Published online Oct 7, 2008. doi: 10.3748/wjg.14.5683
Revised: August 8, 2008
Accepted: August 15, 2008
Published online: October 7, 2008
AIM: To evaluate the protective effects of ilomastat, an exogenous matrix metalloproteinase (MMP) inhibitor, on trinitrobenzenesulfonic acid (TNB)-induced ulcerative colitis (UC) in rats.
METHODS: Male SD rats were randomly divided into model group, protective groups A and B, and normal control group. Rats in the model group received only intra-colonic TNB. Rats in the protective groups A and B received intra-peritoneal ilomastat of 10 mg/kg and 20 mg/kg, respectively, beside TNB. Rats in the normal control group received only intra-colonic normal saline. After 3 wk, segments of colon were obtained. RT-PCR and immunohistochemistry were used to examine the expression of MMP-1 and TIMP-1. Hematoxylin-eosin (HE) staining was used for pathological study.
RESULTS: The model of UC was successfully induced in rats. Inflammation of colonic mucosa greatly improved in protective groups A and B. Expression of MMP-1 and TIMP-1 in the model group, protective groups A and B was significantly higher than that in the normal control group (P < 0.0001) with MMP-1 expression increased more significantly than TIMP-1 expression. Expression of MMP-1 in protective groups A and B was significantly lower than that in the model group (P < 0.0001) . Expression of MMP-1 in protective group B was significantly lower than that in protective group A (P < 0.0001).
CONCLUSION: Ilomastat improves TNB-induced UC in rats by inhibiting the MMP-1 activity.
- Citation: Wang YD, Wang W. Protective effect of ilomastat on trinitrobenzenesulfonic acid-induced ulcerative colitis in rats. World J Gastroenterol 2008; 14(37): 5683-5688
- URL: https://www.wjgnet.com/1007-9327/full/v14/i37/5683.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.5683
Ulcerative colitis (UC) is a chronic nonspecific inflammatory disease of the colon characterized clinically by abdominal pain, diarrhea and mucus-pus stool. It is believed that factors such as heredity, immune disturbance, environment, and inflammatory cytokines, etc. play an important role in the development of UC[1]. Pathologically, UC invades mainly mucosa and sub-mucosa of colon with excessive degradation of extracellular matrix (ECM) by matrix metalloproteinases (MMPs) secreted by interstitial cells and activated by various cytokines[2-4]. MMPs possess many important physiological and pathophysiological functions in the gastrointestinal tract, such as implantation of T cells in mucosa, regeneration of epithelium, aggregation of lymphocytes and production of cytokines in the process of inflammation[5,6]. MMPs are regulated by their specific tissue inhibitors at transcription, pre-enzyme activation, ECM, and inhibition levels[7]. Tissue inhibitors of matrix metalloproteinases (TIMPs) are natural inhibitors of MMPs. TIMP-1, extensively present in the human body, mainly inhibits MMP-1. Mckaig BC et al[8] found that expression of MMP-1 is significantly higher while expression of TIMP-1 is relatively lower in colonic tissues. Therefore, it is quite possible that exogenous MMP inhibitors exert their inhibitory effects on expression of MMPs and subsequently improve colonic tissue damages. Disebatiano et al[9] utilized batimastat (BB-94), an artificial MMPI, in a rat model of UC to study its protective effect on colonic tissue and found that batimastat is water insoluble with a low bio-availability. Ilomastat is thought to be the most powerful MMPI with a promising future and has been used in trauma healing, tumor inhibition and cornea repair[10-12]. However, no study on its protective effect on UC is available. Therefore, in this study, we used ilomastat to study its protective effect on trinitrobenzenesulfonic acid (TNB)-induced UC in rats.
Thirty two male Sprague-Dawley (SD) rats, weighing 180-220 g, were used to establish a Morris model of UC. Briefly, the animals were fasted for 48 h before experiment. A 2 mm catheter was inserted into the anus of rats. One hundred mg/kg TNB dissolved in 0.25 mL of 50% alcohol was injected into the colon through the catheter. The animals were inverted for about 3-5 min to prevent backflow of the solution.
