Published online Aug 21, 2008. doi: 10.3748/wjg.14.4873
Revised: June 10, 2008
Accepted: June 17, 2008
Published online: August 21, 2008
AIM: To investigate the low intensity ultrasound (US)-induced apoptosis in human gastric carcinoma cells and its potential mechanism and to suggest a new therapeutic approach to gastric carcinoma.
METHODS: Human SGC-7901 gastric carcinoma cells were cultured in vitro and irradiated by low intensity US for 10 min at different intensities with different incubation times after irradiation. Morphologic changes were examined under microscope with trypan blue staining and then the percentage of early apoptotic cells was detected by flow cytometry (FCM) with double staining of fluorescein isothiocyanate (FITC)-Annexin V/propidium iodide (PI). Two-dimensional electrophoresis (2DE) was used to get the protein profile and some proteins differently expressed after US irradiation were identified by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS). Functional analysis was performed to investigate the mechanism of US-induced cell apoptosis.
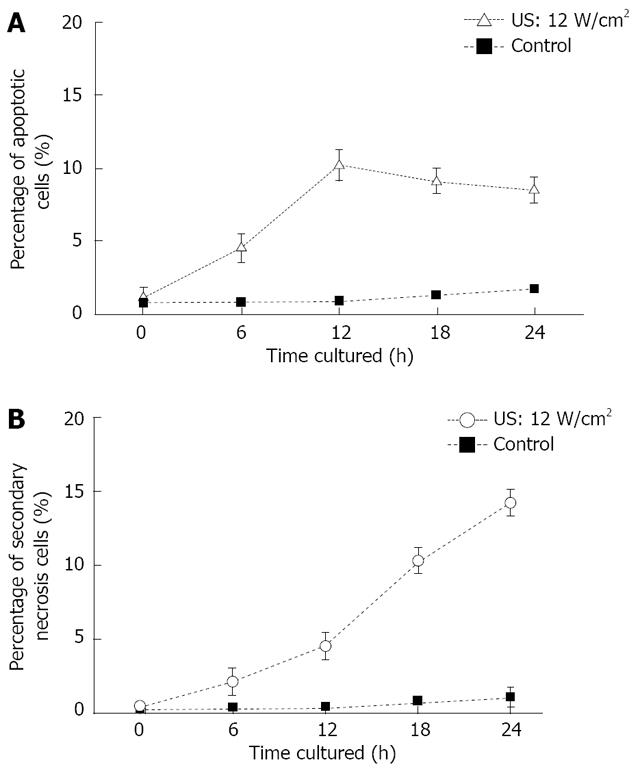
RESULTS: The percentage of apoptotic cells increased about 10% after US irradiation (12.0 W/cm2, 12 h culture). The percentage of early apoptosis and secondary necrosis in the US-irradiated cells increased with the increased US intensity. Moreover, apoptotic cells increased with the increased culture time after US irradiation and reached its maximum at about 12 h. Several new proteins appeared after US irradiation and were up or down regulated more than 2 times. Some heat shock proteins (HSPs) were found to be associated with the signal process simulating the apoptosis of cells.
CONCLUSION: Low intensity US could induce apoptosis in human gastric carcinoma cells. US-induced apoptosis is related to US intensity/culture time. US-induced apoptosis may be caspases-dependent and endoplasmic reticulum (ER) stress-triggered apoptosis may also contribute to it. Proteomic experimental system is useful in finding the protein alteration in carcinoma cells after US irradiation, helping to develop a new cancer therapy.
