Published online Oct 7, 2009. doi: 10.3748/wjg.15.4715
Revised: August 18, 2009
Accepted: August 25, 2009
Published online: October 7, 2009
AIM: To evaluate the efficacy and safety of Traditional Chinese Medicine (TCM) in the treatment of Helicobacter pylori (H pylori) infection.
METHODS: We electronically and manually searched electronic databases, references lists and conferences compilations, and included all randomized clinical trials comparing the treatment of H pylori using TCM with proton pump inhibitor or colloidal bismuth subcitrate-based triple therapy as controls. The Jadad score was used to assess trial quality, H pylori eradication rate and the incidence of side effects were taken as outcome measurements, and heterogeneity analysis, meta-analysis and funnel plot analysis were conducted.
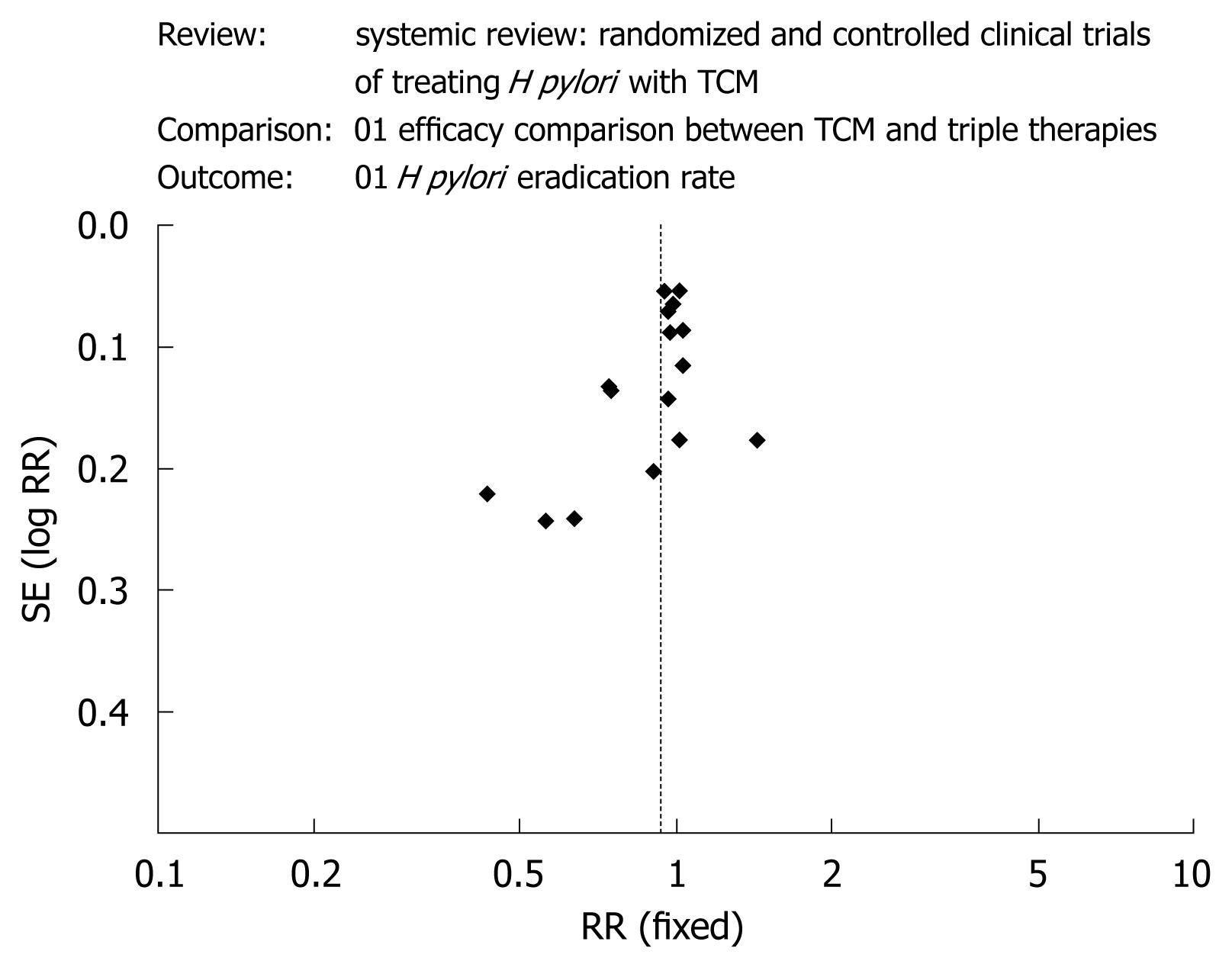
RESULTS: Sixteen trials were included. The Jadad scores of all the trials were not more than 2. Clinical heterogeneity and substantial statistical heterogeneity existed among the trials (P = 0.001, I2 = 59%) and meta-analysis was not conducted. The average eradication rates following TCM and triple therapy were 72% and 78% and the incidence of side effects were 2% and 29%, respectively. The funnel plot was obviously asymmetric.
