Published online Aug 21, 2008. doi: 10.3748/wjg.14.4964
Revised: July 22, 2008
Accepted: July 29, 2008
Published online: August 21, 2008
Small cell carcinoma (SCC) of the pancreas is rare. It has similar histological features to pulmonary small cell carcinoma and is equally aggressive. Most patients with SCC in the pancreas reported in case studies died within 1 year after diagnosis. We present a case of unusually long-term survival after surgery and combined chemotherapy for SCC of the pancreas. A 62-year-old woman presented with epigastric pain and jaundice. Computed tomography revealed dilated common bile duct caused by external compression of the mass in the pancreatic head. Exploratory laparotomy and pancreaticoduodenectomy (PPPD) was performed with histopathological analysis confirming a primary small cell carcinoma of the pancreas. After an uneventful postoperative recovery, the patient was treated with 6 cycles of combined chemotherapy consisting of cisplatin and ectoposide. During the follow-up, there was no evidence of recurrence and the patient has remained in a good health condition for 36 mo since the diagnosis.
- Citation: Chung MS, Ha TK, Lee KG, Paik SS. A case of long survival in poorly differentiated small cell carcinoma of the pancreas. World J Gastroenterol 2008; 14(31): 4964-4967
- URL: https://www.wjgnet.com/1007-9327/full/v14/i31/4964.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.4964
Small-cell carcinoma (SCC) is common malignancy of the lung and represents 20%-25% of all bronchogenic carcinomas[1]. It seldom originated from extrapulmonary sites (2.5%-4% of all SCC). The primary site of extrapulmonary SCC (EPSCC) can be in a variety of organs and the clinical course of these tumors has been found to be aggressive, with early dissemination and frequent recurrence. Primary SCC of the pancreas is rare, comprising about 1% of all pancreatic malignant tumors[2]. Because of its aggressiveness, most of patients with SSC of the pancreas are diagnosed at an advanced stage of disease. According to previous reports, survival of patients with SCC in the pancreas varies between 2 and 5 mo[3]. Here, we report a case of unusually long-term survival after curative surgery and combined chemotherapy for poorly differentiated SCC of the pancreas.
A 62-year-old woman presented to our institute with a 3-wk history of anorexia, dyspepsia, epigastric pain. She had drunken extracts of Hovenia dulcis for 5 mo. There was no history of smoking and regular alcohol consumption. Her family history revealed no abnormalities. She had no relevant previous medical or surgical history. Physical examination was unremarkable except for epigastric tenderness.
Laboratory studies revealed increased total bilirubin (4.9 mg/dL) and direct bilirubin (3.5 mg/dL). Liver enzymes were raised, with serum alkaline phosphatase (1072 U/L), SGOT (233 U/L) and SGPT (101 U/L). The calcium was 9.9 mg/dL and phosphate was 4.0 mg/dL. Serum viral markers, including HIV, HBsAg, and HCV were nonreactive. Tumor markers included carbohydrate antigen 19-9 (CA 19.9, 291.6 U/mL), alpha-fetoprotein (AFP, 3.6 ng/mL), and carcino-embryonic antigen (CEA, 3.2 ng/mL). Serum neuron-specific enolase (NSE) was 12.5 ng/mL (normal, 0.1-16.3 ng/mL).
