Published online Aug 21, 2008. doi: 10.3748/wjg.14.4928
Revised: June 30, 2008
Accepted: July 7, 2008
Published online: August 21, 2008
AIM: To investigate the permeability characteristics of rebamipide across intestinal mucosa, and examine the effects of some absorption enhancers on the permeability across the colonic tissue. Another purpose is to demonstrate the colon-specific delivery of rebamipide with or without absorption enhancers using chitosan capsule as a carrier.
METHODS: The permeability of rebamipide was evaluated using an in vitro diffusion chamber system, and the effects of some absorption enhancers on the permeability via colon were further investigated. The release of rebamipide from chitosan or gelatin capsule was studied by Japan Pharmacopoeia rotating basket method. The colonic and plasma concentrations were analyzed by high performance liquid chromatography (HPLC) to evaluate colon-targeting action after oral administration of various dosage forms, and rebamipide with absorption enhancers in chitosan dosage forms.
RESULTS: The permeability of rebamipide across the jejunal or ileal membranes was higher than the colonic membranes. Both sodium laurate (C12) and labrasol significantly increased permeability across the colon membranes. On the other hand, the release of rebamipide from chitosan capsule was less than 10% totally within 6 h. The area under concentration-time profile of drug in the colon mucosa using chitosan capsules (AUCLI, 1 6011.2 ng·h/g) was 2.5 times and 4.4 times greater than using gelatin capsules and CMC suspension, respectively. Meanwhile, the area under concentration-time profile of drug in the plasma (AUCPL) was 1016.0 ng·h/mL for chitosan capsule, 1887.9 ng·h/mL for CMC suspension p and 2163.5 ng·h/mL for gelatin capsule. Overall, both AUCLI and AUCPL were increased when C12 was co-administrated, but the increase of AUCLI was much greater; the drug delivery index (DDI) was more than 1 compared with simple chitosan capsule group.
CONCLUSION: There was a regional difference in the permeability of Rebamipide across the jejunum, ileum and the colon, and passive diffusion seems to be one of the major transport mechanisms of rebamipide. Absorption enhancers can increase the permeability of rebamipide across the colon tissue significantly. In addition, chitosan capsule may be a useful carrier to deliver rebamipide to the colon specifically and the co-administration of C12 with rebamipide may also be very useful in local treatment.
-
Citation: Huang BB, Li GF, Luo JH, Duan L, Nobuaki K, Akira Y. Permeabilities of rebamipide
via rat intestinal membranes and its colon specific delivery using chitosan capsule as a carrier. World J Gastroenterol 2008; 14(31): 4928-4937 - URL: https://www.wjgnet.com/1007-9327/full/v14/i31/4928.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.4928
Rebamipide (2-(4-chlorobenzoylamino)-3-[2(1H)-quinolinon-4-yl] propionic acid), a novel anti-ulcer drug, has been reported to prevent various acute experimental gastric lesions and accelerate healing of chronic gastric ulcers[1]. This drug has been marketed in Japan since 1990 as a therapeutic agent treating gastric ulcer and acute and/or chronic gastritis. Although the characteristics of rebamipide in preclinical and clinical area[2,3], which is one of key factors to develop its reasonable oral dosage form at the initial stage, has been investigated fully, little was known about the permeability of this drug in different gastrointestinal tissues. On the other hand, recent studies also have shown the beneficial effect of this drug on experimental colitis. It has been demonstrated that the attenuation of colitis indices induced by rebamipide was associated with its inhibition of inflammatory cytokine-mediated granulocyte (neutrophil) infiltration into the colon[4]. Other groups demonstrated that rebamipide can suppress chemically induced colitis in rodents, which appeared to be largely related with the inhibition of the production of reactive oxygen species. In clinical practice, rebamipide has been used to treat patients with proctitis[5]. Therefore, we hope to find some absorption enhancers to augment permeability of rebamipide across the colonic tissues, and in this case, this drug should be specifically localized in the large intestine by its colon-specific delivery to improve its therapeutic effect on colitis and to decrease its side-effects, as well. There are many investigations on absorption enhancers; meanwhile, it has been demonstrated previously that chitosan capsule could act as useful carriers for colon-specific delivery of peptide and anti-inflammatory drugs including insulin, calcitonin, 5-aminosalicylic acid and ridogrel[6-9]. However, the effects of absorption enhancers increasing the distribution on colon and chitosan capsule on the colon-specific delivery of rebamipide ought to be established.
Therefore, we evaluated the permeability of rebamipide across different gastrointestinal membranes in the present study, analyzed potential transport mode, and investigated the effect of some absorption enhancers, such as sodium laurate (C12) and labrasol, on the permeability of rebamipide across colonic tissues. In addition, the effectiveness of chitosan capsule to the colon-specific delivery of rebamipide and the influence of rebamipide chitosan capsules with absorption enhancers on colon specific delivery were evaluated.
Rebamipide was purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO). HEPES (2-[4-(2-hydroxyethyl)-1-piperazinyl] ethanesulfonic acid) and HPMCP (Hydroxypropyl methylcellulose phthalate) were purchased from Wako Pure Chemical Industries Co. C12 was from Tokyo Kasei Kogyo Co. Ltd, while labrasol was from Saint-Priest, France. Chitosan capsules and gelatin capsules were obtained from Aicello Chemical Company Ltd. (Toyohashi, Japan), and the mean diameters and weight of these capsules were 3.5 mm × 1.6 mm and about 1.0 mg, respectively. All other chemicals were of analytical grade.
Diffusion chamber apparatus was purchased from Harvard, US. The Shimadzu LC-10A system (Japan) as high performance liquid chromatography (HPLC) instrument was used in this study. The pH measuring instrument was from DKK.TOA Corporation in Japan. NTR-6000 Dissolution Tester made in Japan was chosen to test the dissolution rate of drug dosage forms.
Male Wistar rats (280 ± 20 g) were purchased from Laboratory Animal Center, Southern Medical University. All of the animal experiments were performed according to the guideline of Experimental Animal Ethics Committee of Southern Medical University.
For in vitro permeability studies, rebamipide was dissolved in oxygenated (O2/CO2, 95/5) HEPES buffer adjusted to pH 7.4, which was prepared daily, to yield final concentration of 80 μmoL/L. In certain experiments, the dosing solutions were added to labrasol (0.4, 1, 2 g/L) or C12 (0.5, 1, 2 mmol/L).
