Published online Oct 7, 2009. doi: 10.3748/wjg.15.4666
Revised: September 17, 2009
Accepted: September 24, 2009
Published online: October 7, 2009
The prevalence of anemia across studies on patients with inflammatory bowel disease (IBD) is high (30%). Both iron deficiency (ID) and anemia of chronic disease contribute most to the development of anemia in IBD. The prevalence of ID is even higher (45%). Anemia and ID negatively impact the patient’s quality of life. Therefore, together with an adequate control of disease activity, iron replacement therapy should start as soon as anemia or ID is detected to attain a normal hemoglobin (Hb) and iron status. Many patients will respond to oral iron, but compliance may be poor, whereas intravenous (IV) compounds are safe, provide a faster Hb increase and iron store repletion, and presents a lower rate of treatment discontinuation. Absolute indications for IV iron treatment should include severe anemia, intolerance or inappropriate response to oral iron, severe intestinal disease activity, or use of an erythropoietic stimulating agent. Four different products are principally used in clinical practice, which differ in their pharmacokinetic properties and safety profiles: iron gluconate and iron sucrose (lower single doses), and iron dextran and ferric carboxymaltose (higher single doses). After the initial resolution of anemia and the repletion of iron stores, the patient’s hematological and iron parameters should be carefully and periodically monitored, and maintenance iron treatment should be provided as required. New IV preparations that allow for giving 1000-1500 mg in a single session, thus facilitating patient management, provide an excellent tool to prevent or treat anemia and ID in this patient population, which in turn avoids allogeneic blood transfusion and improves their quality of life.
- Citation: Muñoz M, Gómez-Ramírez S, García-Erce JA. Intravenous iron in inflammatory bowel disease. World J Gastroenterol 2009; 15(37): 4666-4674
- URL: https://www.wjgnet.com/1007-9327/full/v15/i37/4666.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.4666
| Iron gluconate | Iron sucrose | Iron dextran (LMWID) | Ferric carboxymaltose | |
| Complex type | Type III | Type II | Type I | Type I |
| Labile and weak | Semi-robust and moderately strong | Robust and strong | Robust and strong | |
| Molecular weight (kDa) | 38 | 43 | 73 | 150 |
| Initial distribution volume (L) | 6 | 3.4 | 3.5 | 3.5 |
| Plasma half-life (h) | 1 | 6 | 30 | 16 |
| Labile iron release | +++ | ±1 | - | - |
| Direct iron donation to transferrin (% injected dose) | 5-6 | 4-5 | 1-2 | 1-2 |
| Test dose required | No | Yes/No3 | Yes | No |
| Maximal single dose (mg) | 125 | 300 | TDI | 1000 |
| Premedication | No | No | TDI only | No |
| Life-threatening ADE2 (× 106 doses) | 0.9 | 0.6 | 11.3 | ?? |
| Death rate (× 106 doses)2 | 0.25 | 0.11 | 0.78 | ?? |
| Study (yr) | n | Study design | Compound | Baseline Hb(g/dL) | Total dose, mg (schedule) | Duration (wk) | Response (%) | DCT (%) |
| Gasche et al[31] (2001) | 103 | Multicentre, open-label | Iron sucrose | ≤ 10.5 | 1200 mg (6 × 200 mg) | 4 | 65 | 0 |
| Bodemar et al[32] (2004) | 59 | Retrospective | Iron sucrose | < 12 | Mean 1400 mg (1-2 × 200 mg/wk) | 8 | 60 | 0 |
| 12 | 91 | |||||||
| Schröder et al[14] (2005) | 46 | Multicentre randomized open-label | Iron sucrose (22) | < 10.5 (F) | Mean 1418 mg (7 mg/kg + 5 × 200 mg) | 6 | 55 | 4.5 |
| Ferrous sulfate (24) | < 11 (M) | Mean 5600 mg (100-200 mg/d) | 53 | 20.8 | ||||
| García-López et al[35] (2006) | 70 | Single centre prospective observational | Iron sucrose | < 10.51 | Mean 920 mg (200-1800 mg) (200 mg/1-3 times a week) | Mean 5 (1-9 ) | 67 | 0 |
| Kulnigg et al[37] (2008) | 200 | Multicentre randomized open-label | Ferric carboxymaltose (137) | ≤ 10 | 1000-1500 mg (1-2 infusion of 500-1000 mg) | 12 | 77 | 1.5 |
| Ferrous sulfate (63) | 16 800 mg (200 mg/d) | 68 | 7.9 | |||||
| Lindgren et al[33] (2009) | 91 | Multicentre randomized investigator-blinded | Iron sucrose (45) | < 11.5 | Mean 1700 mg (200 mg/1-2 wk) | 20 | 66 | 7 |
| Ferrous sulfate (46) | Mean 38 400 mg (200-400 mg/d) | 47 | 22 | |||||
| Gisbert et al[34] (2009) | 100 | Multicentre, open-label | Iron sucrose (22) | < 10 | Not reported (2 × 200 mg/wk if Hb < 10) | 26 | 77 | 0 |
| Ferrous sulfate (78) | > 10 | 19 000 mg (106 mg/d) | 89 | 5.1 |
When body iron stores are depleted, iron supplementation seems beneficial, although the optimal route of administration remains controversial. Oral iron supplementation is adequate in some clinical conditions. Administration of oral iron, in the absence of inflammation or significant ongoing blood loss, can correct the anemia, provided significant doses can be tolerated. However, although conventional wisdom “says” that up to 200 mg of elemental iron per day is required to correct iron deficiency anemia (IDA), this is probably incorrect[1]. Early studies indicated that the co-administration of iron with ascorbic acid (vitamin C) might be of benefit in enhancing iron absorption, since, in theory, more ferrous iron is maintained in solution. However reports indicated that such co-administration can induce severe toxicity in the gastrointestinal tract[2]. Moreover, classically, oral iron intake separately from meals is recommended for increasing its absorption but this enhances digestive intolerance and, therefore, decreases compliance. In addition, the absorption of iron salts can be diminished by co-administration of some antibiotics (mainly quinolones, doxycycline, tetracyclines, chloramphenicol or penicillamine); proton pump inhibitors and anti-acid medication (aluminum, bicarbonate, zinc or magnesium salts), levodopa, levothyroxine, cholestyramine, phytates (high fiber diets), soy products, ibandronate, etidronate, tannates, calcium, and phenolic compounds (coffee, tea), whereas amino acids seem to act as enhancers of iron absorption[3].