CONCLUSION: Available evidence is not convincing enough to show that TCM has the same efficacy as triple therapy in H pylori treatment. TCM may be safer than triple therapy. TCM should not be recommended as monotherapy in H pylori infection.
-
Citation: Lin J, Huang WW. A systematic review of treating
Helicobacter pylori infection with Traditional Chinese Medicine. World J Gastroenterol 2009; 15(37): 4715-4719 - URL: https://www.wjgnet.com/1007-9327/full/v15/i37/4715.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.4715
| Trials | No. of cases | Age (yr) | Gender (male) (%) | Jadad score | Regimen of TCM group | Regimen of triple therapies group |
| Chen et al[10] (2001) | 419 | 23-68 | 62.1 | 2 | Fixed formula × 7 d | (PPI + A + F) × 7 d |
| Hua et al[11] (2003) | 155 | 19-65 | 54.2 | 2 | Changweiqing oral liquid × 14 d | (CBS + A + F) × 14 d |
| Fan et al[12] (2006) | 50 | NA | NA | 1 | Anzhong Yin× 28 d | (CBS + A + F) × 14 d |
| Hua et al[13] (2006) | 150 | 23-85 | 61.3 | 1 | Jianwei Mieyou Inspissant × 60 d | (CBS + A + C) × 7 d + CBS × 49 d |
| Ma et al[14] (2006) | 106 | 44.2/43.8 | 79.3 | 2 | Weikang Capsule × 60 d | (RBC + A + M) × 14 d |
| Wang et al[15] (2006) | 77 | NA | 47.7 | 1 | Jianpi Qinghua formula × 30 d | (CBS + A + T) × 14 d |
| Wu[16] (2006) | 71 | 19-65 | 67.6 | 2 | Jiawei Liumo Decoction × 56 d | (PPI + A + M) × 10 d |
| Yang et al[17] (2006) | 80 | 22-65 | 70 | 2 | Weitongning Tab × 28 d | (CBS + A + M) × 14 d |
| Zhou et al[18] (2006) | 56 | 23-70 | 47.2 | 2 | Qingwei Decoction × 14 d | (PPI + A + M) × 14 d |
| Huang[19] (2007) | 320 | 18-77 | 55.6 | 2 | Maimendong Granule × 28 d | (PPI + A + M) × 14 d |
| Jin et al[20] (2007) | 98 | 18-72 | 67.4 | 2 | Maimendong Granule × 28 d | (PPI + A + M) × 7 d |
| Wang et al[21] (2008) | 60 | 20-64 | 61.7 | 1 | Mieyou Decoction × 14 d | (CBS + A + T) × 14 d |
| Ling et al[22] (2008) | 46 | 33.2/35.1 | 71.7 | 2 | Jianwei Yuyang Granule × 6 wk | (PPI + A + M) × 1 wk + PPI × 1 wk |
| Wang[23] (2008) | 149 | 16-66 | 61.7 | 2 | Formulae × 2 w | (CBS + A + M) × 2 wk |
| Xiao et al[24] (2008) | 80 | 19-77 | 62.5 | 2 | Weiyan Decoction × 20 d | (PPI + A + C) × 7 d |
| Xin et al[25] (2008) | 70 | 48.6/44.6 | 60 | 2 | Weikang formula × 1 mo | (PPI + C + T) × 7 d |
| Trials | TCM (n/N) | Triple therapies (n/N) | RR (95% CI) | P | Ref. |
| Fixed formula vs PPI + A + F | 161/211 | 160/204 | 1.02 (0.91, 1.13) | 0.60 | [10] |
| Anzhong Yin vs CBS + A + F | 19/30 | 14/20 | 0.90 (0.61, 1.34) | 0.63 | [12] |