The rats were divided randomly into four groups: UC model group, protective groups A and B, and control group (n = 8). Rats in the model group were given only the mixed solution of TNB and alcohol, rats in protective groups A and B were intra-peritoneally given 10 mg/kg and 20 mg/kg of ilomastat, twice daily beside administration of TNB and alcohol, rats in the control group were given only normal saline. Ilomastat was administered 30 min before enema. All the animals were sacrificed 3 wk after enema.
An abdominal midline incision was made to open the abdomen and separate the colon. The colon was cut open to take the diseased colon samples. The samples were washed in 4°C normal saline to remove stool residues. Then the colon samples were snap frozen in liquid nitrogen for isolation of RNA and RT-PCR, and fixed in 10% formalin and embedded in paraffin for HE staining and immunohistochemistry.
Paraffin-embedded and formalin-fixed colon samples were cut into 4-μm thick sections for HE staining and pathological observation.
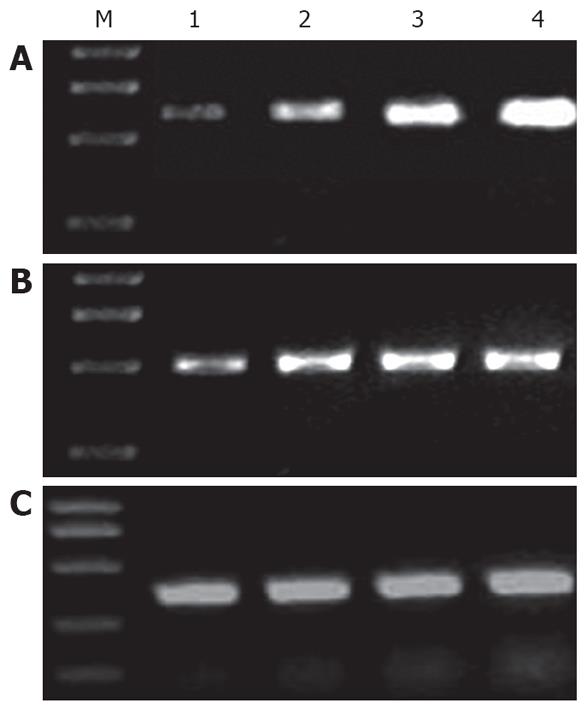
RT-PCR for MMP-1 and TIMP-1 was performed using a TaKaRa RNA PCR kit 3.0 (AMV, supplied by Dalian Baosheng Biotechnology Company) following the manufacturer’s instructions. Five μL of PCR product was run on 2% agarose gel electrophoresis. The sequences of primers[13,14] used are as followings: MMP-1 (639 bp): forward TTGTTGCTGCCCATGAGCTT and reverse ACTTTGTCGCCAATTCCAGG; TIMP-1 (495 bp): forward TTTGCATCTCTGGCCTCTG and reverse AATGACTGTCACTCTCCAG; β-actin (357 bp): forward TAAAGACCTCTATGCCAACAC and reverse TAAAGCCATGCCTAATGTCTC.
Briefly, sample sections were washed 3 times with PBS, 3 min each time after initial treatment. Primary antibodies, mouse anti-human MMP-1 and TIMP-1 monoclonal antibodies (Santa Cruz, USA) were added and incubated at room temperature for about 1.5 h, washed and incubated with peroxidase-conjugated secondary antibody for 15 min and washed. A brown product was developed in diaminobenzidine (DAB) for 10 min.
A bio-imaging system (PALL Company, USA) was employed to analyze the density of PCR product bands. MMP-1 and TIMP-1 mRNAs were semi-quantitatively expressed as the ratio of MMP-1, TIMP-1 and β-actin OD values. All values were expressed as mean ± SD.
