Published online Jan 14, 2009. doi: 10.3748/wjg.15.219
Revised: December 3, 2008
Accepted: December 10, 2008
Published online: January 14, 2009
AIM: To investigate the mechanisms underlying the reduction in gastric blood flow induced by a luminal water extract of Helicobacter pylori (HPE).
METHODS: The stomachs of isoflurane-anesthetized mice were exteriorized, and the mucosal surface exposed. Blood flow was measured with the laser-Doppler technique, and systemic arterial blood pressure monitored. C57BL/6 mice were exposed to water extract produced from H pylori strain 88-23. To investigate the role of a nerve- or iNOS-mediated pathway, we used intraluminal lidocaine and iNOS-/- mice. Blood flow response to the endogenous nitric oxide synthase inhibitor asymmetric dimethyl arginine (ADMA) was also assessed.
RESULTS: In wild-type mice, HPE decreased mucosal blood flow by approximately 30%. This reduction was abolished in iNOS-deficient mice, and by pre-treatment with lidocaine. Luminally applied ADMA resulted in reduction in blood flow similar to that observed in wild-type mice exposed to HPE.
CONCLUSION: A H pylori water extract reduces gastric mucosal blood flow acutely through iNOS- and nerve-mediated pathways.
-
Citation: Henriksnäs J, Atuma C, Phillipson M, Sandler S, Engstrand L, Holm L. Acute effects of
Helicobacter pylori extracts on gastric mucosal blood flow in the mouse. World J Gastroenterol 2009; 15(2): 219-225 - URL: https://www.wjgnet.com/1007-9327/full/v15/i2/219.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.219
Gastric ulcer and cancer of the stomach have been shown to be associated with the bacterium Helicobacter pylori (H pylori), which colonizes up to 50% of the human population[1]. It is not known how an infection with this pathogen causes these lesions; but, disruption of the gastric protection mechanisms is certainly involved. We have previously found that a water extract of H pylori reduces the mucosal blood flow in rats by a mast cell- and platelet activating factor (PAF)-dependent pathway[2].
PAF is a very potent vasoconstrictor, which also mediates early leukocyte recruitment, and can cause gastrointestinal microcirculatory hypoperfusion[34]. PAF is released from a number of inflammatory cells, including mast cells. Mast cells function as “alarm cells” in the gastric mucosa in reaction to infectious material, evoking an inflammatory response[5]. It has been suggested that nerves in the mucosa signal to the mast cells, and inhibition with lidocaine has been found to attenuate mast cell-mediated effects[6].
Gastric mucosal blood flow has a vital role in gastric mucosal protection. A high blood flow is considered good protection against injury, as it dilutes, neutralizes, and removes hazardous substances that have penetrated the gastric mucosal barrier[78]. In previous studies, we have shown that high concentrations of luminal acid alone induce hyperemia without any macroscopic lesions[910]. Furthermore, these results suggested that epithelial inducible nitric oxide synthase (iNOS) is involved in the hyperemic response to acid, possibly signaling to afferent nerves, leading to a blood flow increase.
It is well known that H pylori induces iNOS expression as part of the inflammatory process[11]. Among several other functions, nitric oxide (NO) has antibacterial properties; but, despite this, H pylori is able to survive in the presence of the vast amount of NO that is produced. Several explanations for the survival of these bacteria have been proposed, including the production of an L-arginine analogue, asymmetric dimethyl arginine (ADMA), an endogenous inhibitor of NOS activity. In line with this, the formation of ADMA has been demonstrated in the duodenal mucosa on exposure to a water extract of H pylori[12].
The aim of this study was to further elucidate the acute effects of H pylori on the gastric mucosal blood flow and on distinct signaling pathways. We challenged normal and iNOS-deficient mice with water extracts of H pylori. Furthermore, lidocaine was administered intraluminally to investigate if the blood flow response to H pylori was nerve-mediated. In addition, we assessed the blood flow response to luminally-applied ADMA.
All experimental procedures in this study were conducted in accordance with the guidelines of the Swedish National Board for Laboratory Animals and were approved by the Swedish Laboratory Animal Ethical Committee in Uppsala.
The animals were kept under standardized conditions of temperature (21-22°C) and illumination (12 h light/12 h darkness). They were housed in cages with mesh bottoms, and had free access to tap water and pelleted food (Lactamin, Kimstad, Sweden).