| Jianwei Mieyou Inspissant vs CBS + A + C | 60/100 | 22/50 | 1.43 (1.01, 2.03) | 0.06 | [13] |
| Weikang Capsule vs RBC + A + M | 46/56 | 42/50 | 0.98 (0.82, 1.16) | 0.80 | [14] |
| Jiawei Liumo Decoction vs PPI + A + M | 37/41 | 28/30 | 0.97 (0.84, 1.11) | 0.64 | [16] |
| Weitongning Tab vs CBS + A + M | 32/40 | 31/40 | 1.03 (0.82, 1.30) | 0.78 | [17] |
| Qingwei Decoction vs PPI + A + M | 13/29 | 19/27 | 0.64 (0.40, 1.02) | 0.05 | [18] |
| Maimendong Granule vs PPI + A + M | 166/200 | 98/120 | 1.02 (0.91, 1.13) | 0.76 | [19] |
| Maimendong Granule vs PPI + A + M | 43/50 | 40/48 | 1.03 (0.87, 1.22) | 0.71 | [20] |
| Mieyou Decoction vs CBS + A + T | 22/32 | 19/28 | 1.01 (0.73, 1.43) | 0.88 | [21] |
| Formulae vs CBS + A + M | 81/93 | 49/56 | 1.00 (0.88, 1.13) | 0.94 | [23] |
| Weiyan Decoction vs PPI + A + C | 28/40 | 29/40 | 0.97 (0.73, 1.28) | 0.80 | [24] |
| Changweiqing oral liquie vs CBS + A + F | 53/103 | 36/52 | 0.74 (0.57, 0.96) | 0.03 | [11] |
| Jianpi Qinghua formula vs CBS + A + T | 15/42 | 29/35 | 0.43 (0.28, 0.66) | < 0.01 | [15] |
| Jianwei Yuyang Granule vs PPI + A + M | 11/24 | 18/22 | 0.56 (0.35, 0.90) | 0.01 | [22] |
| Weikang formula vs PPI + C + T | 26/40 | 26/30 | 0.75 (0.57, 0.98) | 0.04 | [25] |
Helicobacter pylori (H pylori) plays a crucial role in the pathogenesis of chronic gastritis, peptic ulcer, gastric mucosa associated lymphoid tissue (MALT) lymphoma and gastric carcinoma. Clinical trials have shown that H pylori eradication can relieve mucosa inflammation, accelerate ulcer healing, reduce complications and recurrence, alleviate MALT and decrease post-operative relapse of early stage gastric carcinoma. The eradication rates of H pylori with proton pump inhibitor (PPI) or colloidal bismuth subcitrate (CBS)-based triple therapy, recommended in the Maastricht consensus report, are around 85%-90%. However, antibiotic resistance in H pylori has increased over the past few years. The incidence of primary resistance to metronidazole is over 50%[1] and to clarithromycin is about 18%[2]. Antibiotic resistance decreases the eradication rates of triple therapies significantly. The eradication rate dropped by 38% for H pylori resistance to metronidazole and by 55% for the strain resistant to clarithromycin[3]. Additionally, the high dosages of antibiotics applied in triple therapies readily induce a high incidence of adverse effects in about 16%-29% of patients, and poor patient compliance decreases the efficacy indirectly[4]. Researchers are exploring more effective and safer treatment regimens.