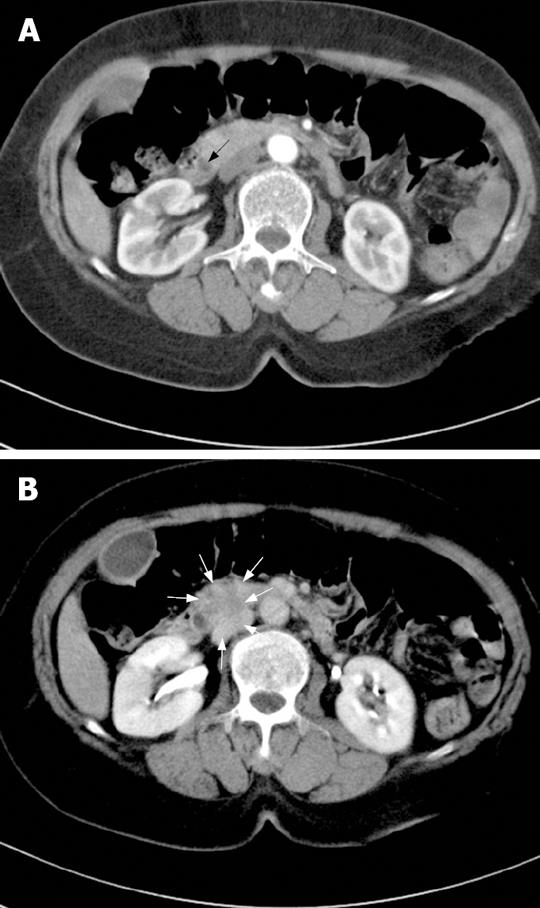
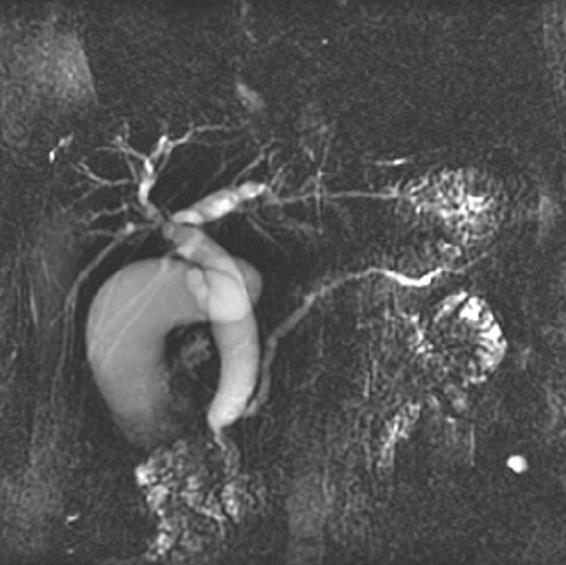
A contrast-enhanced computed tomography (CT) of the abdomen showed dilatation of intrahepatic duct, common bile duct (CBD), and pancreatic duct. There was 1.3 cm sized mass at the head of pancreas compressing the distal CBD (Figure 1). There was no evidence of pancreatitis. Magnetic resonance cholangiopancreatography and endoscopic-retrograde cholangiopancreatography (ERCP) was performed and confirmed abrupt CBD narrowing due to extrinsic compression (Figure 2). During the ERCP, it was possible to obtain tissue from the CBD.
The histopathologic study revealed small uniform nuclei with inconspicuous nucleoli and scanty cytoplasm. The morphology of the cells was similar to that of small cell carcinoma of the lung. The immunohistochemical (IHC) stain results were positive for CD56 and thyroid transcription factor-1 (TTF-1) stain and negative for NSE. Typical microscopic features confirmed small cell carcinoma. Chest X-ray and bone scan were normal.
The tumor was localized in the head of the pancreas and no extension beyond the locoregional boundaries; we performed PPPD (Longmire III operation). During the operation, there was no peritoneal seeding or invasion to adjacent organs. Common hepatic artery (No 8) and para-aortic (No 16) lymph node were enlarged but they were found out to have no metastasis in frozen section biopsy. The patient recovered without any postoperative complications.
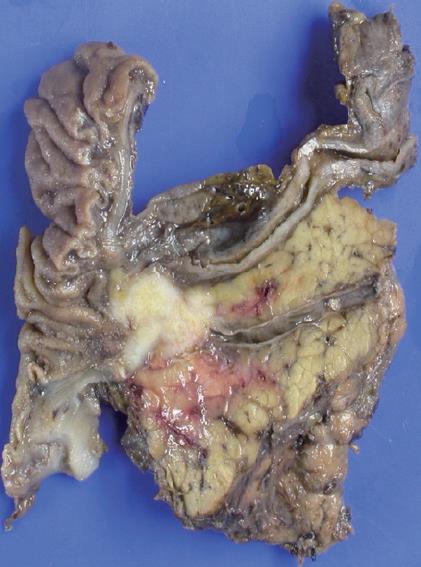
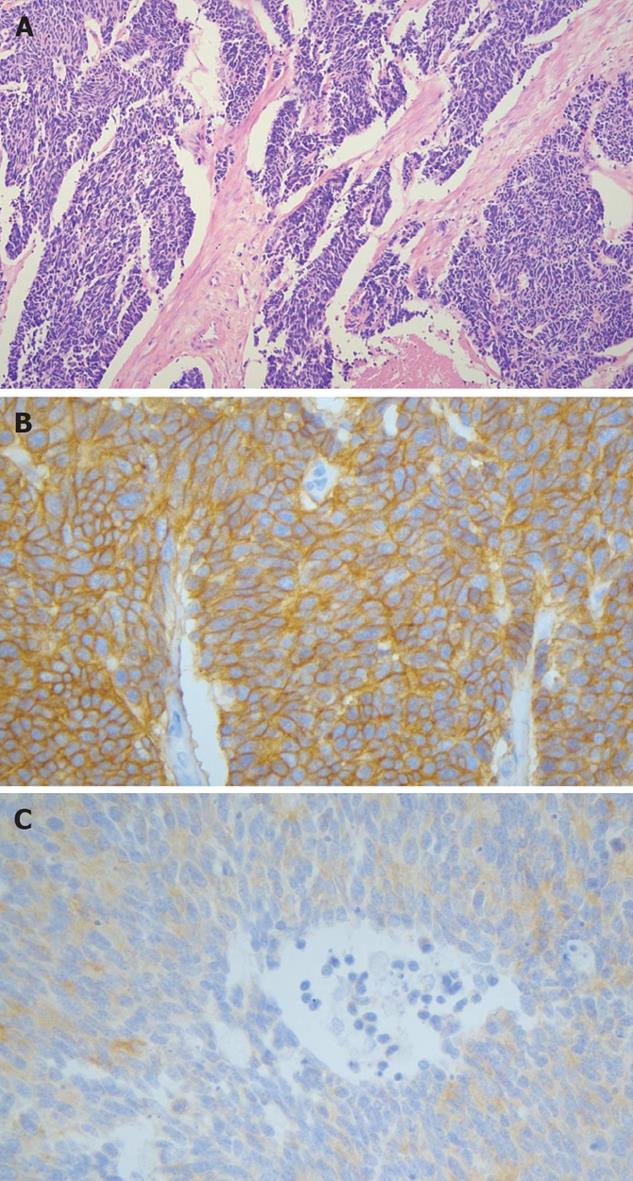
Examination of the surgical specimen showed an ill demarcated grayish white and firm mass measuring 2.0 cm × 1.2 cm in size in the head of the pancreas. 1 out of 16 lymph nodes showed tumor metastasis (Figure 3). There was lymphatic and perineural invasion. The tumor was composed of small monotonous and hyperchromatic poorly differentiated cells with higher nuclear to cytoplasmic ratio, and were positive for CD56, cytokeratin, chromagranin, TTF-1 and CEA, but negative for NSE, CD99 and equivocal for synaptophysin (Figure 4).
