INTRODUCTION
Colorectal cancer liver metastases and hepatocellular carcinomas are the two most common malignancies of the liver, associated with a dismal outcome of zero survival at 5-year if left untreated. Radiofrequency ablation (RFA) is rapidly emerging as one of the most popular, effective, minimally invasive, alternative therapeutic tool to hepatic surgery in the treatment of these liver malignancies. Conventional 2-dimensional (2D) unenhanced ultrasound (US) guidance of RFA is limited due to its lower sensitivity when compared with the referal modalities, such as contrast enhanced computerized tomography (CT) or magnetic resonance imaging (MRI). Recent advances in non-linear imaging modes and developments in 3-dimensional (3D) mechanical and electronic ultrasound probes have led to a marked improvement in the real-time contrast enhanced volumetric imaging with potential impact in the detection, planning and targeting strategy of RFA needle electrodes in the treatment beyond small lesions. The objective of this article is to demonstrate the usefulness of contrast-enhanced 3D ultrasound (CE-3DUS) in the radiofrequency ablation of these liver tumors. The indications for RFA of metastases and hepatocellular carcinomas will be reviewed within the appropriate clinical settings. The applications of CE-3DUS in all the aspects of staging, planning, targeting and follow-up of RFA are described and illustrated.
CLINICAL BACKGROUND
In the Western World, colorectal cancer accounts for 14 and 16 percent of cancer deaths in men and women, respectively, with approximately 25% of patients having liver involvement at the time of initial presentation and up to 50% will develop hepatic metastases during the course of their disease[12]. For patients with colorectal liver metastases, surgical resection is the treatment of choice, but only 10%-20% of patients are initially candidates for potentially curative resection; resection should be considered if there is no unresectable extra-hepatic disease, all liver deposits can be resected with a free clearance margin of 1 cm, and there is adequate liver reserve. The five-year survival rates vary from 25%-40%[34]. Seventy five percent of those who undergo liver resection will develop recurrence and of these, the liver is involved in 50%. Sixty five to eighty five percent of all recurrences appear within the first 2 years[5]. Repeat liver resection in these patients still has a 5-year survival of 30 to 40 percent. Whilst the post-operative mortality or morbidity following repeated hepatectomy is comparable to those of single hepatectomy, they are not entirely negligible and hepatectomy is associated with significant monetary expense[56].
The worldwide incidence of hepatocellular carcinoma is increasing and in particular within North America and Europe, which is progressively affecting younger patients. It is now believed that this is mainly attributed to the rise in hepatitis C viral infection, as the rates associated with alcoholic cirrhosis and hepatitis B virus infection have remained stable[7]. The disease is extremely lethal with median survival rates of untreated symptomatic cases ranging between 4 to 6 mo. Patients with even small tumors also carry a significant mortality as less than 50% will survive 5 years despite undergoing apparently curative resection. Patients with early stage hepatocellular carcinoma should be offered surgical therapeutic options of transplantation or resection[8]. Transplantation offers a 4-year overall survival rate of 75% and a 4-year recurrence free survival rate of 83%. However, few would benefit from transplantation given the shortage of living donors and the eligibility of patients to the “Milan criteria” for transplantation (i.e. decompensated cirrhosis, solitary tumor smaller than 5 cm and up to 3 lesions smaller than 3 cm)[9]. Similarly, less than 5% of cirrhotic patients with hepatocellular carcinoma would be suitable for hepatic resection under current criteria (those with limited tumour burden and relatively well preserved liver function).
Given the shortcomings of current surgical approach, an effective, minimally invasive and repeatable technique for the treatment of liver tumors could potentially impact favorably in the management of these patients.
RADIOFREQUENCY ABLATION
During the last decade, there has been considerable development of the ablative techniques for oncological applications including cryo-, radiofrequency-, microwave- or laser- ablation and high intensity focused ultrasound (HIFU). The development of radiofrequency ablation can be traced back to 1891 through the works of d’Arsonval[10]. In more recent years, with the additional refinements to the design and power of the equipments, it has emerged as the most popular tool for the destruction of hepatic as well as other malignant tissues. During the application of radiofrequency energy, a high frequency alternating current moves from the electrode in the immediate surrounding tissue and as the ions within the tissue attempt to follow the change in the direction of the alternating current, frictional heating of the tissue is generated. Tissue temperature can be elevated beyond 100 degrees centigrade, resulting in coagulation necrosis of the tissue. Precise control of the extent of tissue destruction can be achieved by adjusting local temperature and electrical resistance.