Since Warren and Marshall isolated H pylori in 1982, many in vitro and in vivo studies on the treatment of H pylori with Traditional Chinese Medicine (TCM) have been carried out. The in vitro studies showed that some herbs such as Radix scutellariae, Lonicera, Radix Isatidis Seu Baphicacanthi, Coptis chinensis Franch and Fructus Aurantii Immaturus have obvious inhibitory effects on H pylori[5,6]. The results of clinical studies are, however, variable. There have been no systematic reviews on the efficacy and safety of TCM in the treatment of H pylori infection. Therefore, we reviewed the clinical trials treating H pylori with TCM published between 1982 and 2008 which compared the efficacy and safety of TCM with those of triple therapies, in order to define the status of TCM in H pylori treatment.
Inclusion criteria were as follows: (1) Randomized, positive-controlled and parallel trials, irrespective of whether blinding was adopted; (2) Subjects with chronic gastritis, peptic ulcers, remnant gastritis or gastro-esophageal reflux disease who were H pylori positive. Subjects with bleeding ulcer or gastric cancer were excluded. (3) Determination of H pylori positive status was in accordance with the criteria of the 2007 3rd National Consensus Report on H pylori Infection (Consensus Report)[7]; (4) The treatment groups received TCM including single herb, formulae or Chinese medicine products; (5) The control groups received the first line therapy regimens recommended in the Consensus Report; (6) Outcome measurements included H pylori eradication rate and the incidence of adverse effects. The definition of H pylori eradication must meet the requirements of the Consensus Report.
The electronic databases of MEDLINE (1982-2008), Cochrane Controlled Trials Register (1982-2008), Wei-Pu database (1989-2008) and Wan-Fang database (1998-2008) were searched by using a combination of MESH subject headings of “H pylori”, “Medicine”, “Traditional Chinese” and “clinical trial” without language limitation. Reference lists from trials selected by electronic searching and conference compilations were hand searched.
Two independent reviewers screened all retrieved trials according to the inclusion criteria. If the two reviewers disagreed, the difference was discussed. If a consensus could not be reached, a third reviewer was consulted. The quality of all the eligible trials was scored using the Jadad criteria[8]. Allocation concealment was assessed. Central randomization, pharmacy controlled randomization or random numbers sealed in envelopes were regarded as appropriate concealment. If the method of allocation concealment was not mentioned or the subjects were allocated by the sequence produced by an open random number table, it was regarded as inappropriate concealment[9].
H pylori eradication rate and the incidence of adverse effects were used to evaluate the efficacy and safety of the regimens. The relative risk of H pylori eradication rate and the incidence of adverse effects were calculated using the original data and presented with 95% confidence interval. If there was no heterogeneity among the trials, a fixed effects model was applied in the meta-analysis. If there was heterogeneity among the trials and I2 < 50%, a random effects model was used instead in the meta-analysis. If I2 > 50%, the meta-analysis was aborted. Potential publication bias was analyzed by the funnel plot. All statistics were conducted using RevMan4.2 software.
Three hundred and nine papers were obtained after initial searching. 293 papers were excluded after reading titles, abstracts or texts due to the non-clinical nature of the studies, repetitious publication or failure to meet the inclusion criteria. Sixteen papers[10-25] were finally included with a total number of 1983 subjects (124/per trial). All the clinical trials were conducted in China and published in Chinese language. The characteristics of the included trials are presented in Table 1.
The Jadad scores of all the included trials were not more than two. One trial presented details of the randomization methods (random number table)[22] while the rest did not present any such detail. All the trials were single-centered and non-blinded without reporting allocation concealment and sample size calculation. Three trials[10,17,23] reported dropout cases and one of these trials[23] conducted intention-to-treatment analysis.