Four weeks after the operation, the patient received chemotherapy consisting of cisplantin (100 mg/m2) and ectoposide (60 mg/m2, day 1-3) for 4 wk intervals. After first cycle of chemotherapy CA 19-9 decreased to 19.0 U/mL. The chemotherapy was tolerated well and was continued for 6 cycles. During the follow-up there was no evidence of recurrence and the patient has remained in a good health condition for 36 mo since the diagnosis.
Extrapulmonary small cell carcinomas (EPSCC) are rare with an incidence between 0.1%-0.4% of all cancers[1]. Approximately 2.5% of all SCC’s arise in extrapulmonary sites such as head and neck region, esophagus, stomach, colon, rectum, gallbladder, uterine cervix, breast, urinary bladder, liver, and prostate[4-8].
SCC of the pancreas is a rare entity. Since the earliest case report of pancreatic SCC with clinical and pathologic findings in 1973[9], only a few reports have been published with fewer than 40 cases[3,10-12]. According to the previous studies, SCC of the pancreas is likely to occur in the head of pancreas in patients with average age of 60 and common clinical manifestations are abdominal pain, weight loss and jaundice. Many of the cases reported were diagnosed at autopsy, since most of patients with SSC of the pancreas are diagnosed at an advanced stage of disease and their survival varied between 2-5 mo[1,3].
SCC is thought to originate from neuroendocrine cells, which are found in the epithelium of many mucosal surfaces. Despite evidence of neuroendocrine involvement, the origin of EPSCC is still unclear as development from undifferentiated airway epithelium has also been suggested along with the amine precursor uptake and decarboxylation (APUD) system hypothesis which proposes a common ancestral cell derived from the neural crest, migrating to various epithelial tissues and sites within the body[13,14]. Since SCC cell has similar properties to the endocrine cells of APUD system, EPSCC may also secrete hormones. But in the case of SCC of the pancreas, there is only one report of ACTH-producing tumor by Corrin et al and one case associated with hypercalcemia[9,15], which is different from frequent paraneoplastic syndromes in pulmonary SCC. There were no abnormal findings suggesting paraneoplastic hormone syndromes in the case of our patient. O’Connor et al reported NSE was clearly elevated in SCC of the pancreas. They suggested that serum NSE could be a tumor marker for SCC of the pancreas, but NSE was within the normal range in this case[16].