The in vitro transport of rebamipide across different intestinal membranes was evaluated by a diffusion chamber method using stripped rat intestine for 2 h[10,11]. Male Wistar rats, weighing 280 ± 20 g, were fasted overnight and anesthetized with sodium pentobarbital (32 mg/kg, IP). The intestine of each rat was excised and rinsed in PBS of pH 7.4 and, avoiding Peyer’s patches, experimental segments were obtained. The first 5 cm of the top of small intestine was cut away, the next 10 cm was used as the jejunum and the final 10 cm was considered to be the ileum. The first 2 cm of the large intestine was removed and the next 6 cm was used as the colon. The underlying muscularis from the serosal side of the tissue was removed and the final intestinal segments were mounted in the diffusion chamber in which a surface area of 1.78 cm2 was exposed and preheated to 37°C. Immediately following tissue mounting, 7 mL of HEPES buffer at pH 7.4 was added to serosal side or mucosal side, while an equal volume of the rebamipide solution with or without an absorption enhancer was added to the opposite side. Each side of the chamber was bubbled with a mixture of 95 mL/L O2 and 5 mL/L CO2 to maintain the viability of the membrane. The heating unit was capable of holding six cells and the temperature was kept at 37°C during the whole procedure using a circulating water bath. At predetermined time intervals, 0.4 mL of solution at receiver side was sampled and it was immediately added to an equal volume of the HEPES buffer kept at the same temperature. The concentration of rebamipide in the samples was determined by HPLC. The apparent permeability coefficient (Papp) was calculated by the equation Papp = dC/Dt × (1/A·C0), where Papp is expressed in cm/s, dC/dT is the slope of the linear portion of the permeation curves, A is the diffusion area, and C0 is the initial concentration of rebamipide in the donor side.
One mg of rebamipide or a mixture of C12 (0.05, 0.15 and 0.25 mg) with rebamipide (1 mg) was filled in one chitosan capsule. Then, the surface of the chitosan capsule was coated with hydroxypropylmethylcellulose phthalate (HPMCP) as an enteric coating material. 150 g/L HPMCP dissolved in acetone/ethanol (1/1) solvent was used in the whole procedure. In addition, 1 mg of rebamipide was filled in one gelatin capsule as one control dosage form. The procedure of preparation of this dosage form was the same as that of chitosan capsule. For another control dosage form, 4 mg/mL of rebamipide solution in 5 g/L CMC (Carboxymethylcellulose) containing 10 mmol/L NaOH was also prepared.
The dissolution tests of rebamipide from chitosan capsules and gelatin capsules were carried out using the Japanese Pharmacopoeia (JP) rotating basket method with some slight modifications. Liquid 1 (a model medium of an artificial gastric juice for the Japanese Pharmacopoeia disintegration test) and liquid 2 (a model medium of an artificial intestinal juice for the Japanese Pharmacopoeia disintegration test) were used as media in these experiments. The rotation speed of the baskets was 100 r/min. Samples (0.2 mL) were taken every 60 min and the amount of rebamipide released from the capsules was determined by HPLC.
HPLC conditions: Rebamipide in colonic tissue and plasma was assayed by reversed phase HPLC system containing 5-μm Cosmosil (4.6 mm × 150 mm) particles in an analytical column from Nacalai Tesque, a Shimadzu LC-10 pump system, a Shimadzu SIL-10A autoinjector and a Shimadzu CR-6A integrator. The mobile phase was mixture of 15 mL/L HAc solution (mobile phase A) and acetonitrile containing 100 mL/L tetrahydrofuran (mobile phase B). The gradient system was programmed by linearly increasing the proportion of mobile phase B from 18% to 40% within 55 min. The ultraviolet detector was set at 240 nm.
Preparation of colon tissue sample: Male Wistar rats weighing 280 ± 20 g were fasted for 16 h prior to experiments but allowed water ad libitum. They were anesthetized with sodium pentobarbital (32 mg/kg, ip). The abdomen was opened through a midline incision and the whole colon was removed from the body. After being washed with PBS, the colon tissue was cut into small pieces. The specimens were weighed. Methanol (5 mL) was added and the specimens were homogenated at ice-water bath using a POLYTRON homogenizer. The homogenate was centrifuged at 12 000 r/min for 15 min. The supernatant was evaporated at 60°C under nitrogen flow. The residue was re-dissolved in 0.25 mL of 5 mmol/L NaOH solution by ultrasound for 15 min. The suspension was centrifuged at 12 000 r/min for 15 min. The resulting supernatant was taken as colon tissue sample to be analyzed by HPLC.
Preparation of plasma sample: Male Wistar rats, 280 ± 20 g, were fasted for 16 h prior to experiments but allowed water ad libitum. They were anesthetized with sodium pentobarbital (32 mg/kg, IP). The abdomen was opened through a midline incision and 5 mL blood was collected into heparinized syringes via the abdominal vein. Samples were immediately centrifuged at 10 000 r/min for 5 min to obtain plasma fraction. 5 mL methanol was added in 1 mL plasma, and the mixture was vortexed for 1 min and then centrifuged at 3600 r/min for 15 min. 4.5 mL of supernatant was evaporated at 60°C under nitrogen flow. The residue was re-dissolved in 0.25 mL 5 mmol/L NaOH solution by ultrasound for 15 min. The suspension was centrifuged at 12 000 r/min for 15 min. The resulting supernatant was taken as plasma sample to be analyzed by HPLC.
Male Wistar rats, 280 ± 20 g, were fasted for 16 h before the experiments, and then four chitosan or gelatin capsules (4 mg rebamipide) were administered orally to the stomach via polyethylene tubing under light ether anesthesia. One mL of distilled water was administered after that. For the CMC group, 1 mL of rebamipide CMC solution (4 mg rebamipide) was taken orally under the same conditions. At the predetermined time interval, blood samples and colonic tissue samples were prepared, and then the rebamipide contents in the samples were determined by HPLC. Drug delivery index (DDI) was calculated from the following equation: DDI = (AUC1LI/AUC2LI)/(AUC1PL/AUC2PL), where AUC1LI and AUC1PL represent the area under concentration-time profiles of rebamipide in the large intestinal mucosa and the area under the plasma concentration-time profiles of rebamipide after the oral administration of its chitosan capsules, respectively, while AUC2LI and AUC2PL represent the area under concentration-time profiles of rebamipide in the large intestinal mucosa and the area under the plasma concentration-time profiles of rebamipide, respectively, after the oral administration of its gelatin capsules or its CMC solution. The in vivo absorption experiments were run with C12, and then four chitosan with C12 were orally administered to the stomach, other procedures same as above, DDI = (AUC3LI/AUC1LI)/(AUC3PL/AUC1PL). AUC3LI and AUC3PL represent the area under concentration-time profiles of rebamipide in the large intestinal mucosa and the area under the plasma concentration-time profiles of rebamipide, respectively, after the oral administration of chitosan capsules with different dosage of C12.