On the other hand, non-absorbed iron salts may produce a variety of highly reactive oxygen species including hypochlorous acid, superoxides and peroxides that may lead to digestive intolerance, causing nausea, flatulence, abdominal pain, diarrhea or constipation, and black or tarry stools, and perhaps could activate relapsed inflammatory bowel disease (IBD)[2].
Although preoperative oral iron has shown to be efficacious in uncomplicated IDA, in anemia of chronic disease (ACD) [e.g. Crohn’s disease (CD)], as well as in that associated with acute inflammation (e.g. postoperative period after gastrointestinal surgery), the effectiveness of oral iron administration is rather limited since absorption is down-regulated, and the small amount of iron absorbed is directed to the reticulo-endothelial system (RES), where it is sequestered[4].
In these situations, intravenous (IV) iron has emerged as a safe and effective alternative for IBD anemia management. This takes into consideration factors such as intolerance of or contraindications to oral iron, severe anemia (especially if accompanied by significant ongoing bleeding), short time to surgery, need of a fast recovery or the use of erythropoiesis-stimulating agents (ESAs)[5]. Because daily iron absorption is only 1-2 mg (up to 10 mg in deep ferropenia), for patients presenting with moderate to severe anemia at least three to 4 mo of oral iron administration are needed to correct hemoglobin (Hb) levels and replenish iron stores. As IV iron can allow up to a five-fold erythropoietic response to significant blood-loss anemia in normal individuals[6], Hb starts rising in a few days, the percentage of responding patients is higher and iron stores are replenished. Boosting iron stores is an advantage, particularly for patients receiving ESAs[7].
All IV iron agents are colloids with spheroidal iron-carbohydrate nanoparticles. Each particle consists of an iron-oxyhydroxide core (Fe3+) and a carbohydrate shell that stabilizes the iron-oxyhydroxide core. Differences in core size and carbohydrate chemistry determine pharmacological and biologic differences between the different iron complexes, including clearance after injection, iron release in vitro, early evidence of iron bioactivity in vivo, and maximum tolerated dose and rate of infusion[8,9]. Complexes can generally be classified as labile or robust (kinetic variability), and as weak or strong (thermodynamic variability), with all possible intermediates. Four different products are mostly used in clinical practice: iron gluconate, iron sucrose, iron dextran, and iron carboxymaltose[2,10,11] (Table 1).
Iron gluconate has a core tightly bound to gluconate and weakly associated with sucrose (molecular weight 38 kDa), and is a type III iron complex (labile and weak) with fast degradation kinetics and direct release to plasma proteins (apotransferrin, apoferritin, and others). The potential for acute adverse reactions related to labile iron release after IV injection, which is caused by oversaturation of the transferrin binding capacity, is higher with iron gluconate compared to the other available IV iron preparations. Non-transferrin-bound labile iron may induce acute endothelial cell injury and a transient capillary leak syndrome. Clinical symptoms of iron acute toxicity include nausea, hypotension, tachycardia, chest pain, dyspnea (lung edema), and bilateral edema of the hands and feet, and should not be misread as anaphylaxis[9]. To avoid these side effects, the maximum recommended dose is 125 mg; whereas the administration of total dose is not recommended. The use of iron gluconate for iron deficiency (ID) in patients on dialysis has been found to be efficacious and safe[8,9].
Iron sucrose has a core tightly bound to sucrose (molecular weight 43 kDa), and is a partially stable type with medium degradation kinetics and partial uptake of released iron by plasma proteins such as (apo)-transferrin but also by the RES (Type II: semi-robust and moderately strong). Its half life is relatively short (5-6 h) and the amount of iron transported by transferrin, calculated using the Michaelis-Menten model for a single dose containing 100 mg of iron, is around 30 mg Fe3+/24 h[12]. Following a single IV injection of 100 mg iron sucrose to anemic patients, up to 95% of the injected iron was utilized within 2-4 wk. During the last few years, experience of using iron sucrose in various forms of ID has evolved. In spite of its safety profile, nowadays a test dose is still required at most European countries. Single doses of 100-200 mg as an IV injection[13] or up to 500 mg over an infusion time of 3.5 h seem to be safe[14]. The maximal recommended dosage is 600 mg/wk (200 mg iron as iron sucrose injected or infused intravenously no more than three times a week) but this amount exceeds the physiological needs of the proliferating erythroblast. If the infusion speed is too fast (above 4 mg Fe3+/min) or the single total iron dose too high (above 7 mg Fe3+/kg, with a maximum of 500 mg), non-transferrin bound labile iron may cause transient hypotension, tachycardia, and dyspnea, as described for iron gluconate. Paradoxically diarrhea, epigastric pain or dummy aches could appear within minutes to hours after infusion. Cases of phlebitis have been described, but they are probably secondary to longer lasting administration (rather than larger doses), the need to keep a venous access, or the use of solutions that are too dilute (iron concentration must be at least 1 mg/mL). Overall, iron sucrose is currently considered as the safest IV iron preparation[15].
Iron dextran is a stable parenteral iron product with a molecular weight of 73 kDa (Low molecular weight iron dextran, LMWID) or 156 kDa (High molecular weight iron dextran, HMWID). This type I iron complex (robust and strong) shows high structural homogeneity and only slow and competitive delivery to endogenous iron binding proteins. Complexes are actively phagocytosed by macrophages of the RES before they are released and become available for Hb synthesis. Although the plasma half life of LMWID is 30 h (3 d for HMWID), the full process of iron release from the dextran complex in the RES, storage in ferritin and delivery as TBI to the bone marrow or other tissues may take several months[16]. Iron dextran can be administered as intramuscular (i.m.) or IV injections and as IV infusion, but a test dose is always required before the first administration. The stability of the dextran complex allows administration of high single doses (so called ‘‘total dose infusion’’ which may be given over 4-6 h). In contrast, the bioavailability of iron following i.m. administration has not been studied extensively. There seems to be a risk of incomplete and variable absorption of the iron from the injection site, and a considerable amount (30%-50%) of iron can remain at the i.m. injection site for many months. Therefore i.m. injections are no longer recommended[17]. However, these iron complexes may cause well know dextran-induced anaphylactic reactions, especially in patients receiving HMWID (not commercially available in Europe and considered as an obsolete and dangerous IV iron agent). The exact mechanism of the anaphylactic reaction to iron dextran has not been clarified yet, but it seems to be related to the antibody-mediated release of mediators by mast cells[15].
