Published online Jul 21, 2009. doi: 10.3748/wjg.15.3431
Revised: June 14, 2009
Accepted: June 21, 2009
Published online: July 21, 2009
Goblet cell carcinoid is an uncommon primary tumor of the vermiform appendix, characterized by dual endocrine and glandular differentiation. Whether goblet cell carcinoid represents a morphological variant of appendiceal classical carcinoid or a mucin-producing adenocarcinoma is a matter of conjecture. Rare cases of goblet cell carcinoid with other concomitant appendiceal epithelial neoplasms have been documented. In this report, we describe a rare case of combined appendiceal goblet cell carcinoid and mucinous cystadenoma, and discuss the possible histopathogenesis of this combination.
- Citation: Alsaad KO, Serra S, Chetty R. Combined goblet cell carcinoid and mucinous cystadenoma of the vermiform appendix. World J Gastroenterol 2009; 15(27): 3431-3433
- URL: https://www.wjgnet.com/1007-9327/full/v15/i27/3431.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.3431
Goblet cell carcinoid (GCC) of the vermiform appendix is an uncommon neoplasm. It is characterized by dual endocrine and glandular differentiation, a feature that has led to confusion regarding nomenclature, histogenesis, and clinical management. The origin and histogenesis of GCC are still controversial, and whether GCC represents a histological variant of appendiceal classical carcinoid or a distinct morphological subtype of appendiceal adenocarcinoma with endocrine differentiation is still a matter of debate. Rare cases of GCC combined with other benign and malignant epithelial appendiceal neoplasms have been reported; the relationship between GCC and these neoplasms is not clear. Herein, we report an unusual and rare case of combined GCC and mucinous cystadenoma (MCA) of the vermiform appendix and discuss the possible related histopathogenesis.
A 46-year-old woman presented with severe acute pain in the right iliac fossa and periumbilical region. Ultrasound and a computed tomography scan revealed a mucocele in the vermiform appendix, with a well defined lesion located at the mid zone of the appendix. The patient underwent right hemicolectomy, and her postoperative clinical course was uneventful. Gross examination of the