Radiofrequency ablation of liver tumors can be performed percutaneously, laparoscopically or as part of an open surgical procedure[11–13]. Ultrasound guidance remains the optimal method for accurate targeting of the tumors with the RFA needle electrode as it is mobile, more practical, readily available, rapid and cost-effective compared with CT or MR guidance. Despite the availability of screening facilities with the current advanced CT or MR modalities, RFA guidance with the latter modalities is limited for multiple lesional ablations during the same session requiring repeated large volumes of contrast administration to complete all imaging requirements; as such there is some hampering of the work flow for the whole ablative process. As a result many centers use a combination of both CT and US for the ablative procedure.
INDICATIONS FOR RADIOFREQUENCY ABLATION
Radiofrequency ablation is indicated in patients with disease limited to the liver who do not meet the criteria for surgical resectability for both hepatocellular carcinomas and liver metastases[1415]. RFA is now offered to those who cannot undergo resection because of inadequate surgical margins, inadequate liver reserve, co-existing morbidity or patient choice and is performed with a curative intent as in surgical resection. RFA is also effective in the destruction of lesions localized adjacent to major vascular structures including the hepatic veins confluence with the inferior vena cava which would preclude resection. The blood flow in major vessels acts as a heat sink that protects the vascular endothelium from thermal injury whilst allowing complete coagulation of tissue immediately surrounding the blood vessel wall. However, RFA is avoided for peri-hilar lesions due to potential biliary damage leading to fistulous or stricturing complications. With the increasingly aggressive approach adopted by liver surgeons, open radiofrequency ablation is routinely combined with liver resection in the presence of multi-focal, bilateral metastases or upon the detection of unexpected additional lesion. In these cases hepatectomy is performed to deal with the main tumor bulk and any residual tumors that cannot be resected, is treated with RFA. Nonetheless, standard surgical considerations still apply with no more than 70% of the liver volume is removed and particular attention has to be paid to patients with background liver cirrhosis with limited functional reserve.
The use of new effective systemic chemotherapy for the colorectal liver metastases has increased the potential of obtaining significant response to the point of enabling resectability. Studies have shown that of patients with unresectable disease who received second line neo-adjuvant therapy, up to 22% became resectable[16]. There is also a growing proportion of these patients who have had their disease down-staged, who are subsequently referred for radiofrequency ablation instead of surgical resection. The development of fairly extensive steatosis in 20% to 66% of these patients is among the main reason for the choice of RFA, as there is significant morbidity and mortality associated in these cases following liver resection[1718]. Patients with large hepatocellular carcinomas are also now being considered for trans-arterial chemo-embolisation/trans-arterial embolization followed by RFA of any residual disease. However, there is as yet no evidence that these combination therapies with RFA leads to any survival benefit compared with standard clinical practice. The use of RFA in the treatment of other types of metastatic tumors to the liver have also been advocated; for example stable (6 mo) disease from breast, renal, melanoma or neuro-endocrine metastases[1319].
IMAGING TASKS FOR RFA
Intra-operative ultrasonography (IOUS) has been shown to yield significant new information, not identified on pre-operative imaging, which determines resectability or changes the operative plan in up to 50% of patients; it is considered the gold standard thereby achieving universal usage[20–23]. However, traditionally CT and MRI have been used to routinely stage all patients with hepatocellular carcinomas and metastatic hepatic colorectal disease. Given the well-recognized limitations of conventional unenhanced ultrasound, its role in the liver staging process has been negligible. More recently, there has been increasing interest in the use of ultrasound contrast agents during the sonography of the liver to improve the detection of liver metastases. Ultrasound contrast agents consist of microbubbles of air or gases of low solubility, stabilized by a lipid, surfactant or polymer shell. Analogous to CT or MR, it is the relative distribution of the contrast agents between normal tissue and the lesion, which makes the lesion more visible and easier to characterized[2425]. Recent advances in non-linear imaging in the form of pulse inversion together with power modulation modes, combined with the development of contrast agents with liver specificity, have markedly improved the sensitivity of sonography in the detection of small metastases, which may be equal to or even superior to that of CT or MR in some cases[2627].