Male C57BL/6 mice (n = 29, B&K Universal, Stockholm, Sweden) were used for all experiments except for the iNOS deficient and wild-type (wt) mice (background C57BL/6 × 129SvEv). Breeding pairs of mice deficient in iNOS were kindly provided by J.S. Mudgett (Merck Research Laboratories, Rahway, NJ, USA) and JD MacMicking and C Nathan (Cornell University Medical College, New York, NY, USA). The mice were generated by gene targeting in embryonic stem cells as previously described[13]. Homozygous iNOS-deficient mutants were maintained by interbreeding the F2 generation (n = 11, Animal Department, Rudbeck Laboratory, Uppsala, Sweden). For wild-type controls male C57BL/6 × 129Sv was used (n = 6, Taconic Farms, Germantown, NY).
The procedure for the preparation of HPE is a modification of that of Xiang et al[14], and has been described earlier[15]. HPE were produced from H pylori strain 88-23, wt (kindly provided by M. Blaser, Nashville, TN, USA). The concentrated HPE were diluted twice with a 1.8% saline solution in order to obtain a solution with isotonic properties.
Anesthesia was induced by spontaneous inhalation of isoflurane (Forene®, Abbott Scandinavia AB, Kista, Sweden). The inhalation gas was administered continuously through a breathing mask (Simtec Engineering, Askim, Sweden) and contained a mixture of air, oxygen (total oxygen 40%) and about 2.4% isoflurane. Body temperature was maintained at 37-38°C by means of a heating pad regulated by a rectal thermistor probe.
A catheter containing heparin (12.5 IU/mL) dissolved in isotonic saline was placed in the left carotid artery to monitor blood pressure. The jugular vein was cannulated for continuous infusion of a modified Ringer solution at a rate of 0.35 mL/h. In some experiments, infusion was performed intra-arterially through a Y-catheter.
The preparation of the mouse gastric mucosa for intravital microscopy has been described previously[16]. Briefly, exteriorization of the mucosa through a midline abdominal incision was followed by an incision along the greater curvature in the forestomach. The animal was placed on a Lucite table with part of the corpus of the stomach loosely draped over a truncated cone in the center of the table, with the mucosal surface facing upwards. A “mucosal chamber” with a hole in the bottom corresponding to the position of the cone was fitted over the mucosa, exposing approximately 0.13 cm2 of the gastric mucosa through the hole. The mucosal chamber did not touch the mucosa, so as to avoid impairment of blood flow, and the edges of the hole were sealed with silicone grease. The chamber was filled with 3 mL of unbuffered 0.9% saline, maintained at 37°C by circulation of warm water in a jacket in the bottom of the chamber. The saline was replaced at regular intervals of 10 min and the pH was measured.
Blood flow was measured with laser-Doppler flowmetry (LDF) equipment (Periflux instruments Pf3 and Pf4001, Perimed, Stockholm, Sweden) which had previously been used to study the microcirculatory blood flow of the gastric mucosa in the rat model, as described by Holm-Rutili & Berglindh[17]. In brief, blood flow was recorded as changes in the frequency, that is, the Doppler shift, of monochromatic light from a laser probe (wavelength 633 nm; probe fiber separation 0.5 mm) illuminating a limited area of the tissue. Recorded Doppler-shifted light can be directly and linearly correlated to changes in erythrocyte flux. This flux has been shown to correlate well with gastrointestinal blood flow[17–19]. The laser probe was held in a fixed position in the chamber solution at a distance of 1-2 mm above the mucosa by a micromanipulator. With the type and position of the probe used in these studies, the laser light most likely penetrates through the entire thickness of the gastric wall[20]. However, the recorded blood flow is mainly mucosal, since the amount of back-scattered light decreases exponentially with the depth in the tissue and about 80% of the blood flow of the stomach perfuses the mucosa. Blood flow was monitored continuously throughout the experiment.
The continuously measured blood flow was reported as percent of that during the control period, i.e. the 10 min period, prior to HPE or ADMA application, respectively. Before the experiments, the animals were allowed to stabilize for 45-55 min after surgery. The animals were divided into the following groups: I, Control (n = 5); II, HPE (n = 6): after a 10 min control period, HPE was applied for 2 × 20 min; III, Lidocaine control (n = 6): lidocaine (0.5%) was applied for 3 × 20 min. The last 10 min of the first 20 min period was used as control level; IV, Lidocaine + HPE (n = 6): lidocaine (0.5%) was applied for 20 min (the last 10 min were used as control level), after which HPE mixed with lidocaine (final concentration 0.5%) was applied for 2 × 20 min; V, iNOS control + HPE (n = 6): after a 10 min control period, HPE was applied for 2 × 20 min; VI, iNOS deficient mice + HPE (n = 6): after a 10 min control period, HPE was applied for 2 × 20 min; VII, ADMA (500 &mgr;mol/L) (n = 6): after a 10 min control period, the NOS-inhibitor ADMA was applied for 2 × 20 min; VIII, iNOS deficient mice + ADMA (500 &mgr;mol/L) (n = 5): after a 10 min control period, the NOS-inhibitor ADMA was applied for 2 × 20 min.