Due to the wide variety of TCM treatment applied in each trial and the statistical heterogeneity of the trials (χ2 = 36.97, P = 0.001, I2 = 59.4%), the data could not been pooled to conduct a meta-analysis. The H pylori eradication rates of the TCM treatment in each trial are presented in Table 2. The average H pylori eradication rates following TCM treatment and triple therapy were 72% (36%-90%) and 78% (44%-93%), respectively. The eradication rates following Chang Wei Qing oral liquid and Jian Pi Qing Hua formula were lower than those of CBS-based triple therapies. The eradication rates following Jian Wei Yu Yang granule and Wei Kang formula were lower than those of PPI-based triple therapies. There were no differences in the eradication rates between the remaining TCM treatments and triple therapy. Worst-case scenario was performed in two trials[10,17] which reported dropout cases and did not conduct intention-to-treat analysis. There was no significant difference between the new result and the original result.
Eleven trials[10,11,14,16-20,23-25] reported adverse effects without presenting the methods of acquiring the information on adverse effects. Eight of 11 reported that TCM treatment had adverse effects with an average incidence of 2% (21/903), while 10 of 11 reported that triple therapy resulted in adverse effects with an average incidence of 29% (204/697). The adverse effects associated with TCM included dizziness, loss of taste, nausea/vomiting, poor appetite, belching and loose stool/diarrhea. There were no abnormalities in routine blood tests, liver or kidney function tests in the TCM groups. Twenty subjects withdrew from the trials due to adverse effects and all were in the triple therapy groups[10,11,17,23].
Funnel plot analysis was conducted using the eradication rate as the index. The figure was obviously asymmetrical (Figure 1).
Although the statistical data in this review showed that there were no differences in eradication rate between TCM treatment and triple therapy in the majority of the trials (12/16), there is no powerful evidence to conclude that TCM treatment has the same efficacy as triple therapy in the treatment of H pylori infection due to the following reasons:
All 16 trials were of poor quality and did not meet the requirements of the CONSORT statement[26]. Although these trials claimed to adopt randomization, only one trial presented details of the randomization method and the remaining trials did not give any details of the randomization method. None of the trials described the methods of allocation concealment. Thus, whether the randomization was truly or effectively conducted in these trials is doubtful. Inappropriate randomization or allocation concealment can lead to selection bias. All trials were single-centered and non-blinded, and the treatment courses of the study groups were longer than those of the control groups in some trials, which may have resulted in performance bias. Dropout cases were reported in only three of 16 trials and were not mentioned in the other trials. It is not clear whether these trials had any dropout cases or whether they were simply not reported. Having dropout cases without reporting can result in attrition bias. Selection bias, performance bias and attrition bias may overestimate the efficacy of treatment groups. Additionally, none of the trials showed the calculation of sample size, thus, whether the sample size met the statistical requirement or the reliability of the statistical results cannot be judged. Therefore, confidence in the results obtained from these poor quality trials is questionable.
The asymmetry of the funnel plot may have been caused by the poor quality of the trials and publication bias. All 16 trials were conducted in China and published in Chinese language. Fourteen trials were published in TCM journals, one in a journal of integrated Chinese and Western medicine and one in a college journal. TCM journals are inclined to publish only positive results showing efficacy of TCM treatment. Therefore, when we searched the literature, most of the papers we found reported positive results, with only a few presenting negative results.
The TCM formulae used in the 16 trials were composed of various herbs which can clear heat, strengthen the spleen, regulate Qi or promote blood circulation. The composition of the formula and the dosage of each herb were different in each trial. The treatment courses also varied in each trial, from 1 wk to 2 mo. This resulted in clinical heterogeneity. Heterogeneity analysis also showed that there was substantial statistical heterogeneity. Due to the limited number of trials and the variety of composition and treatment courses, these trials could not be classified into subgroups for meta-analysis. An overall comparison between TCM and triple therapy could not be conducted.
Eight of 11 trials showed that the incidence of adverse effects following TCM treatment was lower than those following triple therapy, while three trials showed that there was no difference between them. Although non-blinded and lacking in detail on how the information on adverse effects was obtained, due to the obvious difference in the incidence of adverse effects between the two groups (2% vs 29%), we speculate that TCM treatment might be safer than triple therapy.