Whilst we are accustomed to viewing cross-sectional imaging in a two dimensional perspective, tumor staging, its treatment planning, targeting and assessment of therapeutic response would clearly benefit from a three dimensional morphological as well as functional imaging aspect. In that respect, as a result of technological innovations in non-linear modes (NLM) and new probe design, real time imaging with CE 3D-US is now a reality.
Liver staging
Almost all patients are referred for RFA, based on the CT and/or MR imaging findings; RFA would only be feasible under ultrasound guidance, if all the lesions identified on the referral imaging modalities could actually be detected on ultrasound itself. However, conventional ultrasound is well recognized to be limited in the detection of liver metastases and in the identification of hepatocellular carcinoma in a multi-nodular cirrhotic background. Furthermore, in patients with colorectal liver metastases who have had neo-adjuvant chemotherapy and subsequently referred for RFA, the difficulty of identifying all metastases is significantly increased due to widespread steatosis. Compared with conventional ultrasound, contrast-enhanced ultrasound (CE-US) has been shown to be highly accurate in determining the extent and distribution of tumor burden within the liver. Recent studies have shown that the sensitivity and accuracy of CE-US are comparable with those of CT and/or MR enhanced with liver specific contrast agents[2627]. Within the RFA clinical setting, the sensitivity of ultrasound in the detection of metastases, HCCs and all lesions combined has been reported to be 42.9%, 66.7% and 51.4%, respectively; in comparison, the sensitivity for contrast enhanced ultrasound was 100% for all 3 groups[28]. Complete ultrasound guided RFA would have been impossible without the use of contrast agent in 60.4% as the lesions remained occult on the unenhanced ultrasound and of these, no lesions could be detected in 15.4%. To identify all lesions as seen on CT/MRI, contrast enhanced ultrasound was required in 88.9% patients with metastases compared with 41.3% of patients with hepatocellular carcinomas. Of the patients with metastases, 44.4% had been on second line systemic chemotherapy and all required contrast enhanced ultrasound to detect all lesions. No patient was subsequently referred for CT/MRI guided RFA. Follow-up CT/MRI confirmed successful targeting in all patients.
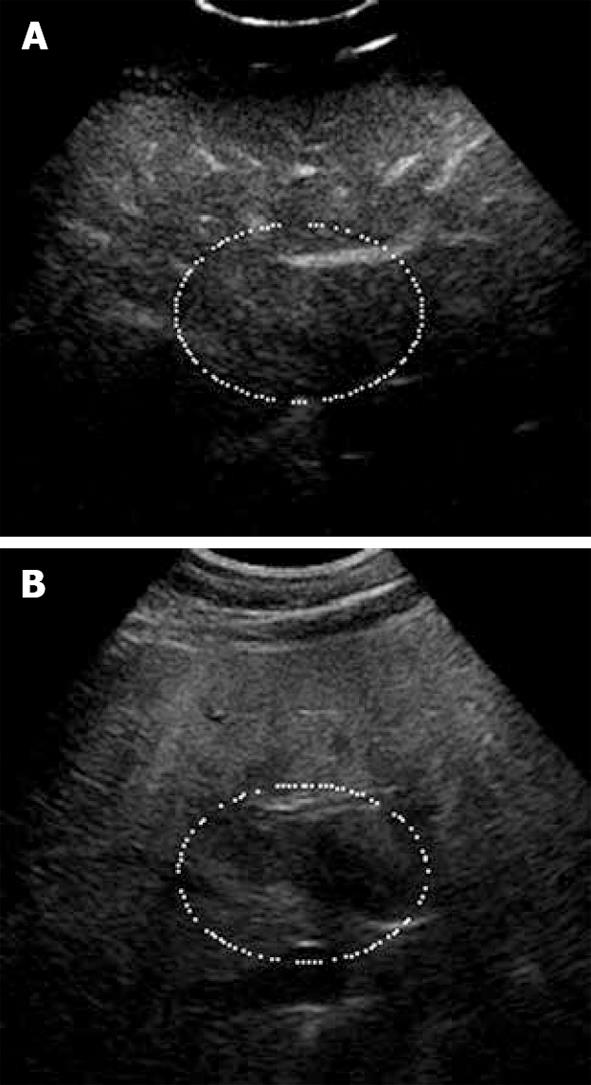
Moreover, in staging the liver for ablation, characterization of all the targeted lesions is important to ensure they are truly malignant; this is particularly so with referrals based on CT scans where hemangiomas may be misdiagnosed as metastases. In patients who have had systemic chemotherapy, there are potential problems with underestimation of the true extent of the disease as well as presence of pseudo-tumors due to diffuse areas of fatty infiltration and focal areas of fatty sparing, respectively. Contrast enhanced ultrasound is particularly useful in confirming the true nature of these benign lesions with real-time evaluation of the lesional micro-vascularization (Figures 1 and 2).