FCM is another stable parenteral iron product with a molecular weight of 150 kDa very similar to iron dextran in terms of stability and structure (Type I, robust and strong). The pharmacokinetic characteristics of FCM are similar but not identical to iron dextran. The distribution volume of both preparations corresponds nearly to that of plasma, but half life is approximately 16 h for FCM as compared to 30 h for LMWID. It seems that FCM is broken down quicker than iron dextran because α-amylase does not affect dextran, or acts at a very slow rate[2]. A study using positron emission tomography has shown that iron from FCM accumulates in the liver, spleen and bone marrow and substantial amounts were found in these organs within minutes. In addition, FCM is able to exchange iron rapidly with transferrin[18]. As a result, the utilization of iron for RBC increased rapidly up to days 6 to 9, after which the utilization increased at a much lower rate. Patients with IDA showed iron utilization over 90% after 24 d compared to 60%-80% utilization for patients with renal anemia[18]. FCM is designed to mimic physiologically-occurring ferritin, providing high iron utilization, without the disadvantageous characteristics associated with iron dextran (anaphylaxis) and iron sucrose (high pH, high osmolarity, dosage limitations, and the long duration of administration). Up to 100-200 mg FCM can be administered as IV injection and up to 1000 mg iron can be infused in at least 15 min and no test dose is required (Table 1). In comparison, the European Union (EU) prescribing information for other IV iron preparations indicates they can be administered only in low doses (e.g. usual recommended dose of LMW iron dextran is 100-200 mg of iron and the maximum EU dose of iron sucrose is 200 mg of iron) over a period of greater than 30 min, which results in the need for frequent infusions to administer the total calculated iron replacement dose[19]. No serious adverse effects, including deaths, were considered related or likely related to FCM by trial investigators; however, the US Food and Drugs Administration has raised concerns about a potential mortality safety signal based on a increased of deaths in comparison to other arms across clinical trials[20]. In contrast, since 2007 the use of FCM corresponds to over 17 000 patient-years (one patient corresponds to 2000 mg iron), and up to September 2008 no anaphylactoid reactions or death have been reported, suggesting a good safety profile for FCM[2]. Therefore, information regarding FCM safety in the clinical setting is somehow conflictive and further post authorization trials to confirm its benefit and safety are needed.
Experience with the use of IV iron therapy is extensive in different clinical settings over the last 60 years. In the late 1980s, the introduction of recombinant human erythropoietin (rHuEPO) led to a revitalized interest in the use of iron therapy, either in combination with rHuEPO therapy, or alone. Intravenous iron therapy can be used in a variety of clinical settings, as long as iron parameters are carefully monitored. In a number of studies, IV iron was shown to be useful for the treatment of anemia associated with a variety of medical [IBD, chronic kidney disease (CKD), chronic inflammatory arthritis, congestive cardiac failure, pregnancy and postpartum, or cancer] and surgical conditions (orthopedic, cardiac, colorectal cancer, and gynecological surgical procedures)[21,22]. Interestingly, in the settings of CKD or cancer related anemia, the use of IV iron resulted not only in a more rapid and complete response to rHuEPO, but also in a reduction of rHuEPO dose, and probably in a reduction of rHuEPO side effects, such as thrombosis[23,24].
Approximately, one third of IBD patients suffer from recurrent anemia across different studies (ranging from 6% to 73%, depending on Hb cut-off for the definition of anemia; patient selection, IBD phenotype and year of publication), and the prevalence of ID is even higher [mean prevalence: 45%, 95% confidence interval (CI): 40%-50%]. A retrospective study found that the prevalence of mild to moderate anemia significantly decreased in the IBD population between 1993 and 2003 (33.8% vs 16.7%, P = 0.013), although the prevalence of severe anemia was similar (6.3% vs 5.6%, P = NS), and the only difference detected between the two cohorts was the increased use of immunosuppressive drugs (mainly azathioprine)[25]. Both ID (due to intestinal blood loss that cannot be matched by duodenal iron absorption, creating a negative iron balance) and ACD (due to the inflammatory nature of the disease) contribute most to the development of anemia in IBD, whereas cobalamin or folate deficiency and various other causes of anemia such as hemolysis occur infrequently. Whatever the underlying mechanism, anemia is universally accepted as a condition having a significant impact on the affected patient’s quality of life[1,26].
Anemia control and recovery in patients with IBD has a beneficial impact on quality of life indices. Our goal is to attain Hb levels above 13 g/dL in males and 12 g/dL in females by the administration of iron supplements, with or without erythropoietin. However, it is worth noting that without an appropriate control of disease activity, the management of anemia associated to IBD is much more difficult. Thus, it is desirable to initiate the pharmacological treatment after adequate inflammation control[27].
According to the recommendations of the Guidelines on the Diagnosis and Management of Iron Deficiency and Anemia in Inflammatory Bowel Diseases (Statement 2B), iron supplementation should be initiated when IDA is present (Grade A). For ID without anemia, different approaches to iron replacement should be considered and discussed with the patient. If patients are likely to develop IDA the monitoring frequency should be increased (Grade D)[26], although it is unknown how often monitoring should be performed.
When body iron stores are depleted, iron supplementation seems beneficial, although the optimal route of administration remains controversial. Total iron deficit (TID) can be calculated using the Ganzoni’s formula: TID (mg) = Weight (kg) × (Ideal Hb - Actual Hb) (g/dL) × 0.24 + depot iron (500 mg).
According to this formula, a person weighing 70 kg with an Hb level of 9 g/dL would have a body iron deficit of about 1400 mg. Nevertheless, Ganzoni’s formula may underestimate iron depot in males, as in them it has been consistently reported to be 700-900 mg[28]. Thus, a TID of 1600-1800 mg may be a more realistic estimation for this subject.