- Citation: Feng Y, Tian ZM, Wan MX, Zheng ZB. Low intensity ultrasound-induced apoptosis in human gastric carcinoma cells. World J Gastroenterol 2008; 14(31): 4873-4879
- URL: https://www.wjgnet.com/1007-9327/full/v14/i31/4873.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.4873
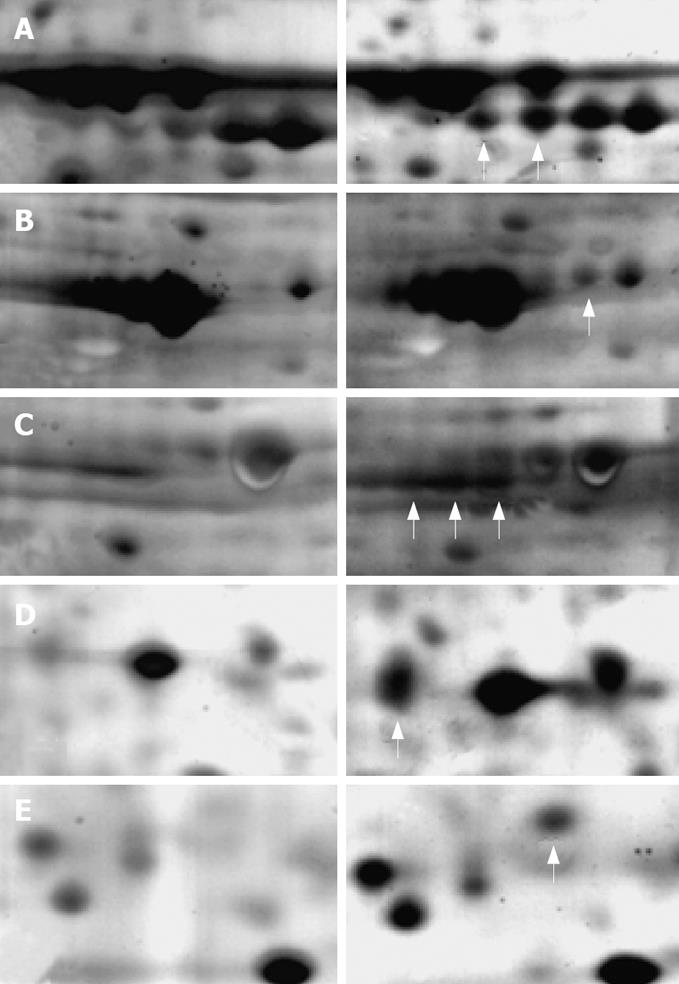
| Swiss-Prot No. | Protein name | Theo. pI/Mw | Exp. pI/Mw | Protein function |
| P11021 | GRP78, Bip | 5.2/71.0 | 5.1/74.4 | ER-stress associated |
| P38646 | HSP70 (Heat shock 70 kDa protein) | 5.9/73.7 | 5.8/72.1 | Activation of pre-caspase 3 |
| P10809 | CH60 (60 kDa HSP) | 5.7/61.2 | 5.5/58.9 | Tumor suppressor and pro-apoptotic protein |
| P27797 | Calreticulin precursor | 4.3/48.2 | 4.5/52.8 | ER-stress triggered apoptosis |
| P35232 | Prohibitin | 5.6/29.9 | 5.4/31.3 | Inhibit the tumor cell growth |
Gastric carcinoma is the second commonest cause of cancer-related deaths worldwide. Although surgical removal of the stomach is the only curative treatment in clinical practice, more studies revealed that chemotherapy and radiation therapy given after surgery could improve the chance of a cure for many patients. To improve the survival rate, new therapeutic methods should be developed.
In non-surgical cancer treatment, induction of apoptosis is a preferred mode of killing cancer cells[1,2]. Several investigators have reported that US irradiation could induce apoptosis in human leukemia cell lines[3-7], including K562, HL-60, Nalm-6 and U937. It was also reported that apoptosis of some solid carcinoma cells, such as human ovarian carcinomas cells[8], SMMC-7721 cells (data not shown) and Walker 256 carcinosarcoma cells[9], are triggered by US irradiation. Different from high intensity focused ultrasound (HIFU) which can thermally ablate tissues via hyperthermia in different carcinomas[10-12], low intensity ultrasound (US) defined as therapeutic US, with a relatively lower intensity than HIFU, has a great potential in apoptosis therapy for cancer and can be relatively easily applied[8,13]. So it is suggested that low intensity US-induced apoptosis in tumor cells could probably offer a new approach to elimination of cancer[4]. To our knowledge, US-induced apoptosis in gastric carcinoma has not yet been reported. Compared with human leukemia cell lines, low intensity US-induced apoptosis in solid carcinoma cells or tissues is still in the process of investigation. The mechanism has also not yet been elucidated.
The aim of this study was to prove low-intensity US-induced apoptosis in human SGC-7901 gastric carcinoma cells and investigate the effects of US intensity and culture time after US irradiation on US-induced apoptosis. Comparative proteomic analysis was performed to examine the alteration in protein profile of gastric carcinoma cells induced by US irradiation. The potential molecular mechanism was suggested via the protein functional analysis.