EPSCC of the pancreas is histologically indistin-guishable from metastatic pulmonary SCC. Therefore, exclusion of pulmonary small cell carcinoma is a prerequisite for the diagnosis of EPSCC. In our case, chest X-ray and PET-CT were negative for the lungs. Since the biopsy from the pancreas is difficult, most of the cases were diagnosed by biopsy from the liver metastasis or lymph node or autopsy and a few cases after surgical removal[3,11,17,18]. To evaluate dilated CBD, our patient received ERCP before surgery. During the procedure it was possible to obtain tissue from the narrowing portion of the CBD. We could confirm the tumor as EPSCC before the surgery.
On gross findings, SCC of the pancreas appears as a poorly demarcated white-gray mass with areas of necrosis and hemorrhage. It usually involves the head of the pancreas with a mean diameter of 4.2 cm[3]. The histopathologic appearance of the tumor consists of nest of small to medium sized round to oval shaped cells with a finely granular and hyperchromatic nucleus, inconspicuous nucleoli and scanty cytoplasm. Primary small cell carcinoma of the pancreas has a varied immunohistochemical profile.
Neuroendocrine markers such as CD56, chromo-granin, TTF-1 and CEA were positive but NSE and synaptophysin was negative in current case.
Unfortunately clinical presentation of EPSCC is usually at an advanced stage due to the aggressive nature of the disease. Therapeutic modalities are determined by the location and extent of disease. Usually chemotherapy remains the treatment of choice and local modalities such as surgery and radiotherapy remain limited in localized disease[19].
In our case, the tumor was localized in the pancreas with regional lymph node involvement. Since there was no extension beyond the locoregional boundaries (limited disease), we could perform curative surgery.
Because of the high incidence of metastasis, chemotherapy should be given after a successful resection of the tumor. Only one case was reported long survival after curative resection of SCC of the pancreas without adjuvant chemotherapy[18]. There are no definite chemotherapeutic regimens for SCC of the pancreas due to the small patient numbers. But the combination cisplatin and etoposide showed best result with response rates reaching 70% in an analysis of the different patients of EPSCC with chemotherapeutic regimens. Doxorubicin-based regimens appear to be less effective[20].
Complete response has been observed with cisplatin-etoposide based treatment in a patient with widespread metastatic disease[11]. The two patients reported by van der Gaast had extensive disease with a survival of 16 and 11 mo after combined chemotherapy with cyclophosphamide, doxorubicin and etoposide[21]. Another patient survived 14 mo with combined chemotherapy and the radiotherapy[22]. Our patient received chemotherapy consisting of cisplantin (100 mg/m2), ectoposide (60 mg/m2, day 1-3) for 4 wk after the surgery. A CT scan of the abdomen after 6 cycles of chemotherapy showed no evidence of metastasis. The patient remains in good health 36 mo after the surgery. The reason for the good prognosis may be associated with an early detection of the tumor and the fact that the tumor was localized and showed no metastasis or dissemination.
Because of the unfavorable prognosis of EPSCC, multimodal therapy was used in most of reported cases with limited disease. In the case of resectable SCC of the pancreas, it is reasonable to perform the extensive surgery followed by chemotherapy. We report a case of primary SCC of the pancreas with unusually long-term survival after multimodal therapy.