Following the administration of oral iron to a patient with uncomplicated IDA, it takes 2-2.5 wk for the Hb to start rising, 2 mo for it to reach normal levels and 6 mo for iron stores to be replete[7]. However, the efficacy of oral iron therapy in IBD patients may be hindered by some IBD specific factors, such as reduced absorption of iron due to inflammation and gastrointestinal side effects of oral ferrous iron (due to the release of activated hydroxyl radicals that may lead to digestive intolerance, causing nausea, flatulence, abdominal pain, diarrhea or constipation, and black or tarry stools)[2]. In addition, oral iron compounds are not all alike, as they may vary in composition, elemental iron concentration, absorption profile, efficiency or tolerance. As a non-written rule, the best tolerated oral agent is usually the one that contains or delivers less iron.
In a crossover study of 19 IBD patients randomly assigned to start treatment with ferrous fumarate 120 mg orally once daily or iron sucrose 200 mg IV three times during a period of 14 d, oral ferrous fumarate, but not IV iron sucrose, increased clinical disease activity in IBD patients[29]. In contrast, iron sucrose, but not ferrous fumarate, increased intravascular oxidative stress[29]. However, a prospective study comparing usage, tolerance, and efficacy of 4 wk therapy with oral iron therapy in patients with ID and IBD and patients with ID of non-inflammatory cause, intolerance to iron was reported in 24% of the patients who had IBD (non-active) and 29% of the patients who did not (P = NS), and only a tiny minority of IBD patients relapse in association with use of oral iron therapy[30]. This data suggest that patients with IBD are no more intolerant to oral iron than other patients and have similar rates of repletion, but the low number of evaluable patients (n = 47) precluded the drawing of definite conclusions. Nevertheless, to avoid the risk of poisoning, other oral iron compounds (such as iron polymaltose which has very low toxicity and meets the requirements for a food supplement) might be used instead of ferrous salt preparations[2] and lower doses (e.g. 50-100 mg of elemental iron) should be recommended[1]. Additionally, the response and tolerance should be monitored and treatment changed to IV iron if necessary (Grade C)[26].
Is this statement supported by the information reviewed above? Because of the limitations of oral iron therapy in IBD patients, parenteral routes of iron administration should be preferred, even though many patients will respond to oral iron. Intravenous iron is more effective, better tolerated, and improves quality of life to a greater extent than oral iron supplements (Grade A)[26]. Absolute indications for IV iron include severe anemia (Hb < 10 g/dL), intolerance or inappropriate response to oral iron (once iron therapy has been initiated the response may be: “complete”, if Hb increases ≥ 2 g/dL; “partial”, if Hb increases 1-1.9 g/dL; or “no-response”, if Hb increases < 1 g/dL), severe intestinal disease activity, concomitant therapy with an erythropoiesis stimulating agent, or patient preference[26]. In a prospective study of 103 patients with severe IBD-associated anemia who received IV iron sucrose for 4 wk (total dose 1200 mg), Gasche et al[31] investigated the parameters that can predict effectiveness. Overall, a complete response at the end of the fourth week was observed in 67 (65%) patients, and the variables significantly associated with response were serum erythropoietin, soluble transferring receptor, transferrin, and IL-6 levels. Once again, these data emphasized the need for an adequate inflammation control in IBD patients. We will review some studies assessing the efficacy of IV iron in anemic IBD patients (Table 2).
In this regard, a retrospective observational study in IBD patients with poor response or intolerance to oral iron, the administration of iron sucrose (200 mg once or twice per week to reach total ID) resulted in a “complete” response (Hb increment ≥ 2 g/dL or correction of anemia) in 60% of patients within 8 wk and in 90% of patients within 12 wk[32]. However, a randomized, controlled, open-label, multicenter trial performed in 46 patients with anemia and transferrin saturation ≤ 20% and/or serum ferritin concentrations ≤ 20 μg/L found no differences in Hb increment within 6 wk between patients receiving IV iron sucrose and those receiving iron sulfate, but resulted in building up iron stores (about ferritin = 200 ng/mL, after 6 wk)[14]. In addition, intractable gastrointestinal adverse events caused permanent study drug discontinuation in five patients (20.8%) receiving iron sulfate, whereas only one patient (4.5%) was withdrawn because of side effects due to IV iron sucrose[14]. Thus, although being equal in short-term efficacy, these results suggest a better gastrointestinal tolerability for iron sucrose.
In a very recent study, 91 patients with IBD and anemia (Hb < 11.5 g/dL) were randomized to oral iron sulfate (n = 46) or IV iron sucrose (n = 45) treatment for 20 wk. More patients in the IV iron group completed the study (93% vs 78%, P = 0.001), increased their Hb ≥ 2 g/dL (66% vs 47%, P = 0.07), raised their ferritin levels to normal (74% vs 48%, P = 0.013), and recovered from anemia (84% vs 59%, P = 0.007) compared to patients in the oral iron group. In addition, treatment with IV iron sucrose improved iron stores faster and more effectively than oral iron (P = 0.002). Only 22 patients (48%) tolerated the prescribed oral dose, and 52% reduced the dose or withdrew from treatment because of poor tolerance[33].
Finally, in a prospective multicenter study of 100 IBD patients with IDA [59 CD, 41 ulcerative colitis (UC)], those with Hb > 10 g/dL were prescribed oral ferrous sulphate (n = 78) and those with Hb < 10 g/dL received IV iron sucrose (n = 22). Hb normalization was achieved in 89% with oral and 77% with IV iron, and was associated with a relevant improvement in the patients’ quality of life. IBD activity increase was not demonstrated in any patient. Four patients (5.1%) showed oral iron intolerance leading to discontinuation of treatment, whereas no adverse events were reported for IV iron[34]. Thus, oral iron treatment was effective and well tolerated in most IBD patients, and did not exacerbate the symptoms of the underlying IBD, whereas IV iron was an effective and safe treatment in more severely anemic or intolerant patients.
