti G, Ricordy R, Perticone P, Cozzi R. Strong antioxidant activity of ellagic acid in mammalian cells in vitro revealed by the comet assay. Anticancer Res. 2001;21:3903-3908. |
| 15. | Boyle SP, Dobson VL, Duthie SJ, Hinselwood DC, Kyle JA, Collins AR. Bioavailability and efficiency of rutin as an antioxidant: a human supplementation study. Eur J Clin Nutr. 2000;54:774-782. |
| 16. | Boots AW, Haenen GR, Bast A. Health effects of quercetin: from antioxidant to nutraceutical. Eur J Pharmacol. 2008;585:325-337. |
| 17. | Govindarajan R, Vijayakumar M, Singh M, Rao ChV, Shirwaikar A, Rawat AK, Pushpangadan P. Antiulcer and antimicrobial activity of Anogeissus latifolia. J Ethnopharmacol. 2006;106:57-61. |
| 18. | Govindarajan R, Vijayakumar M, Rao CV, Shirwaikar A, Mehrotra S, Pushpangadan P. Healing potential of Anogeissus latifolia for dermal wounds in rats. Acta Pharm. 2004;54:331-338. |
| 19. | Janbaz KH, Saeed SA, Gilani AH. Protective effect of rutin on paracetamol- and CCl4-induced hepatotoxicity in rodents. Fitoterapia. 2002;73:557-563. |
| 20. | Janbaz KH, Saeed SA, Gilani AH. Studies on the protective effects of caffeic acid and quercetin on chemical-induced hepatotoxicity in rodents. Phytomedicine. 2004;11:424-430. |
| 21. | Khan S, Pradeep HA, Vijan R. Chemoprotective activity of Anogeissus latifolia bark extract on Paracetamol-induced hepatotoxicity. Pharmacologyonline. 2008;2:303-327. |
| 22. | Mills S, Bone K. Principles and practice of phytotherapy. Churchill Livingstone. 2000;35:220-222. |
| 23. | Sadasivam S, Manickam A. Biochemical methods for Agricultural Sciences. New Delhi: Wiley Eastern Ltd 1992; 187-190. |
| 24. | Woisky R, Salationo A. Analysis of propils: some parameters and procedures for chemical and quality control. J Apicultural Research. 1998;37:99-105. |
| 25. | Kaufman PB, Cseke LJ, Sarawarber , Duke JA, Brielmamm HL. Natural products from plants. New York: CRC Press 1999; 20-22. |
| 26. | Wang X, Lous Z, Mikage M, Namba T. Pharmacognostical studies on the Chinese crude drug da-huang rhubarb II. Botanical origin of three unofficial da-huang. Shoyakugaku Zasshi. 1988;42:302-309. |
| 27. | Vogel G. New natural products and Plant drugs with Pharmacological, Biological and Therapeutical activity. Berlin: Springer Verlag 1977; 249-265. |
| 28. | Mukerjee AB, Dasgupta M. Treatment of viral Hepatitis B an indegenious drug Liv 52. Ind Pract. 1970;6:357. |
| 29. | Tasaduq SA, Singh K, Sethi S, Sharma SC, Bedi KL, Singh J, Jaggi BS, Johri RK. Hepatocurative and antioxidant profile of HP-1, a polyherbal phytomedicine. Hum Exp Toxicol. 2003;22:639-645. |
| 30. | Seglen PO. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29-83. |
| 31. | Tingstrom A, Obrink B. Distribution and dynamics of cell surface-associated cellCAM 105 in cultured rat hepatocytes. Exp Cell Res. 1989;185:132-142. |
| 32. | Kiso Y, Tohkin M, Hikino H. Assay method for antihepatotoxic activity using galactosamine-induced cytotoxicity in primary-cultured hepatocytes. J Nat Prod. 1983;46:841-847. |
| 33. | Ecobichon DJ. The basis of toxicology testing. New York: CRC Press 1997; 43-86. |
| 34. | Niehaus WG, Samuelson B. Formation of malondialdehyde from phospholipids arachidonate during microsomal lipid peroxidation. Eur J Biochem. 1968;17:126-130. |
| 35. | Noroozi M, Angerson WJ, Lean ME. Effects of flavonoids and vitamin C on oxidative DNA damage to human lymphocytes. Am J Clin Nutr. 1998;67:1210-1218. |
| 36. | Afanas’ev IB, Dorozhko AI, Brodskii AV, Kostyuk VA, Potapovitch AI. Chelating and free radical scavenging mechanisms of inhibitory action of rutin and quercetin in lipid peroxidation. Biochem Pharmacol. 1989;38:1763-1769. |
| 37. | Recknagacl R. Carbontetrachloride hepatotoxicity. Pharmacological Reviews. 1967;19:145-196. |
| 38. | Mulander DW, Wrublewski F, La Due JS. Transaminase compared with cholinesterase and alkaline phosphatase an index of hepatocellular integrity. Clinical Research Proceedings. 1955;3:20-24. |
| 39. | Labib R, Turkall R, Abdel-Rahman MS. Endotoxin potentiates cocaine-mediated hepatotoxicity by nitric oxide and reactive oxygen species. Int J Toxicol. 2003;22:305-316. |
| 40. | Sohn DH, Kim YC, Oh SH, Park EJ, Li X, Lee BH. Hepatoprotective and free radical scavenging effects of Nelumbo nucifera. Phytomedicine. 2003;10:165-169. |
| 41. | Gao H, Zhou YW. Anti-lipid peroxidation and protection of liver mitochondria against injuries by picroside II. World J Gastroenterol. 2005;11:3671-3674. |
| 42. | Bear WL, Teel RW. Effects of citrus phytochemicals on liver and lung cytochrome P450 activity and on the in vitro metabolism of the tobacco-specific nitrosamine NNK. Anticancer Res. 2000;20:3323-3329. |