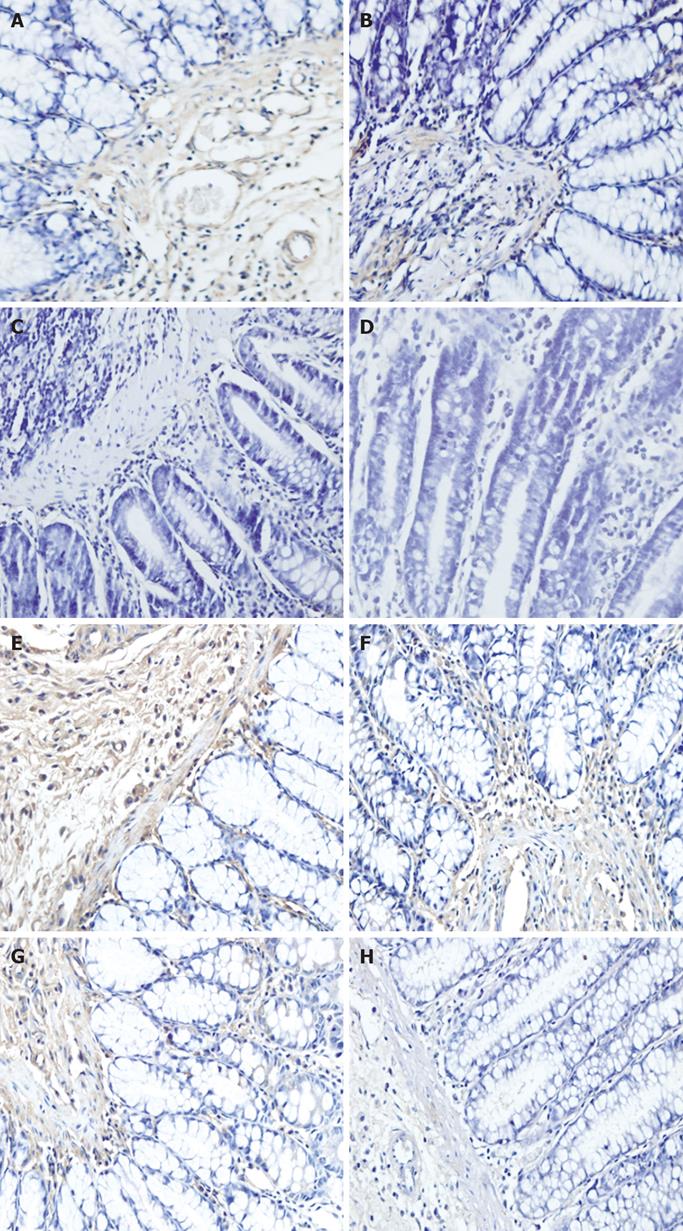
Positive results of immunohistochemistry were determined by the appearance of brown substances in cells after DAB staining. An image-pro-plus 4.5 microscopic image analyzing system (Media Cybernetics Company, USA) was utilized to measure the density of brown substances. Five fields in each section were randomly chosen, and the total density and total area in the fields were measured. The mean density was calculated by the ratio of the total density and total area. A bigger ratio indicates a greater expression of the corresponding proteins.
Student-Neuman-Keuls test was used to compare the expressions of MMP-1 mRNA, TIMP-1 mRNA and their corresponding proteins. P < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS 11.5 for windows.