Figure 1 Focal fat sparing shown as iso-echogenicity as adjacent liver parenchyma.
A: Late phase of CE-US; B: Hypo-echoic area on the fundamental mode.
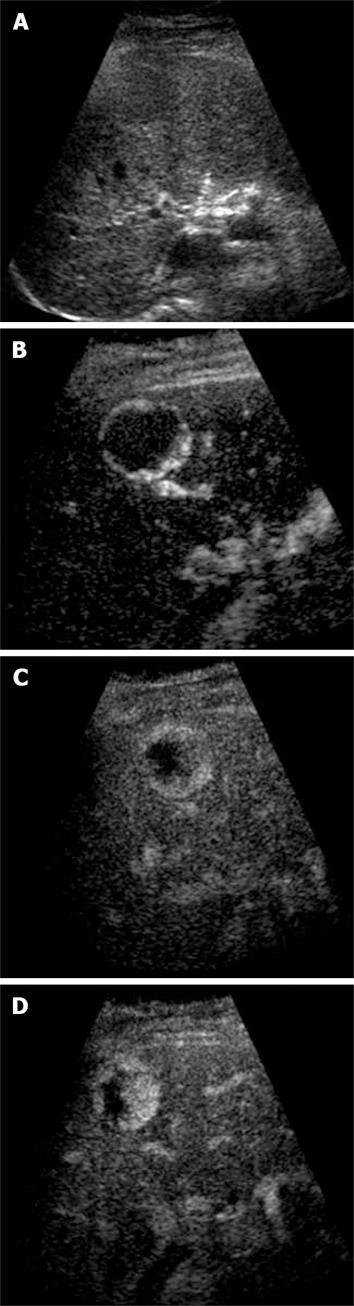
Figure 2 Haemangioma.
A: Baseline focal hypo-echoic lesion; B: Arterial phase showing peripheral rim enhancement; C: Portal venous phase showing peripheral globular rim enhancement; D: Late phase showing progressive centripetal filling-in which is characteristic for hemangioma.