Results were expressed as the mean ± SE and statistical significance was performed by the Student’s t-test or Dunnett’s test for multiple comparisons with the minimum levels of significance, P < 0.05.
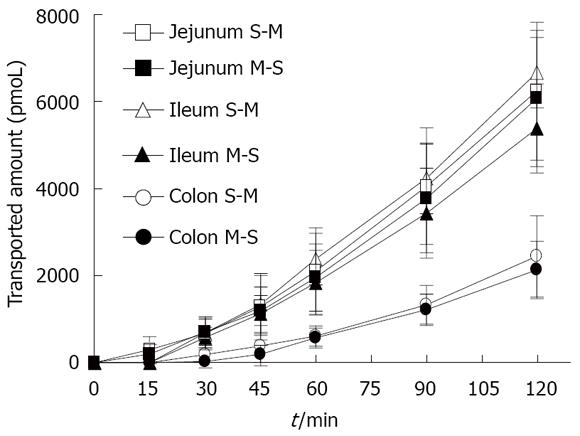
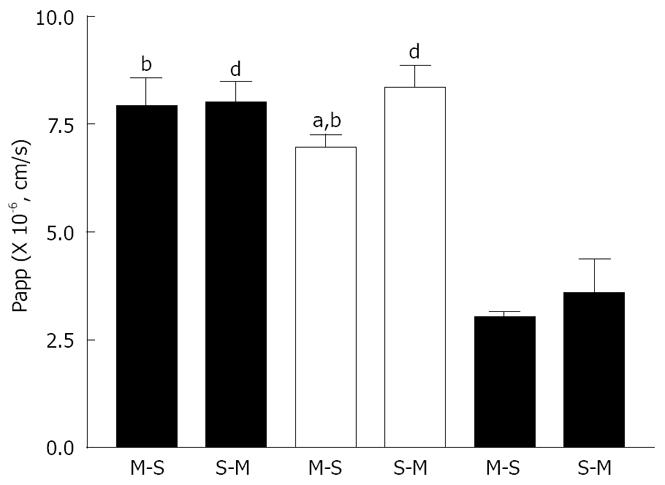
Figure 1 shows the time course of absorptive (mucosal to serosal, M-S) and secretory (serosal to mucosal, S-M) transport of rebamipide across the rat various intestinal membranes. As shown, there were regional differences in the in vitro permeability of rebamipide. The permeability of rebamipide across the jejunal or ileal membranes was higher than that across the colonic membrane. The S-M transport of rebamipide in the jejunum and colon was almost as same as its M-S transport, while the S-M transport of rebamipide across ileal tissues was slightly greater than that from M-S transport. Figure 2 summarizes the Papps of rebamipide across the different intestinal regions. As shown in Figure 2, Papp in jejunum and ileum was higher than that in colon, but no significant difference of drug permeability was observed between jejunal and ileal region. Based on the regionally different absorption studies, we selected colon as a model region to estimate the permeability of rebamipide in the presence of some absorption enhancers in the following, because the colon is the pharmacodynamic position of rebamipide.
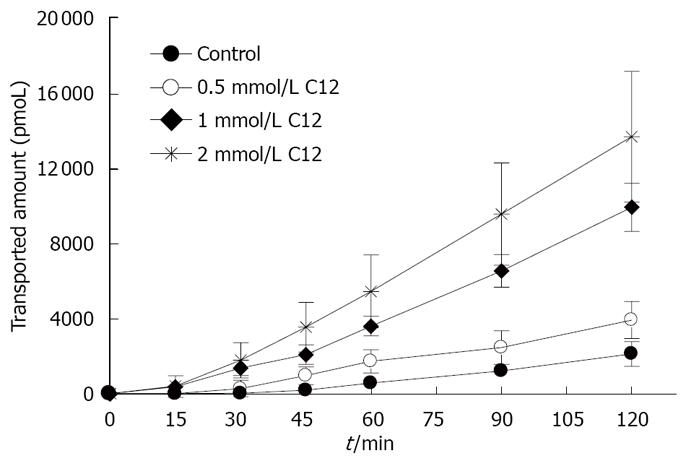
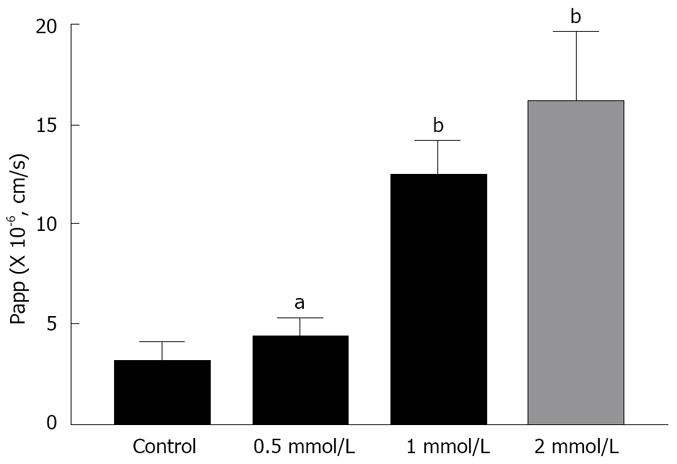
Figures 3 and 4 show the effects of different concentrations of C12 on the cumulative amount and Papp of rebamipide across the colonic region. As showed in Figures 3 and 4, the permeability of rebamipide from the colonic region was remarkably enhanced by the addition of C12. Also, there exists a concentration-dependent effect of C12 on the absorptive transport of rebamipide over the range of 0.5 to 2 mmol/L. In general, the higher concentrations of C12 gave the greater enhancement of rebamipide transport. In this experiment, we also have used 0.4% labrasol to dissolve C12, since C12 itself was not dissolved easily in the HEPES buffer. Therefore, we also investigated the influence of labrasol on the permeability of rebamipide across the colonic membranes.