surgical specimen showed an enlarged appendix, which was filled with thick mucinous material. A distinct lesion which involved the appendiceal wall, and measured 1.5 cm maximally, was identified in the mid-portion of the appendix. There was no evidence of perforation, extravasation of mucin into the periappendiceal tissue, or pseudomyxoma peritonei during surgery.
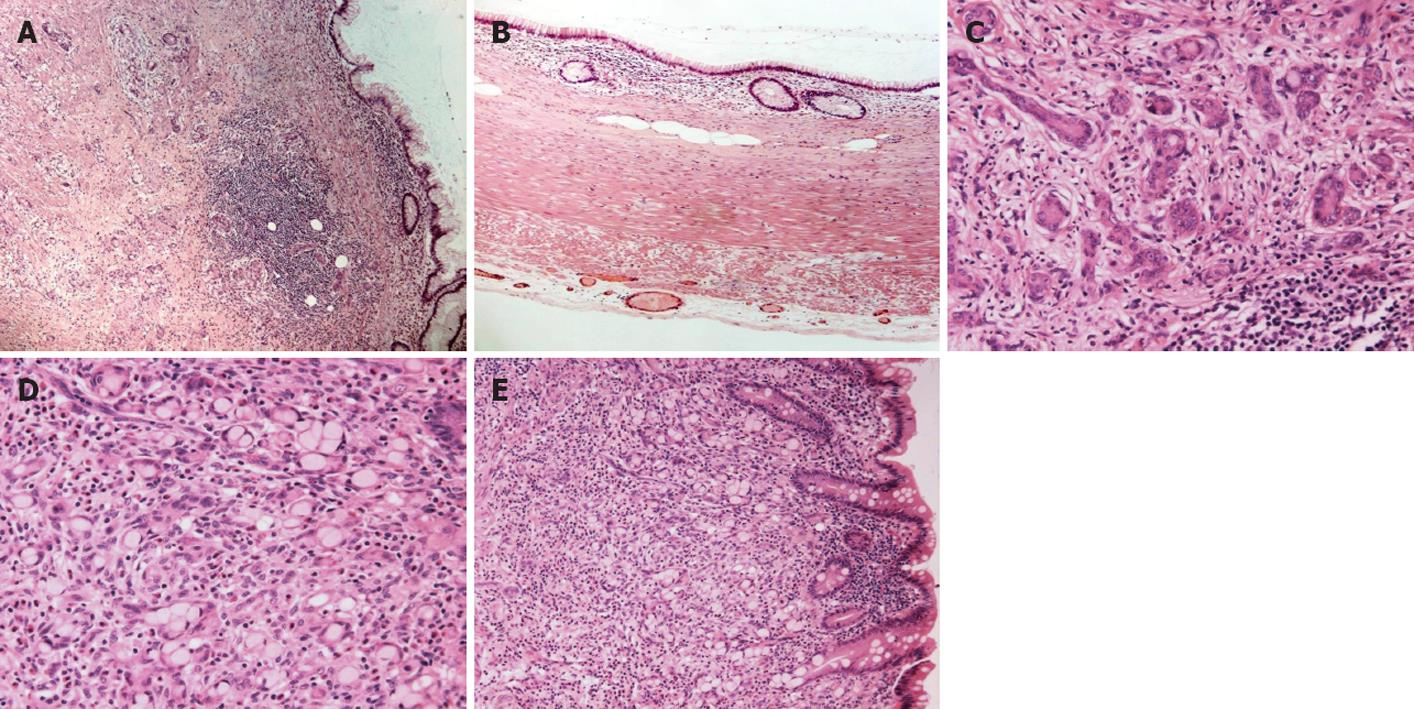
Histopathological examination showed combined GCC and MCA of the vermiform appendix (Figure 1A). The appendiceal lumen was dilated and lined by mucin-containing columnar epithelial cells (Figure 1B). There was no significant cytologic atypia, and no mitotic figures were identified. Focal papillary configurations of the lining epithelium, and mild epithelial pseudostratification were present. In addition, the appendiceal wall was infiltrated by glandular structures of various sizes which were arranged in nests and tubules. These glandular structures comprised 2 distinct types of cells: (1) small to intermediate sized monotonous neuroendocrine cells with a small amount of finely granular eosinophilic cytoplasm, and mild cytonuclear atypia (Figure 1C); (2) mucin-filled intermediate sized cells (goblet cells), with peripherally located small, crescent-like hyperchromatic nuclei, and indistinct nucleoli (Figure 1D). Scattered infiltrating single goblet neoplastic cells were focally present. As previously described[1] the tumor nests appeared to arise from the basiglandular region of the intestinal crypts in close proximity to the MCA (Figure 1E). There was no lymphovascular invasion, although perineural and intraneural invasion was present. The tumor infiltrated the full thickness of the appendiceal wall and extended to the mesoappendix. Ten lymph nodes were histologically identified, of which all were negative for malignancy.
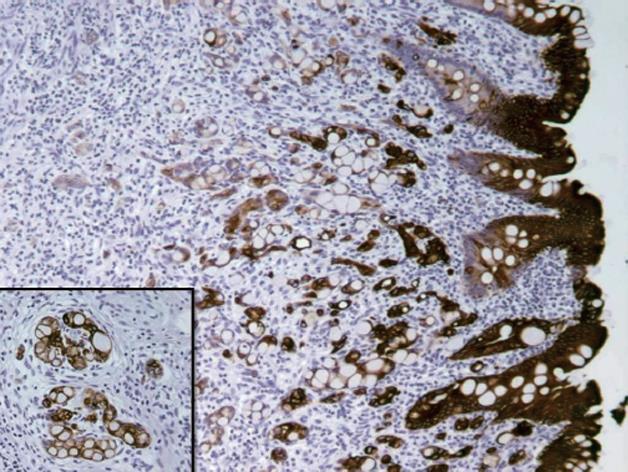
Immunohistochemically (Table 1), the tumor cells of the GCC were positive for chromogranin, synaptophysin, and serotonin, which are neuroendocrine markers. Diffuse staining for cytokeratin (CK) 20 (Figure 2), CK19, and CD99 was also present. The Ki67 proliferating index revealed nuclear staining in approximately 15% of the tumor cells. There was no staining for CK7.