Fluorescein isothiocyanate (FITC)-Annexin V/propidium iodide (PI) kit was purchased from Jingmei Biotechnology Company, China. Immobilized pH gradient (IPG) strips (pH3-10, nonlinear, 13 cm) and IPG buffer (pH3-10, nonlinear) were purchased from Pharmacia, Amersham Biotech. Dithiothreitol (DTT), PMSF and CHAPS were purchased from Sigma Company (USA). All the buffers were made using high purity MilliQ water. Elite ESP flow cytometer was purchased from Coulter Electronics Hialeah, FL. IPGphor isoelectric focusing equipment, Hoefer SE 600 vertical chambers, electrophoresis apparatus, Image Master 2D Elite 4.01 software were purchased from Pharmacia, Amersham Biotech. Voyager-DE MALDI-TOF MS was a product from Applied Biosystems (USA).
SGC-7901 human gastric carcinoma cells (Center of Laboratory Animals of the Forth Military Medical University) were cultured in RPMI 1640 culture medium in a water-saturated atmosphere with 50 mL/L CO2 at 37°C.
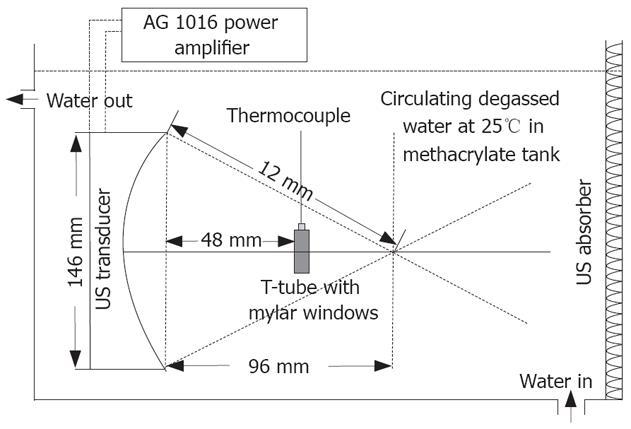
The US irradiation system is shown in Figure 1. It consists of a AWG 2021 arbitrary waveform generator (Sony Tektronix Co., Tokyo, Japan), a AG 1016 power amplifier (T&C Power conversion, Inc., Rochester, NY, USA) and a transducer designed as a semispherical ring with an aperture 146 mm and a radius of curvature 120 mm (Imasonic, Besancon, France), a 750 mm × 750 mm × 500 mm polymethyl methacrylate tank filled with degassed water with 10-cm high water above the transducer. The central frequency is 1.2 MHz. Temperature of water in the tank was controlled at 25°C by circulating. Cell samples were carried in a specially designed inverted “T” tube as previously described[6,9].
The cell samples were divided into ten groups and each treatment was given in three replicates. The first group was used as the control that imitated the whole process with no irradiation. The 2-5 groups were irradiated for 10 min at the intensity of 3.0 W/cm2, 6.0 W/cm2, 9.0 W/cm2 and 12.0 W/cm2 (ISPTA), respectively, and then incubated at 37°C in a humidified air containing 50 mL/L CO2. Apoptosis detection and microscopy observation were performed in each sample. Remained cells were washed 3 times with cold PBS, centrifuged at 1500 ×g for 10 min to get cell pellets, then frozen quickly in liquid nitrogen and stored in a -80°C ultra low temperature freezer (LegaciTM Refregeration system, REVCO Technologies) for subsequent 2D PAGE analysis. The whole process was performed in sterile condition.
To investigate the relationship between culture time after US irradiation and cell apoptosis, the 6-10 groups were irradiated at the intensity of 12.0 W/cm2 and incubated at 37°C in a humidified air containing 50 mL/L CO2 for 0, 6, 12, 18 and 24 h, respectively. The following morphologic observation and apoptosis detection were performed as described above.
The morphologic changes in apoptotic and necrosis cells could be examined under microscope and detected by analysis of a light scatter signal by FCM[14]. SGC-7901 cells were stained with trypan blue and then observed under microscope (Olympus BX40) to detect their structure change after US irradiation. Furthermore, the cells were analyzed with FCM to detect phosphatidylserine (PS) exposure on the cell membrane as a typical marker of early apoptosis.