The ADMA dose was selected from a previously published in vivo study[12], and the pH of the ADMA solution was adjusted with NaOH to that of the saline.
The modified Ringer solution was composed of 120 mmol/L NaCl (Fluka Chemie GmbH, Buchs, Switzerland), 2.5 mmol/L KCl, 25 mmol/L NaHCO3, and 0.75 mmol/L CaCl2 (Merck, Darmstadt, Germany). Other chemicals included heparin (Leo Pharma AB, Sweden), silicone grease (Dow Corning high vacuum grease, Dow Corning GmbH, Weisbaden, Germany), ADMA (NG, NG-Dimethylarginine hydrochloride, Sigma-Aldrich Chemie GmbH, Steinheim, Germany) and lidocaine (Xylocain, AstraZeneca, Södertälje, Sweden).
All values are presented as mean ± SE. Vascular resistance was calculated as the ratio of mean arterial blood pressure (MAP, mmHg) to blood flow (perfusion units). Statistical significance was determined with ANOVA for repeated measurements, followed by Fisher’s protected least significant difference test. The level of significance was set at P < 0.05.
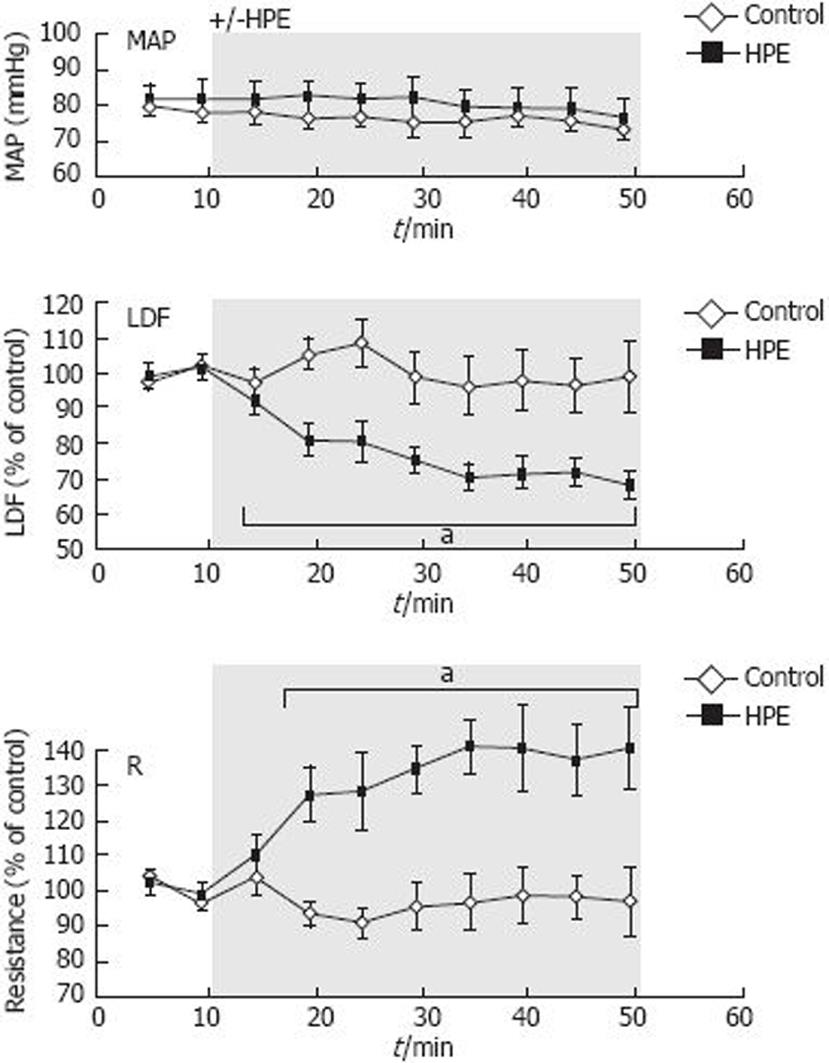
In control animals, the mucosal blood flow was stable during the entire measurement period (Figure 1). Water extract from H pylori was applied to the exposed gastric mucosa in group II. HPE significantly decreased the blood flow to 68% ± 4%, and the resistance increased to 140% ± 12% of the pre-HPE control level (Figure 1). Mean arterial blood pressure was stable around 80 mmHg during the experiments.