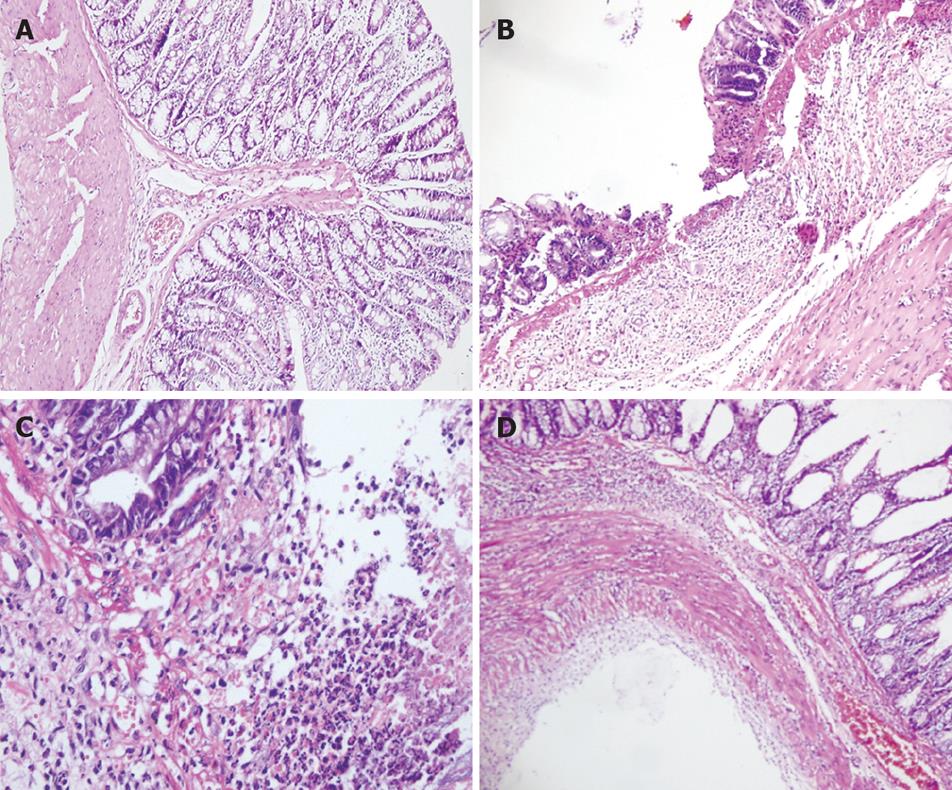
As shown in Figure 1, the colonic mucosa structure in the normal control group was intact. Mucosa and sub-mucosa defects could be clearly seen with infiltrations of inflammatory neutrophils and lymphocytes in the lamina properia of the model group. Tissue necrosis was obvious. Lobed neutrophils were observed in protective group A and proliferation of new blood vessels was found in protective group B. Regenerated epithelia covered the surface of ulcer and the mucosa tended to be complete. Necrotic tissue was absorbed. The base of ulcer was filled with tissues and scars.
As shown in Table 1 and Figure 2, expression of MMP-1 in the model group, protective groups A and B was significantly higher than that in the normal control group (P < 0.0001) and the expression of MMP-1 was more significant than that of TIMP-1. Expression of MMP-1 in protective groups A and B was significantly lower than that in the model group (P < 0.0001). Expression of MMP-1 in protective group B was significantly lower than that in protective group A (P < 0.0001).
Expression of TIMP-1 in the model group was significantly higher than that in protective group B and in the normal control group (P = 0.004). There was no significant difference between protective groups A and B (P > 0.05).
Immunohistochemistry showed that the expression of MMP-1 and TIMP-1 proteins was basically identical to that at transcription level. The expression of MMP-1 and TIMP-1 in the model group, protective groups A and B was significantly higher than that in the normal control group (P < 0.0001, Table 2, Figure 3).
Establishment of an appropriate animal model of UC is essential to the study of pathogenesis and treatment of UC. In this study, we utilized the Morris model of UC induced by TNB[15]. The pathological changes in this model which has been extensively used in recent years mimic those of human UC, revealing dynamic changes at acute and chronic phases. The possible mechanism is that TNB may act as a chemical semi-antigen which combines with keratin protein of the colonic mucosa, thus leading to local cellular immune responses and inflammatory changes when ethanol, an organic solvent, damages the colonic mucosa barrier. Neither TNB nor ethanol is able to produce typical UC[16]. In this study, HE staining revealed erosion and ulceration in the mucosa and sub-mucosa of colon with infiltrations of massive neutrophils and lymphocytes under light microscope. These changes were correspondent to the features of acute pathological lesions in UC. Allgayer et al[17] also suggested that the dividing line between acute and chronic phases in this model is approximately 3 wk after the initiation of TNB administration.