Proteins were extracted from control and irradiated cells, respectively, with the freeze-thaw lysis method. Then, the protein solution (containing 200 μg proteins) was loaded to perform isoelectric focusing and second dimension separation as previously described[15]. Gels were silver stained.
2D gel images of control and irradiated cells were acquired using a Sharp JX-330 scanner, compared and matched via Image Master 2D Elite software. New protein spots and proteins expressing 2 times higher or lower were selected for the following identification based on MALDI-TOF-MS.
The selected protein spots were excised from the gel and in-gel digestion with trypsin was performed. The extracted enzymolyzed peptides were identified by peptide mass fingerprinting based on MALDI-TOF-MS (Voyager-DETM), analyzed using data explore, and matched in ProFound-peptide mapping and SWISS-PROT.
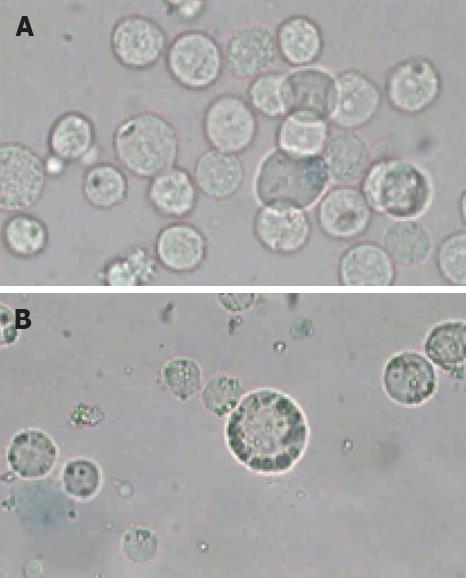
The morphologic observations showed that the distinct morphologic characteristics of apoptotic cells, such as condensation of nucleus, chromatin margination (Figure 2B) were found after the cells were irradiated at 12.0 W/cm2 for 10 min and incubated for 12 h after irradiation. Simultaneously, the characteristics of apoptotic cells were not observed in the control samples incubated at 37°C in a humidified air containing 50 mL/L CO2 (Figure 2A). However, the morphologic observation could not unambiguously give the quantity information about the proportion of cells in different physiological states, such as viable cells, apoptotic cells and secondary necrotic cells. So a further analysis was performed for the detection of functional characteristics such as exclusion of PI or plasma membrane integrity or such dyes as Annexin V-FITC.
FCM with double staining of FITC-Annexin V/PI was performed to detect PS expression on the cell membrane as an end point of early apoptosis[16], showing the exact percentage of cells in different styles, including viable cells (Annexin V-/PI-), early apoptotic cells (Annexin V+/PI-), and secondary necrosis (Annexin V+/PI+).
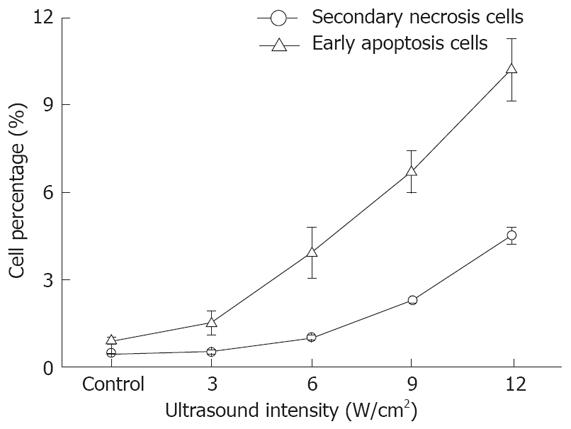
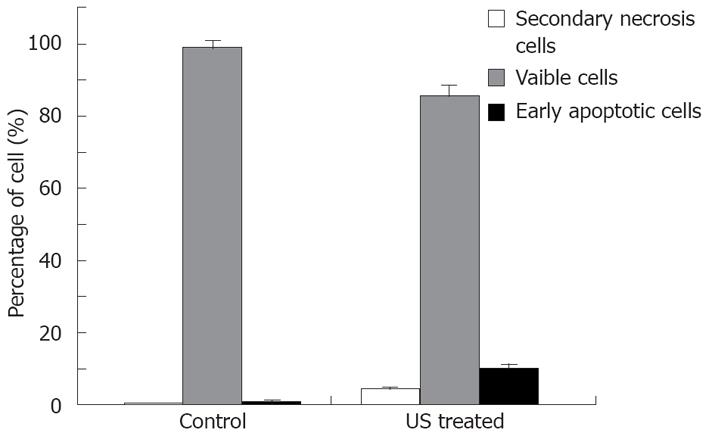
The relationship between cell apoptosis and US intensity/culture time after US irradiation was investigated. The results show that the percentage of early apoptosis and secondary necrosis in the US irradiated cells increased with the increased US intensity (Figure 3). In the mean time, the percentage of viable cells decreased after US irradiation. The percentage of early apoptosis (incubated for 12 h after irradiation at the intensity of 12.0 W/cm2 (ISPTA) for 10 min) increased more than 10% compared with the control cells cultured at 37°C in a humidified air containing 50 mL/L CO2 (Figure 4), suggesting that apoptosis of cells can be induced by US.