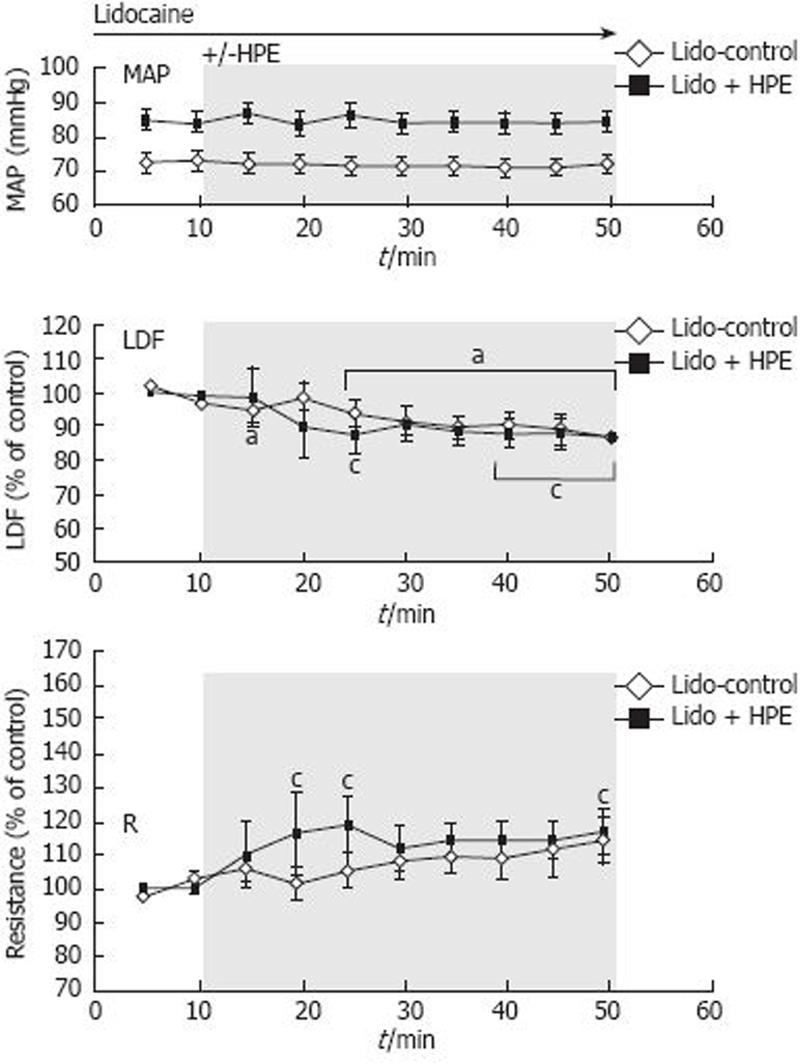
Upon application of lidocaine, LDF decreased slightly, but significantly, to 83% ± 5% of the first control value (last 10-minute period of the first 20 minutes with lidocaine) (Figure 2). The blood flow also decreased slightly, but significantly, to 87% ± 5% during the application of lidocaine + HPE. Thus, the slight blood flow reduction was similar in the two groups, i.e. independently of the application of HPE, suggesting a nerve-mediated blood flow reduction. Lidocaine did not influence blood flow during the first 10 min of application, when LDF was 97% ± 3% of the mean value observed 10 min before the lidocaine application (not shown in the figure). Mean arterial blood pressure was stable during the experiments, and not significantly different between the groups.