On the basis of the above, we cannot conclude that TCM treatment has the same efficacy as triple therapy in the treatment of H pylori infection. TCM should not be recommended as monotherapy in the treatment of H pylori infection until stronger positive evidence is obtained. However, neither can we completely ignore the efficacy of TCM in the treatment of H pylori infection because of the poor quality of the trials. We suggest that a scientific proposal should be designed and clinical trials performed strictly in accordance with the requirements of evidence-based medicine, in order to truly show and objectively assess the efficacy of TCM in H pylori treatment. Additionally, based on the tenet that medical study originates from clinical practice and serves clinical practice, studies on TCM in the treatment of H pylori infection should focus on the problems faced in western medicine, such as antibiotic resistance, the high incidence of adverse effects and high cost of the treatment, in addition to comparing the efficacy between TCM and Western medical treatment.
As antibiotic resistance to Helicobacter pylori (H pylori) has increased over the past few years, the eradication rates following triple therapies have decreased. New approaches to eradicate H pylori are under development.
Some herbs show obvious inhibitory effects on H pylori in vitro. Traditional Chinese Medicine (TCM) alone or integrated with western medicine have been used to treat H pylori infection in many clinical trials. However, the results of these trials are controversial and the efficacy of TCM in H pylori treatment is indefinite.
This is the first systematic review to assess the efficacy of TCM in the treatment of H pylori infection. It truly presents the quality of the clinical trials using TCM in H pylori treatment. The authors objectively concluded that the validity of TCM in the treatment of H pylori infection could not be determined due to the poor quality of the trials.
This study suggested that TCM should not be recommended as monotherapy to treat H pylori infection and the quality of TCM clinical trials need to be improved in the future.
A systematic review is a summary of research that uses explicit methods to perform a thorough literature search and critical appraisal of individual studies to identify valid and applicable evidence.
This is a good paper.
Peer reviewer: Yoshiharu Motoo, MD, PhD, FACP, FACG, Professor and Chairman, Department of Medical Oncology, Kanazawa Medical University, 1-1 Daigaku, Uchinada, Ishikawa 920-0293, Japan
S- Editor Tian L L- Editor Webster JR E- Editor Zheng XM
| 1. | Yakoob J, Fan X, Hu G, Liu L, Zhang Z. Antibiotic susceptibility of Helicobacter pylori in the Chinese population. J Gastroenterol Hepatol. 2001;16:981-985. |
| 2. | Romano M, Iovene MR, Russo MI, Rocco A, Salerno R, Cozzolino D, Pilloni AP, Tufano MA, Vaira D, Nardone G. Failure of first-line eradication treatment significantly increases prevalence of antimicrobial-resistant Helicobacter pylori clinical isolates. J Clin Pathol. 2008;61:1112-1115. |
| 3. | Dore MP, Leandro G, Realdi G, Sepulveda AR, Graham DY. Effect of pretreatment antibiotic resistance to metronidazole and clarithromycin on outcome of Helicobacter pylori therapy: a meta-analytical approach. Dig Dis Sci. 2000;45:68-76. |
| 4. | Guo CY, Wu YB, Liu HL, Wu JY, Zhong MZ. Clinical evaluation of four one-week triple therapy regimens in eradicating Helicobacter pylori infection. World J Gastroenterol. 2004;10:747-749. |
| 5. | Du P, Zhu S, Lü P. [Antibacterial activity of 20 kinds of Chinese medicinal materials for Helicobacter pylori in vitro]. Zhongyaocai. 2001;24:188-189. |