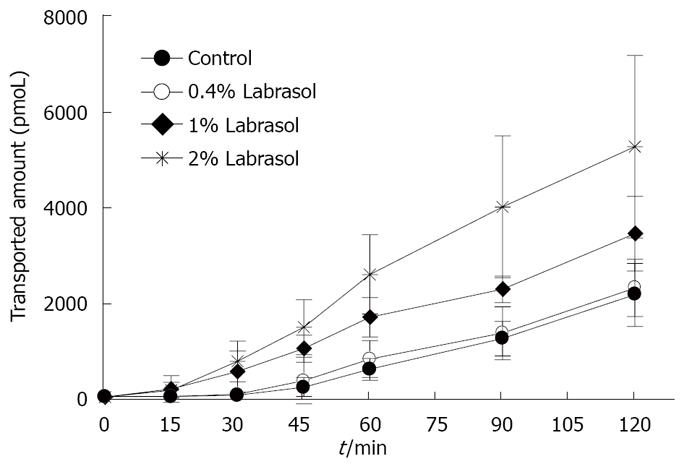
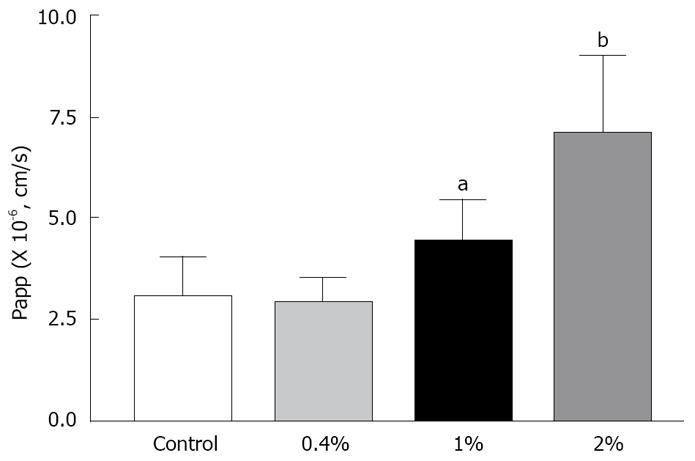
The transport of rebamipide with labrasol across the colonic membranes was examined. We observed a concentration-dependent effect of labrasol on the cumulative amount and Papp of rebamipide across the colonic region, as indicated in Figures 5 and 6. However, there exists no effect of labrasol at lower concentration (0.4 g/L) on the transport of rebamipide. In addition, the enhancement effect of labrasol for the permeability of rebamipide was not as strong as that with C12.
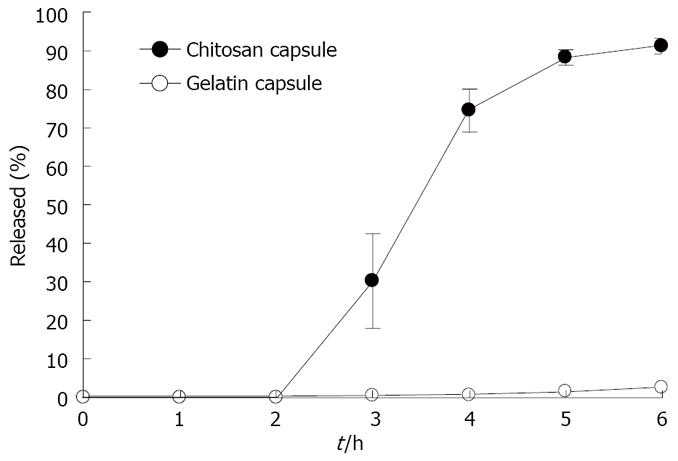
Figure 7 shows the release-time profiles of rebamipide from chitosan capsules and gelatin capsules. We studied drug release in liquid 1, an artificial gastric juice (pH 1), during the period 0-2 h after dosing, and in liquid 2, an artificial intestinal juice (pH 7) during the period 2-6 h after dosing. The release of rebamipide from the chitosan capsules in the protection of HPMCP was less than 10% totally within 6 h, while its release from the gelatin capsules reached about 100% in the same conditions.
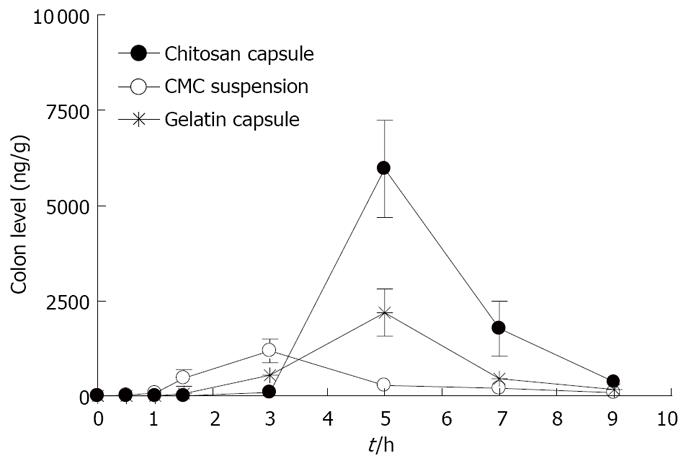
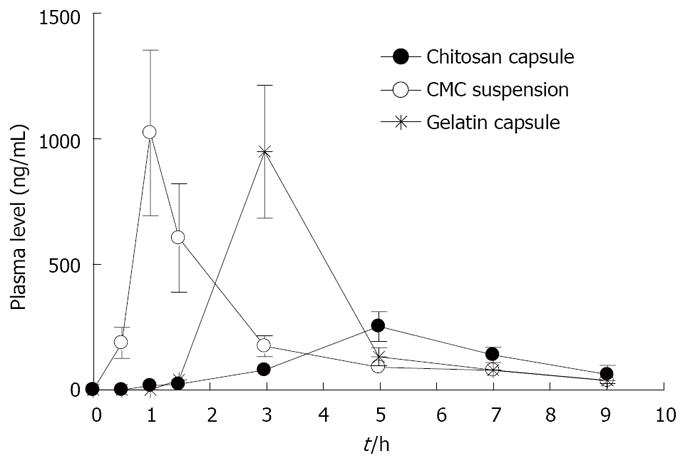
Figure 8 shows the time course of rebamipide content in the large intestine after the oral administration of rebamipide in different dosage forms. The area under concentration-time profile of drug in the large intestinal mucosa (AUCLI,16011.22 ng· h/g) after the oral administration of rebamipide using chitosan capsules was 2.5 times and 4.4 times greater than that of rebamipide using gelatin capsules and CMC solution, respectively. The target site of rebamipide is the large intestine, while the transfer amount of rebamipide to the systemic circulation after the oral administration is an index of the drug level in non-targeted sites and is related to the manifestation of adverse effects. We therefore determined the plasma concentrations of rebamipide after its oral administration with chitosan capsules and gelatin capsules as well as CMC solution. Figure 9 shows the plasma concentration-time profiles of rebamipide after the oral administration of rebamipide in different dosage forms. The area under the curve in the plasma (AUCPL) in CMC solution group was 1887.92 ng· h/mL, and AUCPL in gelatin capsule group was 2163.52 ng· h/mL. On the other hand, we observed an at least 1 h lag time in plasma concentration after the oral administration of chitosan capsules containing rebamipide. The AUCPL was 1016.02 ng· h/mL. Overall, the AUCPL value of rebamipide with chitosan capsule was lower than that seen with gelatin capsule or CMC solution. Thus the absorption of rebamipide from the gastrointestinal tract to the systemic circulation after the oral administration was inhibited by the use of chitosan capsules. In addition, the DDI, which shows the ratio of drug amount in targeted and non-targeted sites in different dosage forms, constitutes a good index for drug efficacy and safety. By using the equation indicated in Materials and Methods, the DDI of rebamipide in chitosan capsules was calculated to be 8.3 and 5.4, compared with CMC solution and gelatin capsules, respectively, and thus confirmed the effectiveness of chitosan capsules in ensuring the colon-specific delivery of rebamipide.