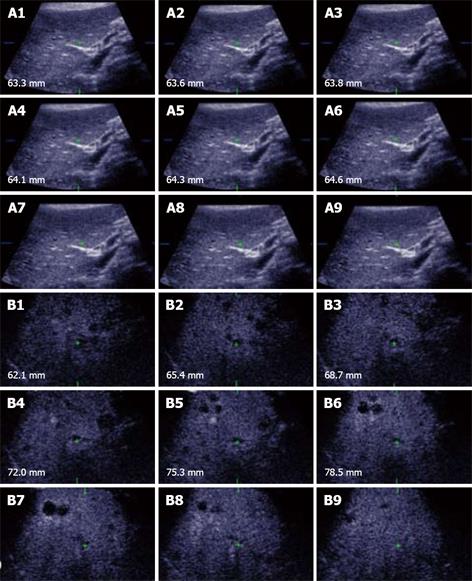
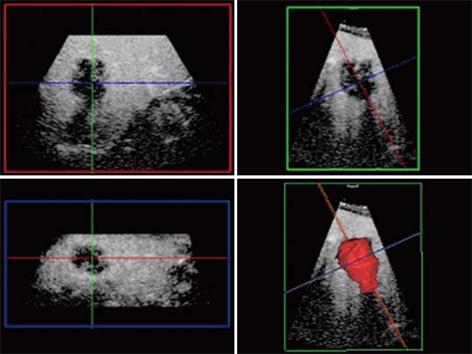
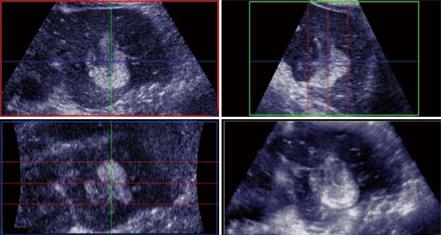
From a practical standpoint, scheduling for CT or MR scans within the 4-6 wk of the RFA procedure may not always be possible because of lack of availability and/or accessibility. With the benefit of its higher accuracy, contrast enhanced ultrasound also offers the flexibility in enabling re-staging immediately prior to the RFA procedure. Using standardized scanning protocols, CE 3D-US may even be superior to CE-US (2D) in the detection and display of these occult liver tumors as well as improving workflow. To ensure complete coverage of the liver, scanning protocols with CE-3DUS include wide angled automated sweeps at the epigastrium, sub-costally and the 3 intercostal spaces in the right upper quadrant of the abdomen, during the arterial, portal venous and late phases of the intravenous bolus injection of the contrast agent. The CE-3DUS set of data may be transferred onto a workstation or a PACS for archiving and reviewed subsequently; it can also be displayed in the same manner as CT or MRI, analyzed and then reported (Figure 3). The whole examination may be performed by the sonologist and the review can be carried out immediately online or subsequently by the sonologist or the Radiologist with improvement in the workflow. There are anecdotal reports of the superiority of CE-3DUS over baseline fundamental unenhanced 2D and 3D ultrasound in the detection and display of occult liver metastases (Figure 3). Furthermore, in dealing with larger tumors and in particular local recurrence, CE-3DUS can define more clearly the biologically active target tumor volume. This is supported by recent preliminary study using a 3D Shape based analysis of CT scans showing that the technique may be useful in assessing RFA ablation results in revealing earlier recurrences unsuspected clinically[29].
Figure 3 Unenhanced ultrasound and contrast enhanced 3D US.
A: Unenhanced Fundamental mode ultrasound showing apparently normal liver; B: Contrast enhanced 3D US displayed as axial scans showing numerous occult metastases appearing as filling defects in the late phase.
Assessment of tumour size and geometry
The selection of the RFA needle electrodes and the appropriate ablation protocols depend on the size of the tumor to be ablated. When the tumor is small (2 to 3 cm), its geometry is usually spherical. However, larger (> 3 cm) tumours may be elliptical. In locally advanced disease there may be aggregates of “daughter” hepatocellular carcinomas or “satellite” metastases merging as they grow resulting into lobular masses. Unenhanced and CE-3DUS assessment of the tumor geometry and determination of the lesional long axis is required to plan for the RFA targeting in order to restrict the number of RFA needle electrode insertions to the bare minimum to avoid seedling and potential complications (Figure 4). If the tumor is elongated, 3D assessment of the tumor geometry and its orientation relative to the probe position enables the planning of the insertion of the RFA needle electrode along the center of the tumor’s long axis to treat the lower half of the lesion first and subsequent withdrawal of the electrode to ablate the proximal residual tumor mass with a single puncture approach (Figure 4B).
Figure 4 Multi-planar reconstruction.
A: Tumor appears to be spherical in tumor modeling; B: Background liver subtraction shows geometry of the tumor model and long axis.