MMPs are a group of zinc-dependent proteases that play an important role in ECM remodeling, connective tissue injury, formation of new blood vessels, and proliferation of inflammatory cells[18]. An excessive degradation of ECM by MMPs is the basic pathophysiological event leading to mucosa ulceration in UC. The activity of MMPs can be inhibited specifically by their natural inhibitor, TIMP. TIMP-1 is the main member of TIMPs inhibiting nearly all MMPs, particularly MMP-1. In this study, we detected the expression of MMP-1 and TIMP-1 at transcription level using RT-PCR. The results showed that expression of both MMP-1 and TIMP-1 was significantly higher than that in the control group and the expression of MMP-1 was significantly increased. Salmela et al[19] have also observed similar results using in situ hybridization and gene sequence analysis. Our immunohistochemistry results showed that expression of MMP-1 and TIMP-1 was identical to that at transcription level, showing that the expression level of MMP-1 is higher than that of TIMP-1. MMP-1/TIMP-1 ratio was 0.02631 in the control group and 1.1164 in the model group, implying that imbalance between MMP-1 and TIMP-1 is closely related to the ulceration of colonic mucosa in UC patients[20]. Therefore, we speculate that exogenous MMP inhibitors may be able to inhibit expression of MMP-1, thus balancing the expression of MMP-1 and TIMP-1 and relieving, at least partially, the pathological changes in the colonic mucosa of UC patients. Sykers et al[21] used marimastat, another artificial MMPI, to treat TNB-induced ulcerative colitis in rats and found that marimastat is capable of relieving colonic injury and decreasing colonic inflammatory score. Medina et al[22] showed that inflammation of the colonic mucosa improves to some extent in rat models of inflammatory bowel disease. Disebatiano et al[9] found that intra-peritoneal injection of batimastat can decrease the activity of MPO and improve colonic tissue damage in TNB-induced ulcerative colitis.
In this study, we used ilomastat, a known most potent artificial MMPI, to observe its protective role in TNB-induced ulcerative colitis in rats. Its intra-peritoneal dosage used was 10 mg/kg in protective group A and 20 mg/kg in protective group B as previously described[9,12]. The pathological changes in the protective groups A and B under light microscope much improved compared with the model group. Infiltration of neutrophils and lymphocytes was greatly lessened and the mucosa tended to be perfect in the protective groups A and B, suggesting that ilomastat is able to relieve ulceration of colonic mucosa in UC patients. RT-PCR and immunohistochemistry showed that expression of MMP-1 in the protective groups A and B was obviously decreased when compared with the model group, suggesting that ilomastat is able to inhibit expression of MMP-1. Meanwhile, there was no significant difference in expression of TIMP-1 between protective groups A and B and the model group, indicating that ilomastat does not influence TIMP-1 expression. Therefore, we speculate that ilomastat exerts its protective effects by inhibiting MMP-1 expression and by restoring the balance between MMP-1 and TIMP-1. The possible mechanisms underlying its protective effect on MMP-1 expression and balance between MMP-1 and TIMP-1 may be as follows: (1) ilomastat inhibits the catalytic activity and hydrolysis of ECM by combining the active site of zinc in MMP-1 molecule; (2) ilomastat inhibits hydrolysis of collagens in a dose-dependent manner in the presence of plasminogen; (3) ilomastat inhibits formation of active MMP-1 by suppressing transfer of MMP-1 precursor.
Comparing protective groups A and B, we found that the morphological improvement was different in different dosage groups. In protective group B receiving a bigger dosage of ilomastat, mucosa ulcer was much shallower and infiltration of neutrophils and lymphocytes was fewer than that in protective group A, with regenerated epithelia cells covering the ulcer and granulation and scar tissues filling the base. Furthermore, expression of MMP-1 was also different. The expression level of MMP-1 was obviously lower in protective group B than in protective group A. These results indicate that the protective effects of ilomastat are dose dependent, which is consistent with the reported findings[21].