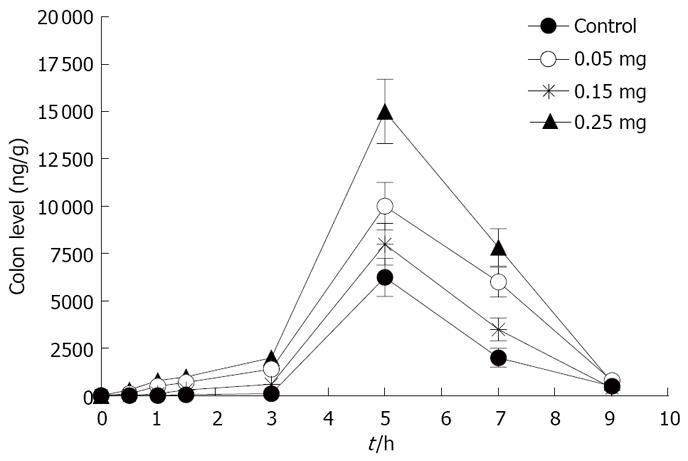
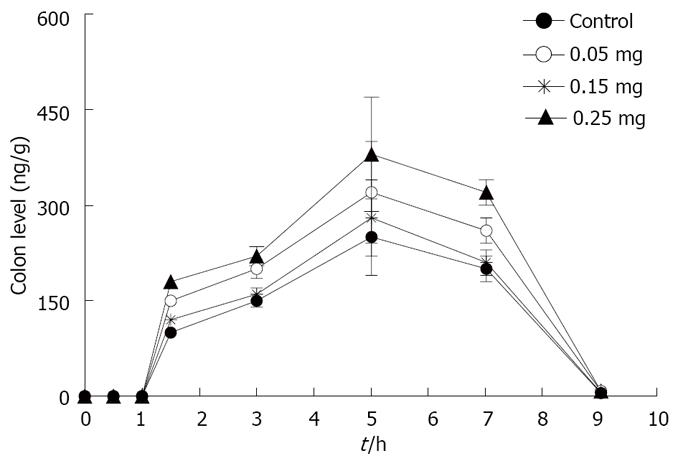
The time course of rebamipide content in the large intestine and plasma of rebamipide after the oral administration of rebamipide with different dosage of C12 is shown in Figures 10 and 11. The area under concentration-time profile of drug in the large intestinal mucosa (AUCLI) increased remarkably after the oral administration of rebamipide with C12, especially the dosage of 0.25 mg; AUCLI of chitosan capsules containing rebamipide was 38 458.2 ng· h/g. Though AUCPL (1536.1 ng· h/mL) also enhanced, the increment of AUCLI was larger than AUCPL statistically. By using the three dosage of C12, the DDI of rebamipide in chitosan capsules was calculated to be 1.8, 1.4 and 1.2, respectively, compared with chitosan capsules. These indicated that absorption enhancer C12 can promote plasma concentration and colonic tissue distribution of rebamipide, but is much more profitable to colonic tissue distribution. In addition, there existed a concentration-dependent effect of C12 on the plasma concentration and colonic tissue distribution over the range of 0.05 to 0.25 mg. In general, the higher dosage of C12 gave the larger enhancement of distribution.
Most drugs (approximately 60%) treat diseases by oral administration, which is the easiest and most useful method for drug delivery, and prediction of drug absorption is therefore very important for the design of an oral preparation. Only a few experimental in vitro methods have so far been established for prediction of drug absorption capability in vivo. Caco-2 monolayers are generally accepted to be a suitable in vitro model for drug transport studies as these cells have been shown to express most of the enzymatic, functional and morphological characteristics of the intestinal and morphological characteristics of the intestinal mucosa[12,13]. However, Caco-2 monolayers cannot be used to predict the regional differences in the permeability of drugs in the gastrointestinal tract. Also, the expression levels of transporters in Caco-2 cells were usually variable and were dependent on the culture condition, which is one of the major disadvantages to estimate the actual permeability of drugs, especially drugs mediated by these transporters. On the other hand, diffusion chamber technique[14-16] is one of the other effective means to predict the absorbability of drugs in humans based on rat intestinal permeability. Recently, Watanabe et al investigated the correlation between the apparent permeability coefficients (Papp) of 15 water-soluble and poorly water-soluble drugs based on the diffusion chamber experiment and the fractions absorbed (Fa) in humans. A good correlation was found between Papp and Fa of the test drugs[17]. Therefore, in this study, we also investigated the permeability of rebamipide across different gastrointestinal membranes using diffusion chamber experiment.
The low solubility and low permeability of rebamipide indicate that it should be classified into Class IV in Biopharmaceutics Classification System (BCS)[18]. It has been known that many drugs categorized into BCS Class IV are P-gp substrates[19]. Also, it has also been demonstrated that the intestinal P-gp, an ATP-dependent multidrug efflux pump, can be an active secretion system or an absorption barrier by transporting some drugs from cells into intestinal lumen. Meanwhile, our results revealed that the permeability of rebamipide across the different gastrointestinal regions in M-S or S-M direction is almost equal, although S-M transport of rebamipide across the ileal tissue was slightly greater than that from M-S transport. Based on these results, it may be inferred that carrier-mediated intestinal transport was not involved in the transport of rebamipide and passive diffusion seems to be one of the major mechanisms for the intestinal transport of rebamipide. However, we have used 80 μmol/L of rebamipide in this study to avoid the limitation of detectable amount by the present HPLC method. For most of carrier-mediated intestinal transports, it has been said that there exists a saturated effect of drug concentration on the transport of drug[20]. Therefore, it is necessary to investigate the characteristics of the permeability of rebamipide across M-S or S-M direction at much lower concentration in order to elucidate the exact mechanism of its transport across the intestinal membrane in the future, as more sensitive determination of rebamipide will have to be set up. In addition, we found in this study that the Papp of rebamipide across the colonic membranes in the absorptive direction was about (3.06 ± 0.07) × 10-6 cm/s (n = 15), while Miyake et al[21] reported the Papp across the same membrane was about (2.93 ± 0.35) × 10-6 cm/s. The slight difference may be result from the different experimental conditions.