Accurate delineation of the viable tumor margins in 3 orthogonal planes is a key in determining the true size of the lesion. Analogous to liver resection, ablation of the 0.5 to 1.0 cm layer of “normal” liver parenchyma around the tumor margin is important to limit subsequent local recurrence. Conventional US may be limited in delineating the true viable tumor margin; on the other hand CE-3DUS can accurately demonstrate the normal liver/tumor margin. Whilst this may not be critical for small lesions, with larger lesions a 5 mm error may significantly increase the risk of leaving residual disease. The importance of the true delineation and geometry is also largely related to the limited ablative capability of current RFA single or cluster needle electrodes which will produce at best a sphere of coagulation necrosis of 5 cm in diameter. Once the 3D data set has been acquired, the measurement of the tumor diameter in all 3 orthogonal planes determines the appropriate RFA needle electrodes.
Volume of the tumor can also be calculated automatically or manually depending on the software available. This can be done automatically following CE-3DUS using an “auto stacked contours” system, which automatically determines the tumor/normal liver border. First all 3 orthogonal planes are aligned into the center of the mass. The borders of the tumor are marked, and the number of slices for tracing the contours can be selected. Each contour is mapped and the tumor model created and its volume calculated automatically. Without the use of contrast, there is no automatic delineation of the tumor/normal liver border and the stacking of the tumor contours needs to be done manually which is more time consuming.
Targeting of tumors
Conventional ultrasound (2D) has been shown to be an excellent real-time tool in the placement of biopsy needles and RFA needle electrodes. Its limitation in the detection of occult liver tumors is well recognized compared with contrast enhanced CT and MR. Without the use of ultrasound contrast agents, these occult tumors could be localized using adjacent anatomical landmarks and then targeted blindly (i.e. without actual visualization of the tumor). CE-US facilitates the identification of these occult tumors for biopsy and/or for ablation. However, CE-US relies on the use of non-linear imaging mode to depict the contrast enhancement of the normal liver parenchyma with the malignant tumors appearing as filling defects in the late phase. Non-linear imaging mode is highly effective in subtracting native tissue linear echoes including those of the target biopsy needle or RFA needle electrodes. Whilst CE-US will identify the occult tumors, visualization of the biopsy needles and RFA needle electrodes is difficult on the non-linear imaging mode at low output power (Mechanical index). In the past, one had to switch between the non-linear imaging mode and fundamental modes screen to identify the position of the tumor and needle, respectively. However, with the advent of the side by side dual screen with the low MI fundamental mode and non-linear imaging mode simultaneously displaying the needle and the occult lesion, respectively, accurate real time targeting of occult lesions has been facilitated and significantly improves the work flow obviating the need to resort to CT or MRI scan guidance (Figure 5).
Figure 5 Side-by-side screen.
Blue arrow points to small occult metastasis only seen on the CE-US non-linear imaging mode (A), whilst the open arrows point to the RFA needle electrode trajectory towards the occult metastasis, which is only visualized on the fundamental US scan (B).
With 2D US data acquisition, it is difficult to visualize mentally the 3D spatial aspects of the tumor and its specific relation to the surrounding hepatic vascular structures. CE-3DUS enables the modelling of the tumor geometry and determination of its long axis as well as its spatial relationship with the adjacent hepatic vascular anatomy and sometimes the extra-hepatic vital structures when the tumor is sub-capsular in location. The placement of the RFA needle electrodes can then be performed with reference to the probe position with the aid of a needle-guide or free-hand control (Figure 4). Furthermore, the added information provided by CE-3DUS also enables a more aggressive approach to the ablation of larger lesions beyond usage of a singular RFA needle electrode. Placement of multiple RFA needle electrodes will create coagulation necrosis even beyond the 7 cm (Figure 6). But the deployment of these multiple RFA needle electrodes needs to be accurate and is facilitated with CE-3DUS planning and guidance.
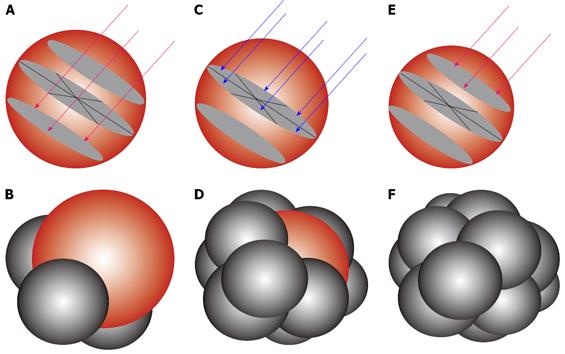
Figure 6 RFA needle electrode.