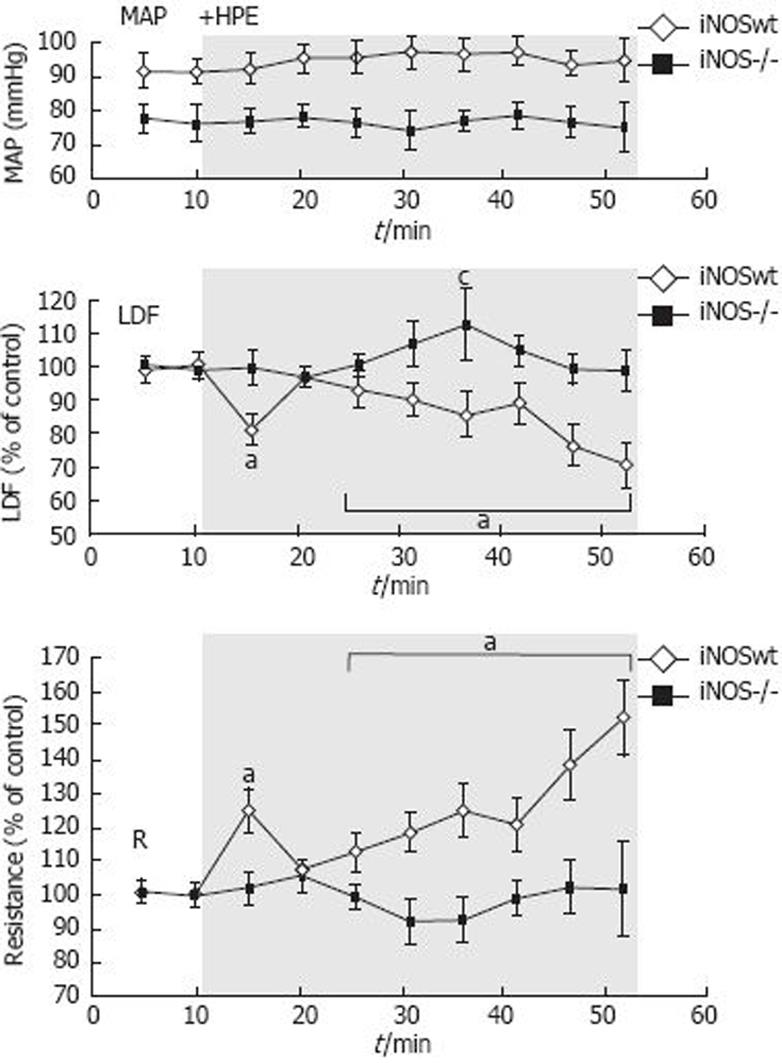
In iNOS wt mice, the blood flow decreased significantly to 71% ± 7% upon exposure to HPE, comparable to the reduction in the control group II. In iNOS-/- mice blood flow did not decrease during application of HPE (99% ± 6%, Figure 3), indicating that iNOS is involved in the HPE induced mucosal blood flow reduction. Mean arterial blood pressure was stable during the experiments, and not significantly different between the groups.
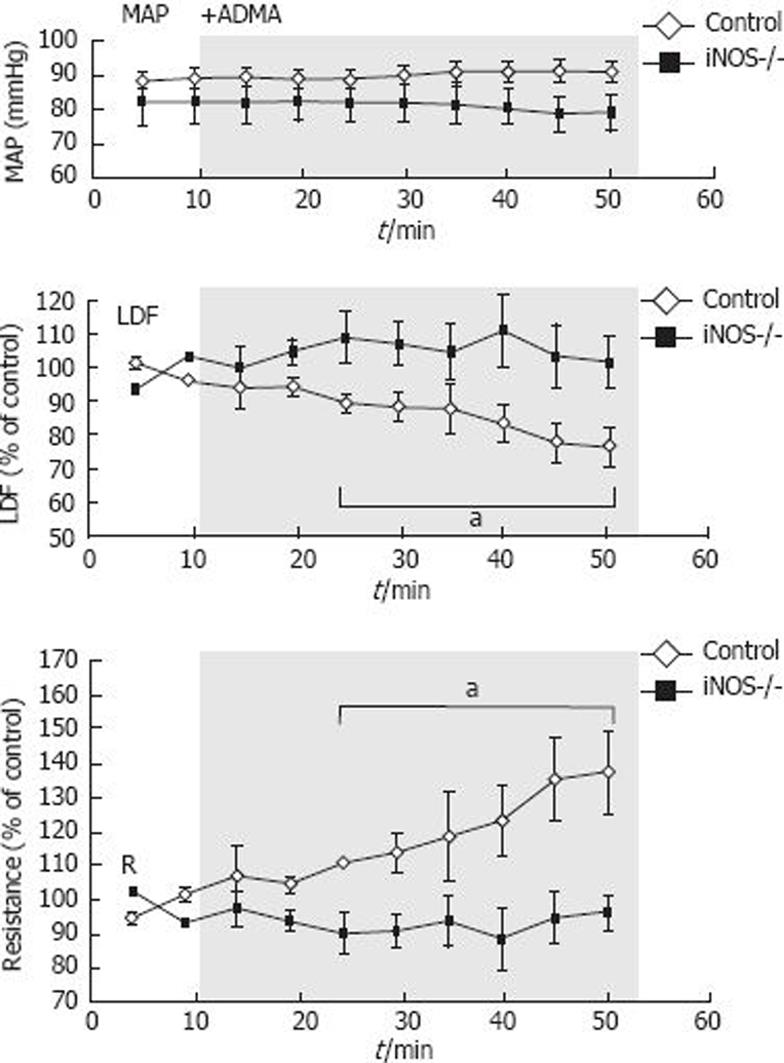
During application of the NOS inhibitor ADMA in normal control mice, the blood flow decreased significantly (to 79% ± 5% of the control level, Figure 4). In iNOS-/- mice, blood flow did not decrease during application of ADMA (102% ± 7%), indicating that ADMA and HPE have the same effect in this setting. Mean arterial blood pressure was stable during the experiments and not significantly different between the groups.