US induced early apoptosis (Figure 5A) and secondary necrosis (Figure 5B) as a function of culture time after US irradiation, are determined by FCM with double staining of FITC-Annexin V/PI. Human SGC-7901 gastric carcinoma cells were irradiated by US at the intensity of 12.0 W/cm2 for 10 min in this study. Apoptotic cells increased with the increased culture time after US irradiation and reached its maximum at 12 h. Then, the percentage of apoptotic cells gradually decreased. However, the secondary necrosis cells kept increasing. The percentage of viable cells kept decreasing after US irradiation.
A total of 1137 protein spots were successfully separated from the human gastric carcinoma SGC-7901 cells, 798 of which had a good match with the proteins in the 2D map of US irradiated cells. The unmatched protein spots amounted to 339. Some of the matched proteins had obviously different expressing characteristics (more than 2 times), indicating that they are obviously up-regulated or down-regulated after US irradiation. The protein expression between control cells (left) and US irradiated cells (right) is also shown in Figure 6.
Proteins were identified by peptide mass fingerprinting based on MALDI-TOF-MS. The identified information about the proteins shown in Figure 6 is summarized in Table 1. The expression of these proteins increased more than 2 times after US irradiation. However, 4 proteins were unmatched although with good spectra. Further identification of them is needed via the tandem MS/MS.
In the present study, we first demonstrated that low intensity US could induce early apoptosis and secondary necrosis in gastric carcinoma cells. US irradiation could trigger apoptosis in both carcinoma and normal cells. However, a series of investigations showed that malignant cells are much more susceptible to US exposure and more prone to being killed than normal cells[17,18]. Lagneaux et al[4] also demonstrated that carcinoma cells are sensitive to ultrasonic treatment with a high percentage of apoptotic cells observed 5 h after treatment, whereas ultrasonic treatment has no effect on normal cells isolated from leukemic patients. This characteristic of carcinoma cells gives the chance to develop US irradiation as a new therapeutic approach to cancer treatment. Furthermore, during the US irradiation, focused US oriented by targeted multi-functional US contrast agents (UCA), could aim at the targeted region, decreasing side-effects on normal tissues around it. In addition, Gene regulation of target cells may be utilized in modifying cellular response to US treatment, thus increasing the sensitivity of diseased cells while making normal cells resistant to the side effects[19]. Therefore, US-induced apoptosis, as a novel cancer therapy, is of practical importance.
Low intensity US-induced apoptosis in gastric carcinoma cells may be an early event that increased with time in this study. The culture time for apoptotic cells to reach their maximum apoptosis is 12 h. Another study on US-induced apoptosis of human hepatocarcinoma cells performed in our laboratory (data not shown) showed that the culture time is 6 h after treatment. This discrepancy may be due to the different sensitivities of carcinoma cells to apoptosis-inducing factors. Similar results have also been reported by other investigators[5,20], suggesting that the sensitivity of carcinoma cells to US irradiation is related to the time when apoptotic cells reach their maximum.