As far as the discordance of the absorbability or bioavailability of rebamipide in human and its in vitro permeability is concerned, it can be inferred that some rebamipide metabolism or any others could be involved in human intestinal tract, as the bioavailability of rebamipide was only about 10%, while the permeabilities of rebamipide across the absorptive jejunal and ileal membranes in our study reached 8.0 × 10-6 cm/s and 6.9 × 10-6 cm/s, respectively. Consistent with our results, Walle et al also showed that the Papp of taxol was 4.4 × 10-6 cm/s in Caco-2 cells, and in general a Papp value in Caco-2 cells of > 1 × 10-6 cm/s is associated with efficient intestinal absorption in humans. Therefore, it was hypothesized that the low oral bioavailability of taxol may be more dependent on presystemic metabolism in the liver than on lack of absorption. Also, the authors pointed out that a novel development involving increased expression of CYP3A4 should be helpful to examine the potential contribution of CYP3A4 to the transport of drugs such as taxol using the Caco-2 cell system[22]. Hence, these factors also should be involved to evaluate the absorption characteristics of rebamipide in human, based on the obtained permeability parameters of rebamipide using diffusion chamber in our present study.
As the improvement of drug permeability by using an absorption enhancer has been very attractive from the aspects of biopharmaceutics, pharmacology, and economics, many researchers investigated the absorption enhancement using various adjuvants[23-25]. However, it has been very difficult to use those adjuvants for practical formulation, because they possibly cause local toxicity. Although many compounds have been reported to have absorption enhancing ability, medium-chain fatty acids and medium-chain glycerides are thought to be relatively safe because they are used as nutritional dietary supplements. Currently, only sodium caprete (C10) has been used and marketed as an absorption enhancer in ampicillin suppository marketed in Japan, Denmark, and Sweden, and in ceftizoxime suppository in Japan[26,27]. Furthermore, Miyake et al demonstrated that C12 was a more effective and safer adjuvant than C10 at the same concentration. Additionally, rebamipide is used to treat the proctitis in colon; therefore, we examined the effect of C12 on the permeability of rebamipide across colonic membranes in the absorptive direction, and we found that the permeability of rebamipide from the colonic region was remarkably enhanced by the addition of C12. Generally, the effects of the absorption enhancing agents are also often intestinal site-dependent. Hence, we also determined the effect of C12 on the permeability of rebamipide across the absorptive ileum membranes. We found the increased Papp ratio of rebamipide with 1 mmol/L of C12, compared with no C12 in this region was 1.92 (data was not shown in the results section), while it was 4.04 in the colon region, where Papp ratio = Papp (with C12)/Papp (without C12). It seems that using C12 as absorption enhancer to increace the absorption of rebamipide is feasible. On the other hand, C12 is not easily dissolved in the experimental HEPES buffer solution; therefore, labrasol was used to increase the solubility of C12 in this test medium. Labrasol is a surfactant that contains saturated polyglycolyzed C6-C14 glycerides, and its NMR characterization indicated that it is a mixture consisting of 30% mono-, di- and triglycerides of C8 and C10 fatty acids, 50% of mono- and diesters of poly (ethylene glycol) (PEG) and 20% of free PEG 400[28]. It shows high tolerance and low toxicity, and has a LD50 of 22 g/kg in rats. Labrasol has been included as a pharmaceutical excipient in European Pharmacopoeia in 1998. As a result, 0.4 g/L labrasol can make 2 mmol/L C12 dissolve in the HEPES buffer. Also, it was found that there was no influence on the permeability of rebamipide at this low concentration, though increased transport of rebamipide with 1 g/L or 2 g/L of labrasol across the colon membrane was found and also showed concentration-dependent effect of labrasol on the permeability of rebamipide.
As the action and some mechanisms of rebamipide on treating colitis in animals and in human beings have been demonstrated, we investigated the colon specific delivery of rebamipide using chitosan capsule. Chitosan is a high molecular weight cationic polysaccharide, which can be prepared by alkaline N-deacetylation of chitin, the second most abundant natural polymer[29]. It has been known that chitosan possesses many advantages including low toxicity, with an oral LD50 in mice of > 16 g/kg, moderate immunostimulating effect, and inert and biodegradable characteristics. In addition, chitosan has been widely applied as a potential formulation excipient in conventional pharmaceutical devices. This polymer also has been investigated as a potential adjuvant for orally controlled release systems[30] and colon targeting[4-7]. In addition, it was reported that chitosan had mucoadhesive properties, which probably was mediated through ionic interactions with negative charges in mucus or on cell surfaces. In this study, the chitosan material with average molecular weight of 43 000 and average deacetyl degree of 83% was chosen to prepare the capsules. As results, we also have demonstrated that this kind of chitosan capsule coated with HPMCP could be a useful carrier to the colon specific delivery of rebamipide, as has been shown in that the DDI of rebamipide chitosan capsule was calculated to be 8.3 or 5.4, compared with CMC solution group or gelatin capsule group, respectively. Based on our findings, we proposed mechanisms for the colon-specific delivery of rebamipide using chitosan capsule. In the case of rebamipide CMC solution, rebamipide has been absorbed from the small intestine region. Additionally, rebamipide in the gelatin capsule also is absorbed from the small intestinal region, though HPMCP coating the surface of gelatin capsule can protect this kind of capsule from the attack of strongly acidic surroundings in the stomach. However, both HPMCP and gelatin material are easily dissolved in the small medium. Therefore, rebamipide is released and absorbed from the small region when HPMCP-coated gelatin capsule reaches this region. On the other hand, when rebamipide was orally administered using HPMCP-coated chitosan capsules, HPMCP first protected the dissolution of chitosan capsule in stomach. Then, the HPMCP is rapidly dissolved and there is little influence of the intestinal medium on the chitosan capsule when HPMCP-coated chitosan capsule enters into the small region. After that, this dosage form reaches the cecum and then the colon. The capsule was disintegrated by microorganisms richly distributed in these regions, and hence rebamipide was released and exerted its local action on colitis. Alternatively, it was reported recently that the pH may actually fall in the colon, when compared with the pH of the small intestine. This low pH, which is caused by acidification of the colonic contents by the products of bacterial fermentation, may be also related to the degradation of chitosan capsule in rat cecal contents and in colon, since chitosan is unstable and easily degraded under acidic conditions. For one thing, rebamipide has weak acidity and can be dissolved in basic solution. Therefore, it can be dissolved in the colonic medium due to the basic property of this medium, which makes rebamipide much easier to distribute into the colon tissue and exert its local action.