The protective effects of ilomastat on experimental rat model of UC observed in our study indicate that exogenous MMPI may be used in treatment of UC. Nevertheless, intervention on up-regulation of endogenous TIMP-1 to restore the balance between MMP-1 and TIMP-1 might be another treatment modality for UC.
In conclusion, expression of MMP-1 and TIMP-1 is significantly increased in TNB-induced ulcerative colitis of rats. Both MMP-1 and TIMP-1 play an important role in the pathogenesis of UC. Ilomastat is able to relieve colonic mucosa damage in TNB-nduced ulcerative colitis of rats in a dose-dependent manner by inhibiting expression of MMP-1. Therefore, ilomastat can be used in treatment of UC.
Matrix metalloproteinase-1 (MMP-1) has been implicated in the development of ulcerative colitis (UC) by the fact that it is over-expressed in this inflammatory bowel disease. Tissue inhibitor of matrix metalloproteinase-1 (TIMP-1), a natural inhibitor of MMP-1, is also believed to be over-expressed, but not parallel to that of MMP-1. Therefore, in this study, ilomastat, an artificial MMP-1 inhibitor (MMPI), was employed to verify its protective effect on the rat model of UC.
In recent years, intense and extensive studies have shown that MMPs, TIMPs and other inflammatory cytokines play an important role in the development of UC, while studies on the effects of exogenous MMPI on UC are not well documented. Therefore, more studies both in animals and in humans are badly needed to verify the protective and therapeutic effects of artificial MMPI on UC.
Ilomastat, a most powerful artificial MMPI, has been used in experimental and clinical treatment of tumors of animal and human beings. Ilomastat can inhibit tumor transfer due to its powerful inhibitory effect of on MMPs, especially on MMP-1. However, its protective effects on UC remain largely unknown. Therefore, in this study, we verified the protective effects of ilomastat on UC in rats and provided a new therapeutic approach to UC.
Up to now, no satisfactory therapy for UC is available. MMPI targeting MMPs may become a new and effective treatment modality for UC.
The authors showed that ilomastat could improve the pathological injuries of colonic mucosa in TNB-induced UC in rats by inhibiting the MMP-1 activity, thus providing a new and effective treatment modality for UC. The study is well designed and interesting.
Peer reviewer: Alastair John Watson, Professor, The Henry Wellcome Laboratory, Nuffield Building, University of Liverpool, Crown St., Liverpool, L69 3GE, United Kingdom
S- Editor Zhong XY L- Editor Wang XL E- Editor Ma WH
| 1. | Kho YH, Pool MO, Jansman FG, Harting JW. Pharmacotherapeutic options in inflammatory bowel disease: an update. Pharm World Sci. 2001;23:17-21. |
| 2. | Pender SL, Tickle SP, Docherty AJ, Howie D, Wathen NC, MacDonald TT. A major role for matrix metalloproteinases in T cell injury in the gut. J Immunol. 1997;158:1582-1590. |
| 3. | Heuschkel RB, MacDonald TT, Monteleone G, Bajaj-Elliott M, Smith JA, Pender SL. Imbalance of stromelysin-1 and TIMP-1 in the mucosal lesions of children with inflammatory bowel disease. Gut. 2000;47:57-62. |
| 4. | von Lampe B, Barthel B, Coupland SE, Riecken EO, Rosewicz S. Differential expression of matrix metalloproteinases and their tissue inhibitors in colon mucosa of patients with inflammatory bowel disease. Gut. 2000;47:63-73. |
| 5. | Naito Y, Yoshikawa T. Role of matrix metalloproteinases in inflammatory bowel disease. Mol Aspects Med. 2005;26:379-390. |