| Antibody | Clone | Dilution | Source |
| CK7 | OV-TL 12/30 | 1:2000 | Dako corporation, carpinteria, CA |
| CK19 | B170 | 1:50 | Vector laboratories, burlingame CA |
| CK20 | KS20.8 | 1:100 | Dako |
| CD99 | O13 | 1:100 | Signet laboratories, dedham, MA |
| Ki67 | MIB1 | 1:100 | Dako |
| Chromogranin | DAK-A3 | 1:200 | Dako |
| Synaptophysin | SY38 | 1:200 | Dako |
| Serotonin | Polyclonal | 1:200 | Dako |
GCC is an uncommon neoplasm of the vermiform appendix with uncertain histopathogenesis and biological behaviour. It is believed that GCC represents an amphicrine tumor, which originates from a single undifferentiated pluripotent intestinal stem cell with divergent neuroendocrine and mucinous differentiation[2], resulting in a composite biphasic neoplasm of 2 distinct populations of endoderm-derived cells. Whether this makes GCC a variant of carcinoid tumor or a subtype of appendiceal adenocarcinoma which exhibits morphological and immunophenotypical features of neuroendocrine differentiation is still a subject of debate. Molecular studies have not elucidated the exact nature of GCC. There is a substantial overlap between GCC and classical carcinoid from a molecular standpoint[34]. Morphological features such as minimal cytologic atypia, presence of non-goblet cells, tubuloacinar neuroendocrine elements, continuity with the basiglandular crypt cells of the mucosal membrane, and the lack of continuity and involvement with the luminal surface mucosa, favour the GCC being related to carcinoid tumor. However, compared to classical carcinoid, the positive immunostaining for CK20[5], the demonstration of IgA staining that is typical of intestinal crypt cells[6], the tendency for regional lymph node and distant metastasis, and tendency for recurrence and more aggressive clinical behaviour are common features for GCC. This suggests that GCC is histologically a form of “crypt cell carcinoma” or more accurately an “amphicrine carcinoma” rather than a variant of appendiceal carcinoid.
Rare cases of GCC coexisting with conventional appendiceal mucinous tumors were reported[178], of which 2 cases were MCA[1]. Similar to our case, both patients were women, aged 54 and 64 years, who presented with clinical features of acute appendicitis and dull ache in the right iliac fossa. Histological examination revealed combined GCC and MCA. If GCC is a true subtype of carcinoid tumor, its coexistence with an appendiceal mucinous neoplasm would support the theory that GCC is derived from a single undifferentiated pluripotent intestinal stem cell with divergent dual neuroendocrine and mucinous differentiation (unitary stem cell hypothesis). The concomitant appendiceal mucinous neoplasm may be considered a coincidental occurrence and raises the possibility of a common etiological factor for both GCC and appendiceal epithelial neoplasms[9]. If GCC is to be considered as an adenocarcinoma of crypt cell origin rather than a carcinoid, then the occurrence of combined GCC and appendiceal mucinous neoplasms may represent an example of adenoma-carcinoma sequence[1]. The nature of GCC and its relation to other neuroendocrine and non-neuroendocrine epithelial tumors of the appendix needs further examination, and more cases of GCC with concomitant appendiceal epithelial neoplasms need to be recorded, which may help in explaining this rare association.
| 1. | al-Talib RK, Mason CH, Theaker JM. Combined goblet cell carcinoid and mucinous cystadenoma of the appendix. J Clin Pathol. 1995;48:869-870. |
| 2. | Kanthan R, Saxena A, Kanthan SC. Goblet cell carcinoids of the appendix: immunophenotype and ultrastructural study. Arch Pathol Lab Med. 2001;125:386-390. |
| 3. | Ramnani DM, Wistuba II, Behrens C, Gazdar AF, Sobin LH, Albores-Saavedra J. K-ras and p53 mutations in the pathogenesis of classical and goblet cell carcinoids of the appendix. Cancer. 1999;86:14-21. |
| 4. | Misdraji J. Neuroendocrine tumours of the appendix. Curr Diagn Pathol. 2005;11:180-193. |
| 5. | Alsaad KO, Serra S, Schmitt A, Perren A, Chetty R. Cytokeratins 7 and 20 immunoexpression profile in goblet cell and classical carcinoids of appendix. Endocr Pathol. 2007;18:16-22. |
| 6. | Isaacson P. Crypt cell carcinoma of the appendix (so-called adenocarcinoid tumor). Am J Surg Pathol. 1981;5:213-224. |
| 7. | Carr NJ, Remotti H, Sobin LH. Dual carcinoid/epithelial neoplasia of the appendix. Histopathology. 1995;27:557-562. |
| 8. | Carr NJ, McCarthy WF, Sobin LH. Epithelial noncarcinoid tumors and tumor-like lesions of the appendix. A clinicopathologic study of 184 patients with a multivariate analysis of prognostic factors. Cancer. 1995;75:757-768. |
| 9. | Pahlavan PS, Kanthan R. Goblet cell carcinoid of the appendix. World J Surg Oncol. 2005;3:36. |