MAP is usually between 70 and 90 mmHg in the C57BL/6 mice anesthetized with isoflurane. There is a tendency to higher blood pressure values in the C57BL/6x129SvEv wt mice, which was not seen in the iNOS deficient mice of the same genetic background. However, we could not find any correlation between perfusion units (LDF signal) and the blood pressure, which could explain our results. Thus, the highest LDF signal was recorded in the mice with the lowest MAP (Group III).
In this study, we have addressed the question of how the gastric pathogen H pylori influences the gastric mucosal blood flow and its regulation on first contact of the mucosa with water extract containing bacterial components.
When the H pylori water extract was applied luminally, the blood flow decreased, in conformity with our previous findings in rats. Results from the present study suggest that the HPE-induced blood flow decrease is nerve-mediated, as inhibition of local nerve activity by application of lidocaine inhibited the reduction in blood flow. In the earlier study in rats, we have also shown that the reduction in blood flow caused by HPE was inhibited both by a mast cell stabilizator, and a PAF receptor antagonist, indicating a possible effect of PAF released from degranulating mast cells[2]. A regulatory relationship between the mucosal nerves and the mast cells has been suggested, as the nerve endings are located in close proximity to the mast cells[21].
We have recently found that a constitutively expressed iNOS in the gastric surface epithelial cells is involved in the regulation of gastric mucosal blood flow[10]. The blood flow increase found upon gastric luminal application of acid was abolished in iNOS deficient mice. Holm et al[22] showed that iNOS played a role in the acid-stimulated HCO3- secretion in the duodenum. In the duodenum, HPE reduced acid-stimulated HCO3- secretion, and it was suggested that this effect was mediated through inhibition of the constitutively expressed iNOS by an endogenous NOS inhibitor, asymmetric dimethyl arginine (ADMA). ADMA is associated with oxidative stress[23] and the presence of HPE in the duodenum leads to increased levels of ADMA[1224]. In the current study, the gastric mucosal blood flow reduction in response to HPE did not occur in iNOS-deficient mice. Furthermore, ADMA applied luminally caused a significant blood flow decrease in the same way as did HPE. However, when ADMA was applied luminally in iNOS-deficient mice no blood flow reduction was seen. Thus, the HPE-induced reduction in mucosal blood flow might involve inhibition of epithelial NO production. We have previously reported that the blood flow reducing effect by HPE is NO-independent. These studies were conducted on rats pretreated with the non-selective NOS inhibitor N-nitro-L-arginine (L-NNA). A reasonable explanation for the contradictory results is that L-NNA has a lower selectivity for iNOS compared with other NOS isoforms[25]. At the time of those experiments, the constitutively expressed epithelial iNOS had not been reported, and its role in blood flow regulation was consequently unheard of.
In addition to the constitutively expressed iNOS in the gastric epithelium, it has been found that H pylori infection induces further iNOS expression in the gastric mucosa[11], suggesting that excessive amounts of NO could be produced. Indeed, Elizalde et al[26] found an increase in the NO level and gastric mucosal blood flow in mice two weeks after H pylori infection. However, within four weeks of the infection, the NO concentration and blood flow had decreased to baseline levels[26]. Other studies have also shown lower or baseline levels of NO in infected patients[2427]. Taken together, these results indicate that H pylori might alter the production of NO. The endogenous NOS inhibitor, ADMA, which is produced when HPE interacts with the mucosa, could inhibit the production of NO. Thus, even though iNOS expression is increased by H pylori, the epithelial NO production might be inhibited by the bacteria. An arginase produced by the bacteria has also been suggested as a strategy to reduce NO production, as it consumes the arginine-substrate for NOS[28].
In the present study, we investigated the effects of an acute exposure of products from H pylori. In a clinical situation it is probably of more interest to investigate the long-term effects of an infection. However, the physiological responses upon first contact with the pathogen are of great importance as it elucidates the early steps of the inflammation. We have recently investigated the effects of a chronic infection with H pylori in mice on different protective mechanisms in the stomach[29]. We found that the hyperemic response to luminal acid, earlier shown to be dependent on epithelial iNOS[10], was abolished in the H pylori infected mice. Thus, both H pylori infection and bacterial products have negative effects on gastric blood flow, which could be a contributing mechanism through which H pylori causes gastro duodenal injury.