At one author’s centre, the safety and efficacy of IV iron sucrose therapy was evaluated in a preliminary study of 70 patients with digestive pathology (54 IBD: 27 CD, 18 UC, nine pouchitis) (34). IV iron sucrose in an “outpatient regimen” was used for patients with IDA due to digestive disorders with at least one of the following criteria: (1) no response or intolerance to oral iron; (2) IBD with severe anemia (Hb < 10.5 g/dL) and/or (3) clinical need of quick recovery of anemia. Average baseline Hb was 9.8 ± 1.7 g/dL, and 11.7 ± 1.5 g/dL at the end of treatment (mean increase 1.9 g/dL, range -2 to 5.5 g/dL), Hb increase exceeded 2 g/dL in 47% of treatments, and anemia was corrected in 67.8% of patients. No severe adverse events were witnessed. The authors concluded that IV iron sucrose should become the standard of care in IBD patients with ID[35].
Therefore, treatment with IV iron sucrose is effective, safe, and well tolerated in correcting Hb and iron stores in patients with IBD, especially in those with severe anemia. The main disadvantage of IV iron sucrose is the need for multiple infusions as the maximun weekly dose should not exceed 600 mg. The availability of stable parenteral iron compounds allowing for TDI infusion may greatly facilitate iron replacement therapy in IBD patients.
As for children, the safety and efficacy of IV iron therapy was retrospectively evaluated in 70 pediatric patients with IBD (50 CD, 20 UC) who received a total of 119 TDI iron dextran infusions between February 1994 and February 2000. The average increase in Hb concentration was 2.9 g/dL. The authors concluded that TDI infusion of iron dextran, when appropriately used, is a safe and potentially efficacious treatment for children with IBD and IDA who are unresponsive to, or noncompliant with, oral iron therapy[36]. However, as mentioned above, iron dextran, especially HMWID, has the disadvantage of potentially life-threatening dextran-associated anaphylactic reactions.
More recently, Kulnigg et al[37] randomized 200 anemic IBD patients (about Hb = 9 g/dL) to receive IV FCM (FCM, n = 173; maximum 1000 mg iron per infusion) at 1-wk intervals until the patients’ calculated TID was reached or oral ferrous sulfate (100 mg bid) for 12 wk. There were no differences between groups in Hb improvement at week 12 (3.8 ± 2.0 g/dL in both groups) or treatment-related adverse events, but response (defined as Hb increase of > 2.0 g/dL) was higher for FCM at week 2 (P = 0.0051) and week 4 (P = 0.0346), with a lower rate of discontinuation of study medication due to adverse events (1.5% and 7.9%, respectively), than for oral iron. Thus, FCM seems to be effective and safe in IBD-associated anemia,
Overall, from data depicted in Table 2, the mean response of IBD-associated anemia to treatment with IV iron (weighted mean) was (281/382) 73.6% and (140/215) 65.1% with oral iron, [odds ratio (OR): 1.49, 95% CI: 1.02-2.17, P = 0.02]. When the analysis was performed for data extracted from prospective randomized trials only, the response to IV iron was 72.5% (143/198) vs 58.2% (71/122) (OR: 1.87, 95% CI: 1.13-3.09, P = 0.0097). In addition, reviewed data strongly suggest that for patients with IBD, treatment with IV iron is effective, safe, well tolerated, provides a fast Hb increase and a sufficient refill of iron stores, and presents a lower rate of treatment discontinuation than oral iron. However, further research is needed to ascertain what is the appropriate timing to start treatment, which are the target Hb and ferritin levels to reach, and how IV iron may affect IBD clinical time course.
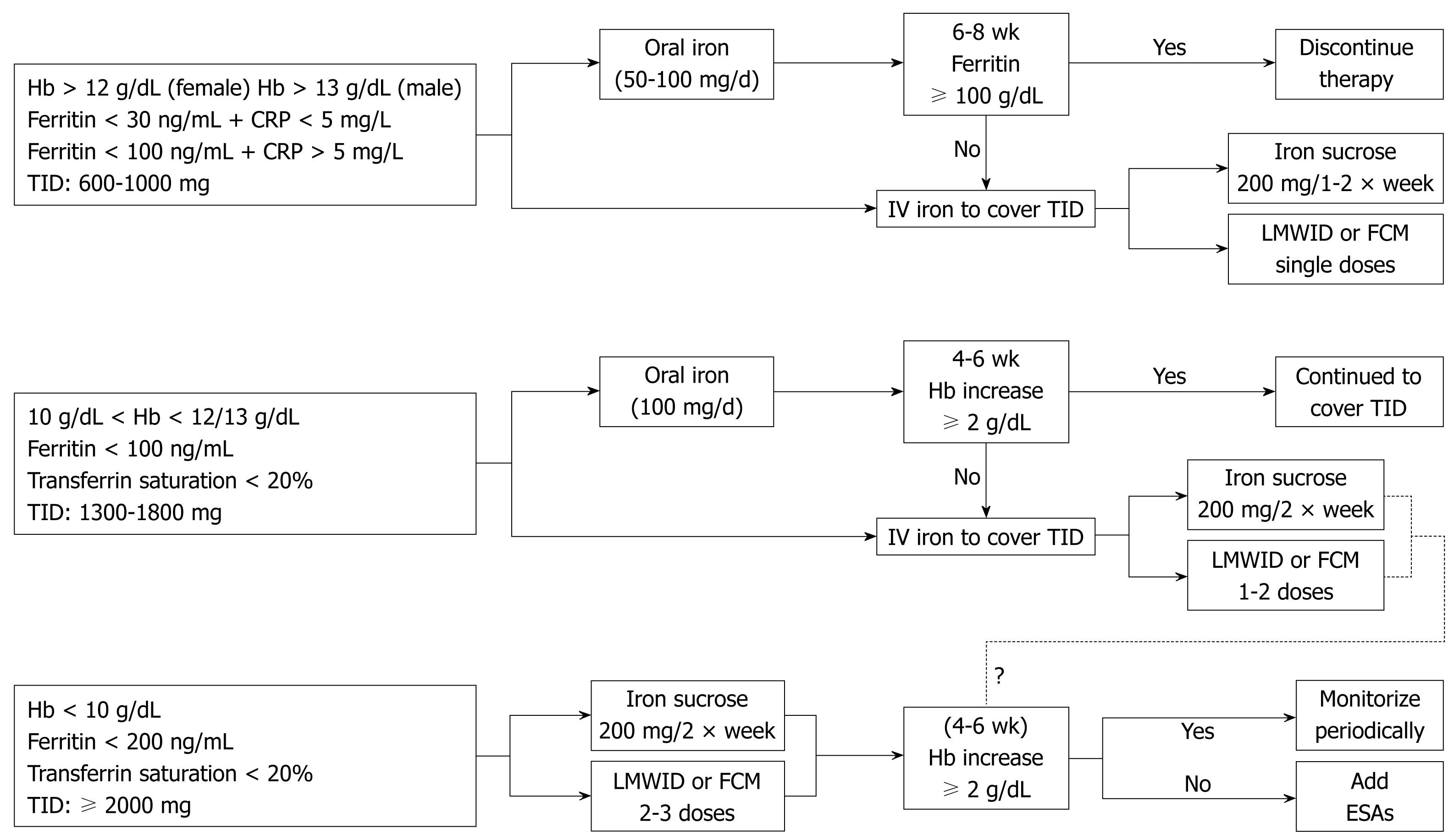
Although further research is needed to ascertain what is the appropriate time to start treatment, which are the target Hb and ferritin levels to reach, or how IV iron may affect the IBD clinical time course, a tentative, easy to follow algorithm for iron replacement in IBD patients is depicted in Figure 1.