Modeling to show the placement of the RFA needle electrodes to enable 12 overlapping spheres to give an equivalent of 7.5 cm diameter target sphere. (A, B) lower pole ablation with 3 RFA needle electrodes (red arrows) to create 3 overlapping ablation spheres (C, D) middle row ablation with 6 RFA needle electrodes (blue arrows) to create 6 overlapping ablation spheres and (E, F) upper pole ablation with retraction of the first 3 RFA electrodes to complete the target sphere of 7.5 cm.
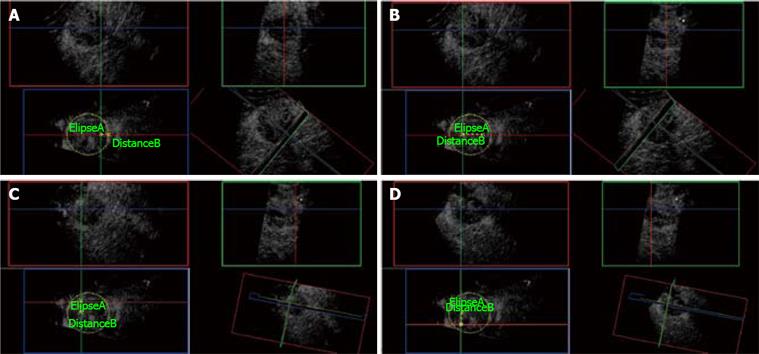
There are reports of improved needle localization and guidance during biopsy using unenhanced 3D-US[30].Earlier studies using unenhanced 3D-US to guide RFA needle electrodes have shown an increase in the confidence of the operator as well as a more accurate positioning of the electrodes in the majority of cases when compared with conventional 2D ultrasound guidance[3132]. With regard to the accurate placement of RFA needle electrodes with expandable multiple antennas (Figure 7), particular attention needs to be observed (1) that the expandable antennas are deployed uniformly and symmetrically within the tumor to ensure margin (2) with lesions adjacent to vascular structures and sub-capsular location, that the latter are not punctured. 3D US is particularly valuable in the depiction of the safe deployment of these expandable antennas with the use of the elevation plane. 3D US enables the visualization of the RFA needle electrodes and the tumor simultaneously in the 3 orthogonal planes, among which the coronal and sagittal planes are aligned along the RFA needle electrode long axis whilst the elevation plane being perpendicular to it (Figures 8,9,10). Through translation or rotation within the acquired 3D volume, all regions of interest can be viewed from arbitrary orientations without any restriction of the transducer axis. The placement of multiple RFA needle electrodes can be planned following acquisition of the 3D volume data and accurate measurement of the distance between the electrodes and the tumor margin can be performed in advanced limiting the danger of unnecessary repeated electrode punctures through “trial and error” (Figure 11).
Figure 7 RFA needle electrode with expandable antennas.
Figure 8 Multi-planar reconstruction.
RFA Needle electrode placement along the long axis of the tumour mass which correspond to the planned “Red” axial plane bisecting the perpendicular the “Green” sagittal plane with reference to the probe position.
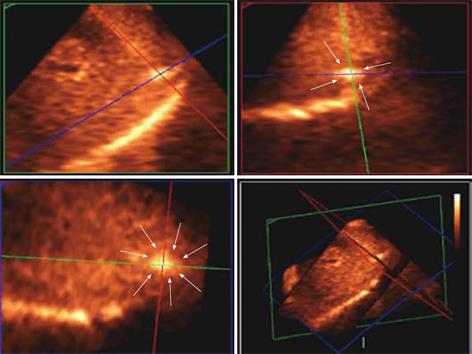
Figure 9 Matrix probe.
3D US showing the hyper-echoic needle electrode tip and its spatial position in the tumour (white arrows delineates the tumour margin) in the subcapsular area and is clearly depicted in 3 orthogonal 2D images confirming the tip being in the center of the tumour.