Based on these mechanisms, it is also necessary to make the model drug released from the chitosan capsule easily dissolve in the colon medium by some means to insure that a large amount of drug is distributed in the tissue or absorbed into the circulation system, if we carry out the colon-specific delivery of some other model drug using chitosan capsule in the future. Since there were many experiments done to demonstrate the possibility of chitosan capsule as a specific colon delivery carrier in animals, we should pay close attention to the large scale production of drug-filled chitosan capsules and its prospects in clinical practice from now on. Further investigation should involve: (1) the difference of colon-specific delivery from other chitosan capsules with different molecular weights and degrees of deacetylation; (2) besides HPMCP, the possibility of some other enteric compounds as the coating material and the effect of the coating thickness on colon specific delivery of drugs; (3) the toxicity of cyanoacrylate adhesive used to seal the body and cap in the preparation of drug-filled chitosan capsules; (4) the evaluation of the advantages and disadvantages of chitosan capsules, compared with other colon specific delivery carriers.
Moreover, it is an important method to co-fill with some absorption enhancer in chitosan capsule to increase the drug absorption when systemic action is expected, while it is doubtful that some absorption enhancer is co-administered if the goal is to exert drug’s local treatment and reduce the systemic side-effects. In our experiment, rebamipide content in the large intestine increasing notably after the oral administration of rebamipide with C12, which may indicate that absorption enhancer can enhance effect of therapeutic on colitis. On the other hand, DDI got raised, but absolutely speaking, the increasing of plasma concentration may produce adverse effects. Therefore, cosidering such factors, we should choose a reasonable dosage of C12, which can improve the distribution in colon of rebamipide and control the level of blood concentration; thus, rebamipide with chitosan capsule and C12 produces the best treatment outcome. In future, we will study the effects on colon absorption of rebamipide with various kinds of absorption enhancers, and the safety and toxicity of combinations of absorption enhancer and chitosan capsule.
In addition, in the case of ulcerative colitis, it is reported[31,32] that rebamipide enema treatment is useful; however, the treatment is mainly effective in local inflammation of rectum and decending colon due to the limitation of enema technique. Using chitosan capsule, rebamipide could be first distributed in the ascending colon, and then in the transverse colon and other colon. Therefore, rebamipide chitosan capsule may be more helpful in treating the extensive colitis or pancolitis.
In conclusion, we demonstrated a regional difference in the in vitro permeability of rebamipide across the small intestine and the colon in rats. Also, absorption enhancers such as C12 and labrasol can increase the permeability of rebamipide across the colon tissue significantly. We demonstrated that rebamipide can be specifically delivered to the colon using HPMCP-coated chitosan capsule. Meanwhile, we also confirm that rebamipide chitosan capsule, when added with C12, is more useful in treating colitis.
Rebamipide has been used to treat patients with proctitis using enema, but this treatment is inconvenient and circumscribed. It has been demonstrated previously that chitosan capsules could act as useful carriers for colon-specific delivery of peptide and anti-inflammatory drugs. However, the effects of absorption enhancement of the distribution on colon and of chitosan capsule on the colon-specific delivery of rebamipide ought to be established.
It is very important to improve therapeutic effects of rebamipide on colitis and decrease its side-effects, so it is suggested to find some absorption enhancers to augment permeability of rebamipide across the colonic tissues, and to localize specifically the drug in the large intestine by colon-specific delivery.
Rebamipide chitosan capsule is more convenient and helpful than enema in treating colitis, especially extensive colitis or pancolitis.
Since there were many experiments done that have demonstrated the possibility of chitosan capsule as a specific colon delivery carrier in animal, the large scale production of drug-filled chitosan capsules and its prospects in clinical practice from now on should be paid much attention. Rebamipide chitosan capsule, added with absorption enhancer, can be using in treating colitis in future.
The apparent permeability coefficient (Papp) is the apparent parameter of permeability (cm/s), was calculated by the equation Papp = dC/dT × (1/A·C0), where dC/dT is the slope of the linear portion of the permeation curves, A is the diffusion area,and C0 is the initial concentration of rebamipide in the donor side.
The authors explained rebamipide delivery using chitosan capsule. The study seems to be interesting and promising.
Peer reviewer: Atsushi Nakajima, Professor, Yokohama City University Hospital, 3-9 Fukuura Kanazawaku, Yokohama 236, Japan
S- Editor Li DL L- Editor Li M E- Editor Lin YP
| 1. | Yamasaki K, Kanbe T, Chijiwa T, Ishiyama H, Morita S. Gastric mucosal protection by OPC-12759, a novel antiulcer compound, in the rat. Eur J Pharmacol. 1987;142:23-29. |
| 2. | Liu YJ, Mei ZC. Progress of Rebamipide in Pharmacological Mechanism and Clinical Applications. Yaoxue Zhuanlun. 2005;14:24-26. |
| 3. | Niwa Y, Nakamura M, Ohmiya N, Maeda O, Ando T, Itoh A, Hirooka Y, Goto H. Efficacy of rebamipide for diclofenac-induced small-intestinal mucosal injuries in healthy subjects: a prospective, randomized, double-blinded, placebo-controlled, cross-over study. J Gastroenterol. 2008;43:270-276. |
| 4. | Kishimoto S, Haruma K, Tari A, Sakurai K, Nakano M, Nakagawa Y. Rebamipide, an antiulcer drug, prevents DSS-induced colitis formation in rats. Dig Dis Sci. 2000;45:1608-1616. |