| 6. | Liu B, Li XT, Xu HL, Wang HY, Zhao JM, Sun Y, Yang XM, Gu YP, Yang YL. Bactericidal action of 5 kinds of traditional herbal drugs for Helicobacter pylori. Zhongguo Xinyao Zazhi. 2002;11:457-459. |
| 7. | Helicobacter Pylori Group/Helicobacter Pylori Research Cooperation Group of Chinese Society of Gastroenterology. 3rd National consensus report on Helicobacter pylori infection. Weichangbingxue. 2008;13:42-46. |
| 8. | Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1-12. |
| 9. | Wang JY. Evidence based medicine and clinical practice. 1st ed. Beijing: Science Press 2002; 68-69. |
| 10. | Chen PH, Lu XJ. Eradication of Hp infection by compound Chinese formula: an observation of 215 cases. Shanghai Zhongyiyao Zazhi. 2001;35:20-21. |
| 11. | Hua GC, Fan ZZ, Sun J, Cao Q, Zhu MH, Li CH. Clinical observation on treating Helicobacter pylori positive chronic gastritis with Chang Wei Qing oral liquid. Shanghai Zhongyiyao Zazhi. 2003;37:9-11. |
| 12. | Fan HZ, Sheng JW. Study on clinical efficacy of An Zhong Yin in treating Helicobacter pylori related gastritis. Zhongguo Zhongyi Jichu Yixue Zazhi. 2006;12:544-545. |
| 13. | Hua Y, Ma H, Yan W. Clinical study on treating Hp related gastritis with Jian Wei Mie You inspissant. Beijing Zhongyi. 2006;25:484-485. |
| 14. | Ma XJ, Yu SL, Chen P, Chen XT, Ren YC, Zhao AC. Clinical observation on treating 56 cases of Helicobacter pylori related peptic ulcer with Wei Kang capsule. Zhongyi Zazhi. 2006;47:187-189. |
| 15. | Wang HB, Zhang SS, Li QG. Treating 34 cases of Helicobacter pylori related gastritis with combination of Jian Pi Qing Hua formula and western medicine. Zhongguo Zhongxiyi Jiehe Xiaohua Zazhi. 2006;14:390-391. |
| 16. | Wu YN. Observation on the efficacy of Jia Wei Liu Mo decoction in treating bile reflux gastritis with Helicobacter pylori infection. Henan Zhongyi. 2006;26:31-32. |
| 17. | Yang XG, Zheng XG. Observation on the efficacy of Wei Tong Ning in treating Helicobacter pylori related gastropathy. Hubei Minzu Xueyuan Xuebao. 2006;23:52-53. |
| 18. | Zhou ZH, Yang Q, Chen DQ, Li T, Mu J, Wang Y, Shi LJ, Yue Y, Wang W. Clinical study on treating Helicobacter pylori related gastropathy of dampness and heat with Qing Wei decoction. Zhonghua Zhongyiyao Zazhi. 2006;21:504-505. |
| 19. | Huang PY. Observation on the efficacy of Mai Men Dong granule in treating 200 cases of Helicobacter pylori positive gastritis. Xinzhongyi. 2007;39:37-39. |
| 20. | Jin T, Qiao YS, You YG. Clinical observation on the treatment of 50 cases of Hp related peptic ulcer by Yukui composition. Zhongyiyao Daobao. 2007;13:32-34. |
| 21. | Wang XJ, Guo JS, Li Q, Guo X, Jiang JL. Analysis on the efficacy of Mie You decoction in treating Helicobacter pylori related gastritis of dampness and heat. Zhongguo Shiyan Fangjixue Zazhi. 2008;14:67-68. |
| 22. | Ling JH, Huang LP, Li JB, Pan YZ, Huang YQ, Shen DZ. Anti-inflammation effect of Jianwei Yuyang granule on Helicobacter Pylori positive peptic ulcer patients. Zhongyi Zazhi. 2008;49:131-134. |
| 23. | Wang XW. Clinical observation on eradicating Helicobacter pylori with Chinese medicine and western medicine. Zhongwai Jiankang Wenzhai. 2008;5:59. |
| 24. | Xiao LD, Ye R, Wu XD, Zhou B, Xu HY. Clinical observation on eradicating Helicobacter pylori with Wei Yan decoction. Zhonghua Zhongyiyao Xuekan. 2008;26:804-805. |
| 25. | Xin H, Wang XY, Zhang JQ. Clinical observation on treating Hp related gastritis with Wei Kang formula. Sichuan Zhongyi. 2008;26:65-66. |
| 26. | Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel group randomized trials. BMC Med Res Methodol. 2001;1:2. |