Peer reviewers: Claudio Bassi, Professor, Policlinico B. Roma, Piazza LA Scuro, Verona 37134, Italy; Kiichi Tamada, MD, Department of Gastroenterology, Jichi Medical Shool, 3311-1 Yakushiji, Minamikawachi, Kawachigun, Tochigi 329-0498, Japan
S- Editor Zhong XY L- Editor Ma JY E- Editor Yin DH
| 1. | Remick SC, Ruckdeschel JC. Extrapulmonary and pulmonary small-cell carcinoma: tumor biology, therapy, and outcome. Med Pediatr Oncol. 1992;20:89-99. |
| 2. | Cubilla AL, Fitzgerald PJ. Morphological lesions associated with human primary invasive nonendocrine pancreas cancer. Cancer Res. 1976;36:2690-2698. |
| 3. | Berkel S, Hummel F, Gaa J, Back W, Hofheinz R, Queisser W, Singer MV, Lohr M. Poorly differentiated small cell carcinoma of the pancreas. A case report and review of the literature. Pancreatology. 2004;4:521-526. |
| 4. | Kim JH, Lee SH, Park J, Kim HY, Lee SI, Nam EM, Park JO, Kim K, Jung CW, Im YH. Extrapulmonary small-cell carcinoma: a single-institution experience. Jpn J Clin Oncol. 2004;34:250-254. |
| 5. | Kitakata H, Yasumoto K, Sudo Y, Minato H, Takahashi Y. A case of primary small cell carcinoma of the breast. Breast Cancer. 2007;14:414-419. |
| 6. | Lee SS, Lee JL, Ryu MH, Chang HM, Kim TW, Kim WK, Lee JS, Jang SJ, Khang SK, Kang YK. Extrapulmonary small cell carcinoma: single center experience with 61 patients. Acta Oncol. 2007;46:846-851. |
| 7. | Hojo T, Kinoshita T, Shien T, Terada K, Hirose S, Isobe Y, Ikeuchi S, Kubochi K, Matsumoto S, Sadako AT. Primary small cell carcinoma of the breast. Breast Cancer. 2009;16:68-71. |
| 8. | Choi SJ, Kim JM, Han JY, Ahn SI, Kim JS, Kim L, Park IS, Chu YC. Extrapulmonary small cell carcinoma of the liver: clinicopathological and immunohistochemical findings. Yonsei Med J. 2007;48:1066-1071. |
| 9. | Corrin B, Gilby ED, Jones NF, Patrick J. Oat cell carcinoma of the pancreas with ectopic ACTH secretion. Cancer. 1973;31:1523-1527. |
| 10. | Reyes CV, Wang T. Undifferentiated small cell carcinoma of the pancreas: a report of five cases. Cancer. 1981;47:2500-2502. |
| 11. | Morant R, Bruckner HW. Complete remission of refractory small cell carcinoma of the pancreas with cisplatin and etoposide. Cancer. 1989;64:2007-2009. |
| 12. | Kinoshita K, Minami T, Ohmori Y, Kanayama S, Yoshikawa K, Tsujimura T. Curative resection of a small cell carcinoma of the pancreas: report of a case of long survival without chemotherapy. J Gastroenterol Hepatol. 2004;19:1087-1091. |
| 13. | Pearse AG. The APUD cell concept and its implications in pathology. Pathol Annu. 1974;9:27-41. |
| 14. | Remick SC, Hafez GR, Carbone PP. Extrapulmonary small-cell carcinoma. A review of the literature with emphasis on therapy and outcome. Medicine (Baltimore). 1987;66:457-471. |
| 15. | Hobbs RD, Stewart AF, Ravin ND, Carter D. Hypercalcemia in small cell carcinoma of the pancreas. Cancer. 1984;53:1552-1554. |
| 16. | O'Connor TP, Wade TP, Sunwoo YC, Reimers HJ, Palmer DC, Silverberg AB, Johnson FE. Small cell undifferentiated carcinoma of the pancreas. Report of a patient with tumor marker studies. Cancer. 1992;70:1514-1519. |
| 17. | Reyes CV, Wang T. Undifferentiated small cell carcinoma of the pancreas: a report of five cases. Cancer. 1981;47:2500-2502. |
| 18. | Kinoshita K, Minami T, Ohmori Y, Kanayama S, Yoshikawa K, Tsujimura T. Curative resection of a small cell carcinoma of the pancreas: report of a case of long survival without chemotherapy. J Gastroenterol Hepatol. 2004;19:1087-1091. |
| 19. | Casas F, Ferrer F, Farrus B, Casals J, Biete A. Primary small cell carcinoma of the esophagus: a review of the literature with emphasis on therapy and prognosis. Cancer. 1997;80:1366-1372. |
| 21. | Van Der Gaast A, Verwey J, Prins E, Splinter TA. Chemotherapy as treatment of choice in extrapulmonary undifferentiated small cell carcinomas. Cancer. 1990;65:422-424. |
| 22. | Wahid NA, Neugut AI, Hibshoosh H, Brunetti JC, Fountain KS, Rubin M. Response of small cell carcinoma of pancreas to a small cell lung cancer regimen: a case report. Cancer Invest. 1996;14:335-339. |