Figure 10 The 3 orthogonal planes showing the RFA needle electrodes with the multiple antennas deployed and clearly visualised on the (Blue: left lower quadrant) elevation plane following alignment of the Green and Red planes along the axis of the RFA needle electrode.
Figure 11 3D CE-US.
A: Ellipse marks the tumor border at its maximal diameter (5 cm). First needle electrode insertion is planned from the elevation plane (Blue box) at 1 cm from the edge of the tumor. Green and Red orthogonal planes mark the trajectory of the needle electrode. B: Distance B measures 2.5 cm from the first needle electrode insertion and plans the plane (Green) of insertion for the next two needle electrodes. C: The second needle electrode is placed at the Green and Red planes intersection measuring 1.5 cm superior to the last position. D: Third needle insertion is placed at the Red and Green plane intersection, 3 cm below the second needle electrode as shown on the Blue plane.
Monitoring of response
To assess the initial response to treatment, CE-3DUS can be performed only at 7 to 10 min after the end of RFA to allow for the dissipation of the native gas produced during the ablation process. Acquisition of 3D data set is carried out during the hepatic arterial, portal venous and late phase. Absence of any intra-lesional enhancement or moving microbubbles is consistent with complete coagulation necrosis and is easily depicted in hypervascular tumors. Residual viable tumor tissue is suspected when a portion of the original lesion maintains its micro-vascularity during the vascular phases. In enabling immediate further RFA of the residual disease, unnecessary delay may be avoided ensuring complete treatment within a single session. However, non-visualization of vascularity is not always a reliable indicator in the case of hypovascular tumors as in the case of most colorectal liver metastases. Complete coagulation necrosis may then be assessed through the side-by-side comparison of pre-RFA lesion size, volume and location, with those of the post-treatment coagulation necrosis. Peri-lesional “safety” margin adequacy must be evaluated at the same time. Clearly the advantage of CE-3DUS data acquisition during the vascular (arterial and portal) and late phases is evident to ascertain complete assessment of the volume of coagulation necrosis or presence of residual disease (Figure 12). Evaluation of the post RFA ablation volume relative to the pre RFA tumor volume may also be a particular additional important parameter in the assessment of response. A similar technique can also be used in the surveillance of these patients in the post ablative periods at 3, 6 or 12 mo; whilst this may not be optimal for patients with metastases who would benefit from CT in the surveillance for extra-hepatic disease, it may be adequate for the surveillance of patients with hepatocellular carcinomas due to the much lower incidence of extra-hepatic dissemination.
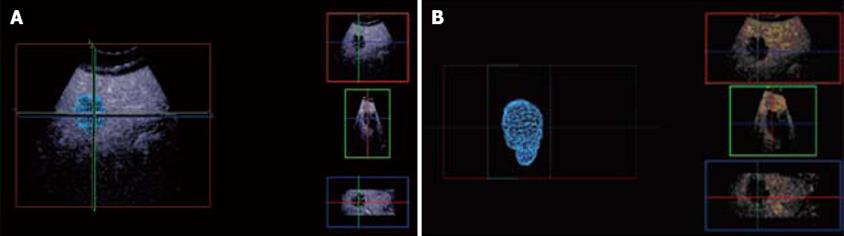
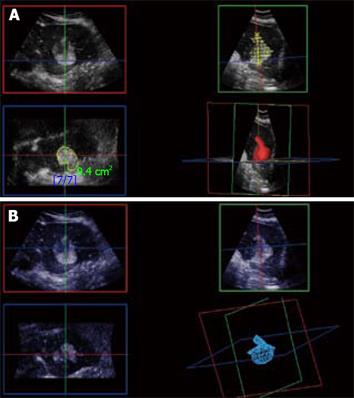
Figure 12 3D CE-US.
Arterial phase 3D acquisition showing recurrent enhancing HCC in 3 orthogonal planes and the volume rendering analysis (left lower quadrant).
Once local recurrence has been identified, CE-3DUS evaluation in the arterial phase and multi-planar reconstruction modelling are important in the planning for further RFA in that the geometry of the mass is usually non-spherical (Figure 13).
Figure 13 3D reconstruction of local recurrence geometry in the planning for further ablation.
A: With normal liver background; B: Without normal liver background.