Apoptosis is always a special cellular response to stress stimuli, such as exposure to chemotherapeutic drugs, oxidative stress, free radicals, X-rays, ultraviolet radiation and shear stress. US irradiation belongs to the physical stress factor. Ashush et al[3] first reported that apoptosis is induced by high-intensity pulsed US in human leukemia cell lines, Lagneaux and Meulenaer demonstrated that low intensity US could also trigger apoptosis in human leukemia cell lines and low intensity US-induced apoptosis in tumor cells could probably offer a new approach to the elimination of cancer[4]. Feril et al[6] also pointed out that US without increasing its temperature and time can increase hyperthermia-induced apoptosis. It was reported that early apoptosis and necrosis induced by US are mainly caused by inertial cavitation[5-7]. During US cavitation, collapsing bubbles could create drastic conditions in an extremely short time, with a temperature of 2000 to 5000 K and a pressure up to 1800 atm inside it. Shear forces, jets and shock waves are also produced outside the bubbles. The inertial cavitation also induces free radical formation via the thermal dissociation of water into ·OH and ·H radicals. Any kind of these stress factors from US cavitation might cause apoptosis of tumor cells. Apoptosis may be triggered as a response to cell membrane and DNA damage induced by inertial cavitation or via the action of residual hydrogen peroxide[21,22]. It is further proved when introduction of dissolved gases or UCA, OptisonTM and YM454 to the system enhanced the US-induced cell killing in vitro[5,7]. Moreover, intracellular ROS generated from mitochondria rather than from extracellular ROS (directly produced by inertial cavitation in the medium), are involved in the regulation of apoptosis induced by US[23]. However, Lagneaux and Meulenaer claimed that US-induced apoptosis is probably related to the oxidative stress[4].
To investigate the molecular mechanism of US-induced apoptosis in carcinoma cells, comparative proteomic analysis is a novel method that was introduced in this study. Because the biologic processes are directly executed by proteins that are dynamically modified and processed at multiple levels during or after sonication, the alteration in protein profile can reflect the status of differentiation and physiological conditions of cells. Protein functional analysis can also help reveal the molecular signal processes triggered by US irradiation in tumor cells or tissues.
Apoptosis is well-known to require the activation of caspases, a group of enzymes involved in apoptotic cascade events[24]. Apoptotic stimulus can activate apoptosis-related proteins to enter mitochondria, then induce mitochondria membrane to form pores to release molecules into cytosol, such as cytochrome C (CytC). The released molecules can activate caspase-9, which cleaves procaspase-3 to caspase-3, finally inducing apoptosis[25,26]. The activity of different caspases was not detected in this study, but some proteins, such as CH60, related to the activation of caspases were up-regulated in the cells after sonication. CH60 is regarded as a protective protein induced by the response process of mammalian cells under stress, which promotes apoptosis by helping maturation of precaspase-3[27,28]. Precaspase-3 is the key enzyme involved in the caspases-dependent apoptotic process, indicating that the US-induced apoptosis may be caspases-dependent. It is known that members of the heat shock protein (HSP) family function at multiple points in the apoptotic signal pathway. Sometimes, due to their cytoprotective role, they inhibit the apoptotic response by inhibiting the key steps in the apoptotic process. However, in other times, they serve as chaperones of a key signaling protein or directly promote apoptosis. Vykhodtseva reported that focused US sonication close to the thermal threshold exposures could induce apoptosis, suggesting that the mechanism leading to apoptosis is the production of HSPs[29]. In this study, CH60, HSP70 and Bip were found belonging to the HSP family. They participate in the apoptosis induced via different ways. Bip and calreticulin participate in the press-induced reactions on the endoplasmic reticulum (ER), both of them are pivotal molecules mediating ER-initiated apoptosis involved in ER stress. Prohibitin is an antiproliferative protein and functions as a tumor suppressor. Commonly, it is involved in blocking DNA synthesis and negative regulation of cell proliferation. In US-induced apoptosis of tumor cells, the increased expression of prohibitin would help inhibit the growth of tumor cells.
In summary, low intensity US can induce apoptosis in human gastric carcinoma cells. The percentage of early apoptosis and secondary necrosis in the US-irradiated cells are increased with increased US intensity; Moreover, apoptotic cells increase with increased culture time after US irradiation and reach their maximum at about 12 h. Group of HSPs participates in the US-induced apoptosis. US-induced apoptosis is caspases-dependent, and ER stress-triggered apoptosis may also contribute to it. Moreover, experimental system can also be introduced into the research of bio-effects caused by other physical and chemical factors on tumor cells, which would be significant in investigating tumor cell apoptotic mechanism and cancer therapy.