According to this algorithm, in which we assume that the severity of ID and anemia correlates with disease activity, the total iron dose and the route of administration rely upon baseline Hb, serum ferritin level and transferrin saturation. For male IBD patients (70-90 kg) with Hb > 13 g/dL and ferritin < 30 ng/mL, TID is estimated to be around 800-1000 mg. The corresponding value for TID in women (60-80 kg) with Hb > 12 g/dL would be 600-800 mg. If there are no contraindications, oral iron would be the simplest replacement therapy (50-100 mg/d, for 2-3 mo), and iron complexes are preferred to iron salts. If there is a contraindication for, or a non adequate response (ferritin < 100 ng/mL after 6-8 wk) to, oral iron, an IV iron preparation should be administered: iron sucrose (200 mg IV, 1-2 times/wk), LMWID (up to 1000 mg IV, single dose), or FCM (up to 1000 mg IV, single dose).
As for patients with Hb between 10 g/dL and 12/13 g/dL, ferritin < 100 ng/mL and transferrin saturation < 20%, TID is estimated to be 1300-1800 mg. Oral iron supplements could still be indicated (100 mg/d for 4-6 mo) but the IV route is preferred, as it will provide a faster Hb recovery (iron sucrose 200 mg IV, twice a week; LMWID or FCM, two doses one week apart). Nevertheless, IV iron should be administered when there is intolerance to, or a non complete response to, oral iron, as defined by an Hb increase ≥ 2 g/dL or Hb normalization.
For patients with Hb < 10 g/dL and transferrin saturation < 20%, a higher ferritin trigger is selected (< 200 ng/mL), and a TID ≥ 2000 mg is estimated. These patients should receive IV iron (iron sucrose 200 mg IV, twice a week; LMWID or FCM, 2-3 doses). Patients should be re-evaluated after 4-6 wk; if there is not a complete Hb response, adjuvant treatment with erythropoiesis stimulating agents (e.g. epoetin, darbepoetin) should be considered.
Nausea, abdominal pain, constipation, diarrhea, injection site reactions (pain, superficial phlebitis), metallic taste, headache, dizziness and rash may occur with all IV preparations, and were observed in clinical trials with an incidence of 1%-3%. However, the incidence of life-threatening adverse drug events (ADEs) associated with parenteral iron is much smaller.
The numbers of non-CKD patients receiving IV iron are not large enough to draw definitive conclusions regarding the safety of IV iron agents in these clinical settings. Therefore, we will focus on ADEs associated with parenteral iron in CKD patients, as they are the largest collective receiving these drugs. According to data from the United States Food and Drug Administration (FDA) on ADEs attributed to the provision of four formulations of IV iron (HMWID, LMWID, iron gluconate and iron sucrose) during 2001-2003, the total number of reported parenteral iron-related ADEs was 1141 amongst approximately 30 million doses administered (approx. 38 ADEs per million), with 11 deaths (seven iron dextran, three iron gluconate, one iron sucrose)[15]. Relative to lower molecular weight iron dextran, total and life-threatening ADEs were significantly more frequent among recipients of higher molecular weight iron dextran and significantly less frequent among recipients of sodium ferric gluconate complex and iron sucrose. The absolute rates of life-threatening ADEs were 0.6, 0.9, 3.3 and 11.3 per million for iron sucrose, sodium ferric gluconate complex, lower molecular weight iron dextran and higher molecular weight iron dextran, respectively, whereas absolute rates of death were 0.11, 0.25, 0.75 and 0.78 per million, respectively (Table 1). However, there were no significant differences in mortality rates between LMWID and iron gluconate (OR: 0.3, 95% CI: 0.1-1.3) or iron sucrose (OR: 0.2, 95% CI: 0.1-1.0), and there are no conclusive data available regarding the safety of FCM. Therefore, the frequency of IV iron-related ADEs reported to the FDA has decreased, and overall, the rates are extremely low (Table 1). In addition, the rates of ADES associated with IV iron, including iron-related deaths, are much lower than that of ABT-related severe side effects (10 per million) and ABT-related deaths (four per million)[38].
Current information on the relationship between IV iron and infection, and between IV iron and oxidative stress deserves special consideration. Elemental iron is an essential growth factor for bacteria with many species expressing iron transport proteins that compete with transferrin, and it has long been suggested that patients with iron overload are at increased risk of infection[39]. In contrast, in the peritoneal dialysis population, no increased risk of peritonitis was found in patients receiving IV iron with respect to those not receiving IV iron[40]. In addition, a meta-analysis of six observational studies (807 patients) revealed that the administration of IV iron to patients undergoing major orthopedic surgery led to a significant decrease in both transfusion rate [relative risk (RR): 0.60, 95% CI: 0.50-0.72, P < 0.001] and infection rate (RR: 0.45, 95% CI: 0.32-0.63, P < 0.001)[41]. Nevertheless, despite this absence of definitive clinical data, it seems sensible to avoid IV iron administration in the setting of acute infection, and to withhold IV iron in patients with pre-treatment ferritin values > 500 ng/mL[5].