In conclusion, we have shown that the reduction in gastric mucosal blood flow caused by a water extract of H pylori is mediated through iNOS- and nerve-dependent pathways. Our working hypothesis is that the epithelial iNOS is constitutively expressed and involved in the regulation of gastric blood flow in response to luminal contents. The NO produced by iNOS could, among other things, stabilize mast cells, be a signal to nerves or directly dilate blood vessels. HPE contains and/or produces ADMA, and when either of the solutions is applied onto the gastric mucosa, the NO production by iNOS is inhibited. This will remove the signal to maintain an adequate blood flow. Furthermore, reduction in NO-production will destabilize the mast cells, which can degranulate and release, for example, PAF, leading to vasoconstriction. The involvement of nerves is probably more complex, and could include both direct regulation of the blood flow and regulation of the mast cells. Further studies are needed to elucidate which nerves are important in the blood flow effects induced by HPE.
The stomach is frequently exposed to hazardous agents, and to resist this harsh environment, several protective mechanisms exist. Of special interest is the gastric pathogen Helicobacter pylori (H pylori), which causes gastritis, ulcers and cancer. However, the mechanism leading to these diseases is still unclear. It is very likely that H pylori negatively influences the protection mechanisms that exist in the stomach. The aim of the present study was to investigate the mechanisms underlying the reduction in gastric blood flow induced by a luminal water extract of H pylori (HPE).
The authors studied the mechanism by which a water extract of H pylori reduces the gastric mucosal blood flow.
This study shows that a H pylori water extract reduces gastric mucosal blood flow acutely through iNOS- and nerve-mediated pathways.
The physiological responses upon the first contact with the pathogen are of great importance as they elucidate the early steps of the pathogen-associated inflammation.
The authors showed that a H pylori water extract reduces gastric mucosal blood flow acutely through iNOS- and nerve-mediated pathways. The results of this research might be important for the understanding of the mechanisms of gastric inflammation.
| 1. | Helicobacter and Cancer Collaborative Group. Gastric cancer and Helicobacter pylori: a combined analysis of 12 case control studies nested within prospective cohorts. Gut. 2001;49:347-353. |
| 2. | Atuma C, Engstrand L, Holm L. Helicobacter pylori extracts reduce gastric mucosal blood flow by a nitric oxide-independent but mast cell- and platelet-activating factor receptor-dependent pathway in rats. Scand J Gastroenterol. 1999;34:1183-1189. |
| 3. | Whittle BJ, Morishita T, Ohya Y, Leung FW, Guth PH. Microvascular actions of platelet-activating factor on rat gastric mucosa and submucosa. Am J Physiol. 1986;251:G772-G778. |
| 4. | Kubes P, Ibbotson G, Russell J, Wallace JL, Granger DN. Role of platelet-activating factor in ischemia/reperfusion-induced leukocyte adherence. Am J Physiol. 1990;259:G300-G305. |
| 5. | Bonavida B, Mencia-Huerta JM. Platelet-activating factor and the cytokine network in inflammatory processes. Clin Rev Allergy. 1994;12:381-395. |
| 6. | Castagliuolo I, LaMont JT, Letourneau R, Kelly C, O’Keane JC, Jaffer A, Theoharides TC, Pothoulakis C. Neuronal involvement in the intestinal effects of Clostridium difficile toxin A and Vibrio cholerae enterotoxin in rat ileum. Gastroenterology. 1994;107:657-665. |
| 7. | Holzer P, Livingston EH, Guth PH. Neural, metabolic, physical, and endothelial factors in the regulation of the gastric circulation. Physiology of the gastrointestinal tract. 3rd ed. New York: Raven Press 1994; 1311-1329. |
| 8. | Kawano S, Tsuji S. Role of mucosal blood flow: a conceptional review in gastric mucosal injury and protection. J Gastroenterol Hepatol. 2000;15 Suppl:D1-D6. |
| 9. | Synnerstad I, Johansson M, Nylander O, Holm L. Intraluminal acid and gastric mucosal integrity: the importance of blood-borne bicarbonate. Am J Physiol Gastrointest Liver Physiol. 2001;280:G121-G129. |