Low intensity ultrasound (US) irradiation could trigger apoptosis in carcinoma cells, and therefore has distinct potential as a technique for cancer therapy. To investigate US-induced apoptosis in different carcinoma cells is significant to better understand its mechanism and develop a new therapeutic approach.
The present study demonstrated low intensity US-induced apoptosis in gastric carcinoma cells. Based on protein functional analysis, the molecular mechanism of US-induced apoptosis is suggested.
Some previous studies have proved that US can induce apoptosis in human leukemia cells and solid carcinoma cells. The present study first demonstrated that low intensity US could induce early apoptosis and secondary necrosis in gastric carcinoma cells. The effects of US intensity and culture time after treatment on US-induced apoptosis are revealed. The potential molecular mechanism of US-induced apoptosis is suggested via comparative proteomic analysis.
The present study would help to make better analysis of US-induced apoptosis in gastric carcinoma cells, and promote new therapeutic schemes. Further investigation of its mechanism would supply more useful information about US- induced apoptosis in solid carcinoma cells.
The authors demonstrated that low intensity US could induce early apoptosis and secondary necrosis in gastric cell line SGC-7901. As suggested by the authors, low intensity US irradiation may help to develop new cancer therapies. Therefore, this study may provide important information about gastric cancer.
Peer reviewers: Yoshio Yamaoka, MD, PhD, Associate Professor, Department of Medicine/Gastroenterology, Baylor College of Medicine and VA Medical Center (111D), 2002 Holcombe Blvd, Houston, Texas 77030, United States; Kalpesh Jani, PhD, Sigma, 102, Abhishek House, Vadodara 390011, India; Yogesh K Chawla, PhD, Professor, Department of Hepatology, Postgraduate Institute of Medical Education and Research, Chandigarh 160012, India; Aydin Karabacakoglu, PhD, Assistant Professor, Department of Radiology, Meram Medical Faculty, Selcuk University, Konya 42080, Turkey
S- Editor Li DL L- Editor Wang XL E- Editor Yin DH
| 1. | Sun SY, Hail N Jr, Lotan R. Apoptosis as a novel target for cancer chemoprevention. J Natl Cancer Inst. 2004;96:662-672. |
| 2. | Fulda S, Debatin KM. Apoptosis pathways in neuro-blastoma therapy. Cancer Lett. 2003;197:131-135. |
| 3. | Ashush H, Rozenszajn LA, Blass M, Barda-Saad M, Azimov D, Radnay J, Zipori D, Rosenschein U. Apoptosis induction of human myeloid leukemic cells by ultrasound exposure. Cancer Res. 2000;60:1014-1020. |
| 4. | Lagneaux L, de Meulenaer EC, Delforge A, Dejeneffe M, Massy M, Moerman C, Hannecart B, Canivet Y, Lepeltier MF, Bron D. Ultrasonic low-energy treatment: a novel approach to induce apoptosis in human leukemic cells. Exp Hematol. 2002;30:1293-1301. |
| 5. | Honda H, Zhao QL, Kondo T. Effects of dissolved gases and an echo contrast agent on apoptosis induced by ultrasound and its mechanism via the mitochondria-caspase pathway. Ultrasound Med Biol. 2002;28:673-682. |
| 6. | Feril LB Jr, Kondo T, Zhao QL, Ogawa R. Enhancement of hyperthermia-induced apoptosis by non-thermal effects of ultrasound. Cancer Lett. 2002;178:63-70. |
| 7. | Feril LB Jr, Kondo T, Zhao QL, Ogawa R, Tachibana K, Kudo N, Fujimoto S, Nakamura S. Enhancement of ultrasound-induced apoptosis and cell lysis by echo-contrast agents. Ultrasound Med Biol. 2003;29:331-337. |
| 8. | Yu T, Wang Z, Mason TJ. A review of research into the uses of low level ultrasound in cancer therapy. Ultrason Sonochem. 2004;11:95-103. |