| 6. | Pender SL, MacDonald TT. Matrix metalloproteinases and the gut - new roles for old enzymes. Curr Opin Pharmacol. 2004;4:546-550. |
| 7. | Brew K, Dinakarpandian D, Nagase H. Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta. 2000;1477:267-283. |
| 8. | McKaig BC, McWilliams D, Watson SA, Mahida YR. Expression and regulation of tissue inhibitor of metallo-proteinase-1 and matrix metalloproteinases by intestinal myofibroblasts in inflammatory bowel disease. Am J Pathol. 2003;162:1355-1360. |
| 9. | Di Sebastiano P, di Mola FF, Artese L, Rossi C, Mascetta G, Pernthaler H, Innocenti P. Beneficial effects of Batimastat (BB-94), a matrix metalloproteinase inhibitor, in rat experimental colitis. Digestion. 2001;63:234-239. |
| 10. | Winding B, NicAmhlaoibh R, Misander H, Hoegh-Andersen P, Andersen TL, Holst-Hansen C, Heegaard AM, Foged NT, Brunner N, Delaisse JM. Synthetic matrix metalloproteinase inhibitors inhibit growth of established breast cancer osteolytic lesions and prolong survival in mice. Clin Cancer Res. 2002;8:1932-1939. |
| 11. | Garton KJ, Gough PJ, Blobel CP, Murphy G, Greaves DR, Dempsey PJ, Raines EW. Tumor necrosis factor-alpha-converting enzyme (ADAM17) mediates the cleavage and shedding of fractalkine (CX3CL1). J Biol Chem. 2001;276:37993-38001. |
| 12. | Witte MB, Thornton FJ, Kiyama T, Efron DT, Schulz GS, Moldawer LL, Barbul A. Metalloproteinase inhibitors and wound healing: a novel enhancer of wound strength. Surgery. 1998;124:464-470. |
| 13. | Chen H, Li D, Saldeen T, Mehta JL. TGF-beta 1 attenuates myocardial ischemia-reperfusion injury via inhibition of upregulation of MMP-1. Am J Physiol Heart Circ Physiol. 2003;284:H1612-H1617. |
| 14. | McLennan SV, Kelly DJ, Cox AJ, Cao Z, Lyons JG, Yue DK, Gilbert RE. Decreased matrix degradation in diabetic nephropathy: effects of ACE inhibition on the expression and activities of matrix metalloproteinases. Diabetologia. 2002;45:268-275. |
| 15. | Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96:795-803. |
| 16. | Little JR, Eisen HN. Preparation and characterization of antibodies specific for the 2,4,6-trinitrophenyl group. Biochemistry. 1966;5:3385-3395. |
| 17. | Allgayer H, Deschryver K, Stenson WF. Treatment with 16,16‘-dimethyl prostaglandin E2 before and after induction of colitis with trinitrobenzenesulfonic acid in rats decreases inflammation. Gastroenterology. 1989;96:1290-1300. |
| 18. | Woessner JF Jr. Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991;5:2145-2154. |
| 19. | Salmela MT, MacDonald TT, Black D, Irvine B, Zhuma T, Saarialho-Kere U, Pender SL. Upregulation of matrix metalloproteinases in a model of T cell mediated tissue injury in the gut: analysis by gene array and in situ hybridisation. Gut. 2002;51:540-547. |
| 20. | Wang YD, Yan PY. Expression of matrix metalloproteinase-1 and tissue inhibitor of metalloproteinase-1 in ulcerative colitis. World J Gastroenterol. 2006;12:6050-6053. |
| 21. | Sykes AP, Bhogal R, Brampton C, Chander C, Whelan C, Parsons ME, Bird J. The effect of an inhibitor of matrix metalloproteinases on colonic inflammation in a trinitrobenzenesulphonic acid rat model of inflammatory bowel disease. Aliment Pharmacol Ther. 1999;13:1535-1542. |
| 22. | Medina C, Videla S, Radomski A, Radomski M, Antolin M, Guarner F, Vilaseca J, Salas A, Malagelada JR. Therapeutic effect of phenantroline in two rat models of inflammatory bowel disease. Scand J Gastroenterol. 2001;36:1314-1319. |