| 10. | Phillipson M, Henriksnäs J, Holstad M, Sandler S, Holm L. Inducible nitric oxide synthase is involved in acid-induced gastric hyperemia in rats and mice. Am J Physiol Gastrointest Liver Physiol. 2003;285:G154-G162. |
| 11. | Fu S, Ramanujam KS, Wong A, Fantry GT, Drachenberg CB, James SP, Meltzer SJ, Wilson KT. Increased expression and cellular localization of inducible nitric oxide synthase and cyclooxygenase 2 in Helicobacter pylori gastritis. Gastroenterology. 1999;116:1319-1329. |
| 12. | Fändriks L, von Bothmer C, Johansson B, Holm M, Bolin I, Pettersson A. Water extract of Helicobacter pylori inhibits duodenal mucosal alkaline secretion in anesthetized rats. Gastroenterology. 1997;113:1570-1575. |
| 13. | MacMicking JD, Nathan C, Hom G, Chartrain N, Fletcher DS, Trumbauer M, Stevens K, Xie QW, Sokol K, Hutchinson N. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell. 1995;81:641-650. |
| 14. | Xiang Z, Censini S, Bayeli PF, Telford JL, Figura N, Rappuoli R, Covacci A. Analysis of expression of CagA and VacA virulence factors in 43 strains of Helicobacter pylori reveals that clinical isolates can be divided into two major types and that CagA is not necessary for expression of the vacuolating cytotoxin. Infect Immun. 1995;63:94-98. |
| 15. | Atuma C, Engstrand L, Holm L. Extracts of Helicobacter pylori reduce gastric mucosal blood flow through a VacA- and CagA-independent pathway in rats. Scand J Gastroenterol. 1998;33:1256-1261. |
| 16. | Henriksnäs J, Phillipson M, Petersson J, Engstrand L, Holm L. An in vivo model for gastric physiological and pathophysiological studies in the mouse. Acta Physiol Scand. 2005;184:151-159. |
| 17. | Holm-Rutili L, Berglindh T. Pentagastrin and gastric mucosal blood flow. Am J Physiol. 1986;250:G575-G580. |
| 18. | Kvietys PR, Shepherd AP, Granger DN. Laser-Doppler, H2 clearance, and microsphere estimates of mucosal blood flow. Am J Physiol. 1985;249:G221-G227. |
| 19. | Ahn H, Lindhagen J, Nilsson GE, Salerud EG, Jodal M, Lundgren O. Evaluation of laser Doppler flowmetry in the assessment of intestinal blood flow in cat. Gastroenterology. 1985;88:951-957. |
| 20. | Johansson K, Ahn H, Lindhagen J, Lundgren O. Tissue penetration and measuring depth of laser Doppler flowmetry in the gastrointestinal application. Scand J Gastroenterol. 1987;22:1081-1088. |
| 21. | Stead RH, Dixon MF, Bramwell NH, Riddell RH, Bienenstock J. Mast cells are closely apposed to nerves in the human gastrointestinal mucosa. Gastroenterology. 1989;97:575-585. |
| 22. | Holm M, Powell T, Casselbrant A, Johansson B, Fandriks L. Dynamic involvement of the inducible type of nitric oxide synthase in acid-induced duodenal mucosal alkaline secretion in the rat. Dig Dis Sci. 2001;46:1765-1771. |
| 23. | Leiper J, Vallance P. Biological significance of endogenous methylarginines that inhibit nitric oxide synthases. Cardiovasc Res. 1999;43:542-548. |
| 24. | von Bothmer C, Edebo A, Lonroth H, Olbe L, Pettersson A, Fandriks L. Helicobacter pylori infection inhibits antral mucosal nitric oxide production in humans. Scand J Gastroenterol. 2002;37:404-408. |
| 25. | Boer R, Ulrich WR, Klein T, Mirau B, Haas S, Baur I. The inhibitory potency and selectivity of arginine substrate site nitric-oxide synthase inhibitors is solely determined by their affinity toward the different isoenzymes. Mol Pharmacol. 2000;58:1026-1034. |
| 26. | Elizalde JI, Mendez A, Gomez J, del Rivero M, Gironella M, Closa D, Quintero E, Pique JM. Gastric mucosal blood flow changes in Helicobacter pylori infection and NSAID-induced gastric injury. Helicobacter. 2003;8:124-131. |
| 27. | Shiotani A, Yanaoka K, Iguchi M, Saika A, Itoh H, Nishioka S. Helicobacter pylori infection reduces intraluminal nitric oxide in humans. J Gastroenterol. 1999;34:668-674. |
| 28. | Gobert AP, McGee DJ, Akhtar M, Mendz GL, Newton JC, Cheng Y, Mobley HL, Wilson KT. Helicobacter pylori arginase inhibits nitric oxide production by eukaryotic cells: a strategy for bacterial survival. Proc Natl Acad Sci USA. 2001;98:13844-13849. |
| 29. | Henriksnäs J, Phillipson M, Storm M, Engstrand L, Soleimani M, Holm L. Impaired mucus-bicarbonate barrier in Helicobacter pylori-infected mice. Am J Physiol Gastrointest Liver Physiol. 2006;291:G396-G403. |