Biologically active iron, which is released by all IV iron agents, also plays a role in inflammation, oxidative stress and the propensity for accelerated atherosclerosis. Persistent oxidative stress in CKD patients promotes inflammation and, in turn, atherogenesis, and increased cardiovascular morbidity and mortality. However, available evidence relating IV iron administration to atherogenesis is indirect, and there is little evidence that IV iron adversely affects survival in patients with dialysis-dependent CKD. Nevertheless, the evidence argues for caution, not complacency, in prescribing IV iron[9].
The association between iron overload with cancer risk in humans has been under increased scrutiny in recent decades, although epidemiological studies on the association of iron with cancer remain inconclusive. The concerns are mostly focused on a possible risk associated with dietary iron in colorectal cancer, the increased risk of developing hepatocellular carcinoma in hereditary hemochromatosis and related hepatic iron overload and cirrhosis, and association between occupational exposure to iron and kidney, lung and stomach cancers. The risk of iron-induced sarcoma by repeated i.m. injections of iron dextran has also been raised. However, IV iron therapy has not been associated with an increase in tumor incidence[42].
The prevalence of anemia across the studies on patients with IBD is high (30%) and that of ID is even higher (45%). However, the prevalence of anemia is decreasing, and this seems to be related with the availability and use of IV iron[43].
Iron replacement therapy should start as soon as anemia or ID is detected (Grade D), and its goal is to attain a normal level of Hb, ferritin and transferrin saturation (Grade D)[26]. Importantly, our efforts to correct anemia should rely on adequate inflammation control, in the absence of which no proper approach to this condition is feasible[27].
Although many IBD patients will respond to oral iron, IV iron is more effective, better tolerated, and improves the quality of life to a greater extent than oral iron supplements (Grade A)[26]. Absolute indications for IV iron include severe anemia, intolerance or inappropriate response to oral iron, severe intestinal disease activity, use of ESAs, or patient preference[26].
The use of ESAs should be restricted to those patients presenting with Hb < 10 g/dL and who do not appropriately respond to IV iron replacement for 4 wk (Grade B)[26].
After the initial resolution of anemia and the repletion of iron stores, patients should be closely monitored, and maintenance iron treatment should be provided as required. New IV preparations that allows for giving up to 1000-1500 mg in a single session, provide an excellent tool to avoid or treat anemia and ID in the IBD patient population.
Peer reviewer: Bruno Annibale, Professor, Digestive and Liver Disease Unit, University “La Sapienza” II School of Medicine, Via di Grottarossa 1035, Roma 00189, Italy
S- Editor Tian L L- Editor O’Neill M E- Editor Zheng XM
| 1. | Gisbert JP, Gomollón F. Common misconceptions in the diagnosis and management of anemia in inflammatory bowel disease. Am J Gastroenterol. 2008;103:1299-1307. |
| 2. | Crichton RR, Danielsson BG, Geisser P. Iron therapy with special emphasis on intravenous administration. 4th ed. Bremen: UNI-Med Verlag AG 2008; . |
| 3. | Cook JD. Diagnosis and management of iron-deficiency anaemia. Best Pract Res Clin Haematol. 2005;18:319-332. |
| 5. | Beris P, Muñoz M, García-Erce JA, Thomas D, Maniatis A, Van der Linden P. Perioperative anaemia management: consensus statement on the role of intravenous iron. Br J Anaesth. 2008;100:599-604. |
| 6. | Goodnough LT, Skikne B, Brugnara C. Erythropoietin, iron, and erythropoiesis. Blood. 2000;96:823-833. |
| 7. | Maniatis A. The role of iron in anaemia management: can intravenous iron contribute to blood conservation? ISBT Sci Ser. 2008;3:139-143. |
| 8. | Aronoff GR. Safety of intravenous iron in clinical practice: implications for anemia management protocols. J Am Soc Nephrol. 2004;15 Suppl 2:S99-S106. |
| 9. | Van Wyck DB. Labile iron: manifestations and clinical implications. J Am Soc Nephrol. 2004;15 Suppl 2:S107-S111. |
| 10. | Silverstein SB, Rodgers GM. Parenteral iron therapy options. Am J Hematol. 2004;76:74-78. |
| 11. | Fishbane S, Kowalski EA. The comparative safety of intravenous iron dextran, iron saccharate, and sodium ferric gluconate. Semin Dial. 2000;13:381-384. |
| 12. | Danielson BG. Structure, chemistry, and pharmacokinetics of intravenous iron agents. J Am Soc Nephrol. 2004;15 Suppl 2:S93-S98. |
| 13. | Macdougall IC. Experience with intravenous iron in nephrology. Semin Hematol. 2006;43:S9-S12. |
| 14. | Schröder O, Mickisch O, Seidler U, de Weerth A, Dignass AU, Herfarth H, Reinshagen M, Schreiber S, Junge U, Schrott M. Intravenous iron sucrose versus oral iron supplementation for the treatment of iron deficiency anemia in patients with inflammatory bowel disease--a randomized, controlled, open-label, multicenter study. Am J Gastroenterol. 2005;100:2503-2509. |
| 15. | Chertow GM, Mason PD, Vaage-Nilsen O, Ahlmén J. Update on adverse drug events associated with parenteral iron. Nephrol Dial Transplant. 2006;21:378-382. |
| 16. | Grau PW. Intravenous iron therapy. Disorders of iron homeostasis, erythrocytes, erythropoiesis. Paris: European School of Haematology 2006; 420-434. |