| 5. | Makiyama K, Takeshima F, Kawasaki H, Zea-Iriarte WL. Anti-inflammatory effect of rebamipide enema on proctitis type ulcerative colitis: a novel therapeutic alternative. Am J Gastroenterol. 2000;95:1838-1839. |
| 6. | Tozaki H, Komoike J, Tada C, Maruyama T, Terabe A, Suzuki T, Yamamoto A, Muranishi S. Chitosan capsules for colon-specific drug delivery: improvement of insulin absorption from the rat colon. J Pharm Sci. 1997;86:1016-1021. |
| 7. | Tozaki H, Fujita T, Odoriba T, Terabe A, Suzuki T, Tanaka C, Okabe S, Muranishi S, Yamamoto A. Colon-specific delivery of R68070, a new thromboxane synthase inhibitor, using chitosan capsules: therapeutic effects against 2,4,6-trinitrobenzene sulfonic acid-induced ulcerative colitis in rats. Life Sci. 1999;64:1155-1162. |
| 8. | Tozaki H, Fujita T, Odoriba T, Terabe A, Okabe S, Muranishi S, Yamamoto A. Validation of a pharmacokinetic model of colon-specific drug delivery and the therapeutic effects of chitosan capsules containing 5-aminosalicylic acid on 2,4,6-trinitrobenzenesulphonic acid-induced colitis in rats. J Pharm Pharmacol. 1999;51:1107-1112. |
| 9. | Tozaki H, Odoriba T, Okada N, Fujita T, Terabe A, Suzuki T, Okabe S, Muranishi S, Yamamoto A. Chitosan capsules for colon-specific drug delivery: enhanced localization of 5-aminosalicylic acid in the large intestine accelerates healing of TNBS-induced colitis in rats. J Control Release. 2002;82:51-61. |
| 10. | Yodoya E, Uemura K, Tenma T, Fujita T, Murakami M, Yamamoto A, Muranishi S. Enhanced permeability of tetragastrin across the rat intestinal membrane and its reduced degradation by acylation with various fatty acids. J Pharmacol Exp Ther. 1994;271:1509-1513. |
| 11. | Wallon C, Braaf Y, Wolving M, Olaison G, Soderholm JD. Endoscopic biopsies in Ussing chambers evaluated for studies of macromolecular permeability in the human colon. Scand J Gastroenterol. 2005;40:586-595. |
| 12. | Wang YR, He Y. The application of Caco-2 cell model in the research of natural drug absorption. Zhongguo Shenghua Yaowu Zazhi. 2007;28:66-69. |
| 13. | Sadeghi AM, Dorkoosh FA, Avadi MR, Weinhold M, Bayat A, Delie F, Gurny R, Larijani B, Rafiee-Tehrani M, Junginger HE. Permeation enhancer effect of chitosan and chitosan derivatives: Comparison of formulations as soluble polymers and nanoparticulate systems on insulin absorption in Caco-2 cells. Eur J Pharm Biopharm. 2008;70:270-278. |
| 14. | Bollmann A, Lewis K, Epstein SS. Incubation of environmental samples in a diffusion chamber increases the diversity of recovered isolates. Appl Environ Microbiol. 2007;73:6386-6390. |
| 15. | Yang H, Finaly R, Teitelbaum DH. Alteration in epithelial permeability and ion transport in a mouse model of total parenteral nutrition. Crit Care Med. 2003;31:1118-1125. |
| 16. | Li GF, Chen JH, Yang J, Guo D, Ren F, Wang CX, Hou LB. [Using chamber technique for studying the permeability of dexamethasone sodium phosphate liposome through rabbit colon mucosa in vitro]. Di Yi Jun Yi Da Xue Xue Bao. 2004;24:11-14. |
| 17. | Watanabe E, Takahashi M, Hayashi M. A possibility to predict the absorbability of poorly water-soluble drugs in humans based on rat intestinal permeability assessed by an in vitro chamber method. Eur J Pharm Biopharm. 2004;58:659-665. |
| 18. | Amidon GL, Lennernas H, Shah VP, Crison JR. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res. 1995;12:413-420. |
| 19. | Martinez MN, Amidon GL. A mechanistic approach to understanding the factors affecting drug absorption: a review of fundamentals. J Clin Pharmacol. 2002;42:620-643. |
| 20. | Lin JH. Drug-drug interaction mediated by inhibition and induction of P-glycoprotein. Adv Drug Deliv Rev. 2003;55:53-81. |
| 21. | Miyake M, Oka Y, Minami T, Toguchi H, Odomi M, Ogawara K, Higaki K, Kimura T. Combinatorial use of sodium laurate with taurine or L-glutamine enhances colonic absorption of rebamipide, poorly absorbable antiulcer drug, without any serious histopathological mucosal damages. J Pharm Sci. 2003;92:911-921. |
| 22. | Walle UK, Walle T. Taxol transport by human intestinal epithelial Caco-2 cells. Drug Metab Dispos. 1998;26:343-346. |
| 23. | Gao Y, He L, Katsumi H, Sakane T, Fujita T, Yamamoto A. Improvement of intestinal absorption of insulin and water-soluble macromolecular compounds by chitosan oligomers in rats. Int J Pharm. 2008;359:70-78. |
| 24. | Morimoto K, Fukushi N, Chono S, Seki T, Tabata Y. Spermined dextran, a cationized polymer, as absorption enhancer for pulmonary application of peptide drugs. Pharmazie. 2008;63:180-184. |
| 25. | Jeong JM, Choi CH. Enhancement of paclitaxel transport and cytotoxicity by 7,3',4'-trimethoxyflavone, a P-glycoprotein inhibitor. J Pharm Pharm Sci. 2007;10:547-553. |
| 26. | Lindmark T, Kimura Y, Artursson P. Absorption enhancement through intracellular regulation of tight junction permeability by medium chain fatty acids in Caco-2 cells. J Pharmacol Exp Ther. 1998;284:362-369. |
| 27. | Motohiro T, Aramaki M, Tanaka K, Koga T, Shimada Y, Tomita N, Sakata Y, Fujimoto T, Nishiyama T, Kuda N. [Fundamental study on ceftizoxime suppositories in adults and children]. Jpn J Antibiot. 1985;38:3013-3056. |
| 28. | Kreilgaard M, Pedersen EJ, Jaroszewski JW. NMR characterisation and transdermal drug delivery potential of microemulsion systems. J Control Release. 2000;69:421-433. |
| 29. | Singla AK, Chawla M. Chitosan: some pharmaceutical and biological aspects--an update. J Pharm Pharmacol. 2001;53:1047-1067. |
| 30. | Aksungur P, Sungur A, Unal S, Iskit AB, Squier CA, Senel S. Chitosan delivery systems for the treatment of oral mucositis: in vitro and in vivo studies. J Control Release. 2004;98:269-279. |
| 31. | Miyata M, Kasugai K, Ishikawa T, Kakumu S, Onishi M, Mori T. Rebamipide enemas-new effective treatment for patients with corticosteroid dependent or resistant ulcerative colitis. Dig Dis Sci. 2005;50 Suppl 1:S119-S123. |
| 32. | Furuta R, Ando T, Watanabe O, Maeda O, Ishiguro K, Ina K, Kusugami K, Goto H. Rebamipide enema therapy as a treatment for patients with active distal ulcerative colitis. J Gastroenterol Hepatol. 2007;22:261-267. |