| 17. | Auerbach M, Ballard H, Glaspy J. Clinical update: intravenous iron for anaemia. Lancet. 2007;369:1502-1504. |
| 18. | Beshara S, Sörensen J, Lubberink M, Tolmachev V, Långström B, Antoni G, Danielson BG, Lundqvist H. Pharmacokinetics and red cell utilization of 52Fe/59Fe-labelled iron polymaltose in anaemic patients using positron emission tomography. Br J Haematol. 2003;120:853-859. |
| 19. | Lyseng-Williamson KA, Keating GM. Ferric carboxymaltose: a review of its use in iron-deficiency anaemia. Drugs. 2009;69:739-756. |
| 20. | Food and Drugs Administration Center for Drug Evaluation and Research. Summary minutes of the Drug Safety and Risk Management Advisory Committee. February 1, 2008 [online]. Available from: URL: http://www.fda.gov/ohrms/dockets/ac/08/minutes/2008-4337m1-Final.pdf. |
| 21. | Auerbach M, Goodnough LT, Picard D, Maniatis A. The role of intravenous iron in anemia management and transfusion avoidance. Transfusion. 2008;48:988-1000. |
| 22. | Muñoz M, Breymann C, García-Erce JA, Gómez-Ramírez S, Comin J, Bisbe E. Efficacy and safety of intravenous iron therapy as an alternative/adjunct to allogeneic blood transfusion. Vox Sang. 2008;94:172-183. |
| 23. | Kapoian T, O'Mara NB, Singh AK, Moran J, Rizkala AR, Geronemus R, Kopelman RC, Dahl NV, Coyne DW. Ferric gluconate reduces epoetin requirements in hemodialysis patients with elevated ferritin. J Am Soc Nephrol. 2008;19:372-379. |
| 24. | Henry DH, Dahl NV, Auerbach M. Is thromboembolism in cancer patients treated with erythropoietic stimulating agents related to thrombocytosis and iron restricted erythropoiesis [Abstract]. Blood. 2007;110:1625. |
| 25. | Vijverman A, Piront P, Belaiche J, Louis E. Evolution of the prevalence and characteristics of anemia in inflammatory bowel diseases between 1993 and 2003. Acta Gastroenterol Belg. 2006;69:1-4. |
| 26. | Gasche C, Berstad A, Befrits R, Beglinger C, Dignass A, Erichsen K, Gomollon F, Hjortswang H, Koutroubakis I, Kulnigg S. Guidelines on the diagnosis and management of iron deficiency and anemia in inflammatory bowel diseases. Inflamm Bowel Dis. 2007;13:1545-1553. |
| 27. | de la Morena F, Gisbert JP. [Anemia and inflammatory bowel disease]. Rev Esp Enferm Dig. 2008;100:285-293. |
| 28. | Walters GO, Miller FM, Worwood M. Serum ferritin concentration and iron stores in normal subjects. J Clin Pathol. 1973;26:770-772. |
| 29. | Erichsen K, Ulvik RJ, Nysaeter G, Johansen J, Ostborg J, Berstad A, Berge RK, Hausken T. Oral ferrous fumarate or intravenous iron sucrose for patients with inflammatory bowel disease. Scand J Gastroenterol. 2005;40:1058-1065. |
| 30. | de Silva AD, Tsironi E, Feakins RM, Rampton DS. Efficacy and tolerability of oral iron therapy in inflammatory bowel disease: a prospective, comparative trial. Aliment Pharmacol Ther. 2005;22:1097-1105. |
| 31. | Gasche C, Waldhoer T, Feichtenschlager T, Male C, Mayer A, Mittermaier C, Petritsch W. Prediction of response to iron sucrose in inflammatory bowel disease-associated anemia. Am J Gastroenterol. 2001;96:2382-2387. |
| 32. | Bodemar G, Kechagias S, Almer S, Danielson BG. Treatment of anaemia in inflammatory bowel disease with iron sucrose. Scand J Gastroenterol. 2004;39:454-458. |
| 33. | Lindgren S, Wikman O, Befrits R, Blom H, Eriksson A, Granno C, Ung KA, Hjortswang H, Lindgren A, Unge P. Intravenous iron sucrose is superior to oral iron sulphate for correcting anaemia and restoring iron stores in IBD patients: A randomized, controlled, evaluator-blind, multicentre study. Scand J Gastroenterol. 2009;39:1-8. |
| 34. | Gisbert JP, Bermejo F, Pajares R, Pérez-Calle JL, Rodríguez M, Algaba A, Mancenido N, de la Morena F, Carneros JA, McNicholl AG. Oral and intravenous iron treatment in inflammatory bowel disease: Hematological response and quality of life improvement. Inflamm Bowel Dis. 2009;15:1485-1491. |
| 35. | García-López S, Gomollón F, García-Erce JA, Araméndiz R, Sicilia B, Vicente R. Intravenous iron sucrose: a simple, safe, and quick method to treat anemia secondary to digestive diseases [Abstract]. Gastroenterology. 2006;130:A84. |
| 36. | Mamula P, Piccoli DA, Peck SN, Markowitz JE, Baldassano RN. Total dose intravenous infusion of iron dextran for iron-deficiency anemia in children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2002;34:286-290. |
| 37. | Kulnigg S, Stoinov S, Simanenkov V, Dudar LV, Karnafel W, Garcia LC, Sambuelli AM, D'Haens G, Gasche C. A novel intravenous iron formulation for treatment of anemia in inflammatory bowel disease: the ferric carboxymaltose (FERINJECT) randomized controlled trial. Am J Gastroenterol. 2008;103:1182-1192. |
| 38. | Stainsby D, Jones H, Asher D, Atterbury C, Boncinelli A, Brant L, Chapman CE, Davison K, Gerrard R, Gray A. Serious hazards of transfusion: a decade of hemovigilance in the UK. Transfus Med Rev. 2006;20:273-282. |
| 39. | Weiss G. Iron and immunity: a double-edged sword. Eur J Clin Invest. 2002;32 Suppl 1:70-78. |
| 40. | Vychytil A, Haag-Weber M. Iron status and iron supplementation in peritoneal dialysis patients. Kidney Int Suppl. 1999;69:S71-S78. |
| 41. | García-Erce JA, Cuenca J, Gómez-Ramírez S, Villar I, Herrera A, Muñoz M. [Therapeutic options for anemia management in orthopedic surgery]. Anemia. 2009;2:17-27. |
| 42. | Huang X. Iron overload and its association with cancer risk in humans: evidence for iron as a carcinogenic metal. Mutat Res. 2003;533:153-171. |
| 43. | Gasche C, Kulnigg S. Intravenous iron in inflammatory bowel disease. Semin Hematol. 2006;43:S18-S22. |