| 9. | Tian ZM, Wan MX, Lu MZ, Wang XD, Wang L. The alteration of protein profile of Walker 256 carinosarcoma cells during the apoptotic process induced by ultrasound. Ultrasound Med Biol. 2005;31:121-128. |
| 10. | Huber PE, Jenne JW, Rastert R, Simiantonakis I, Sinn HP, Strittmatter HJ, von Fournier D, Wannenmacher MF, Debus J. A new noninvasive approach in breast cancer therapy using magnetic resonance imaging-guided focused ultrasound surgery. Cancer Res. 2001;61:8441-8447. |
| 11. | Blana A, Walter B, Rogenhofer S, Wieland WF. High-intensity focused ultrasound for the treatment of localized prostate cancer: 5-year experience. Urology. 2004;63:297-300. |
| 12. | Wang XJ, Yuan SL, Lu YR, Zhang J, Liu BT, Zeng WF, He YM, Fu YR. Growth inhibition of high-intensity focused ultrasound on hepatic cancer in vivo. World J Gastroenterol. 2005;11:4317-4320. |
| 13. | Abdollahi A, Domhan S, Jenne JW, Hallaj M, Dell'Aqua G, Mueckenthaler M, Richter A, Martin H, Debus J, Ansorge W. Apoptosis signals in lymphoblasts induced by focused ultrasound. FASEB J. 2004;18:1413-1414. |
| 14. | Darzynkiewicz Z, Bruno S, Del Bino G, Gorczyca W, Hotz MA, Lassota P, Traganos F. Features of apoptotic cells measured by flow cytometry. Cytometry. 1992;13:795-808. |
| 15. | Feng Y, Tian ZM, Wan MX, Zheng ZB. Protein profile of human hepatocarcinoma cell line SMMC-7721: identification and functional analysis. World J Gastroenterol. 2007;13:2608-2614. |
| 16. | van Engeland M, Nieland LJ, Ramaekers FC, Schutte B, Reutelingsperger CP. Annexin V-affinity assay: a review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry. 1998;31:1-9. |
| 17. | Lejbkowicz F, Salzberg S. Distinct sensitivity of normal and malignant cells to ultrasound in vitro. Environ Health Perspect. 1997;105 Suppl 6:1575-1578. |
| 18. | Lejbkowicz F, Zwiran M, Salzberg S. The response of normal and malignant cells to ultrasound in vitro. Ultrasound Med Biol. 1993;19:75-82. |
| 19. | Feril LB Jr, Kondo T. Biological effects of low intensity ultrasound: the mechanism involved, and its implications on therapy and on biosafety of ultrasound. J Radiat Res (Tokyo). 2004;45:479-489. |
| 20. | Firestein F, Rozenszajn LA, Shemesh-Darvish L, Elimelech R, Radnay J, Rosenschein U. Induction of apoptosis by ultrasound application in human malignant lymphoid cells: role of mitochondria-caspase pathway activation. Ann N Y Acad Sci. 2003;1010:163-166. |
| 21. | Miller DL, Thomas RM, Frazier ME. Ultrasonic cavitation indirectly induces single strand breaks in DNA of viable cells in vitro by the action of residual hydrogen peroxide. Ultrasound Med Biol. 1991;17:729-735. |
| 22. | Juffermans LJ, Dijkmans PA, Musters RJ, Visser CA, Kamp O. Transient permeabilization of cell membranes by ultrasound-exposed microbubbles is related to formation of hydrogen peroxide. Am J Physiol Heart Circ Physiol. 2006;291:H1595-H1601. |
| 23. | Honda H, Kondo T, Zhao QL, Feril LB Jr, Kitagawa H. Role of intracellular calcium ions and reactive oxygen species in apoptosis induced by ultrasound. Ultrasound Med Biol. 2004;30:683-692. |
| 24. | Yuan J. Molecular control of life and death. Curr Opin Cell Biol. 1995;7:211-214. |
| 25. | Majno G, Joris I. Apoptosis, oncosis, and necrosis. An overview of cell death. Am J Pathol. 1995;146:3-15. |
| 26. | Wyllie AH, Kerr JF, Currie AR. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251-306. |
| 27. | Samali A, Cai J, Zhivotovsky B, Jones DP, Orrenius S. Presence of a pre-apoptotic complex of pro-caspase-3, Hsp60 and Hsp10 in the mitochondrial fraction of jurkat cells. EMBO J. 1999;18:2040-2048. |
| 28. | Xanthoudakis S, Roy S, Rasper D, Hennessey T, Aubin Y, Cassady R, Tawa P, Ruel R, Rosen A, Nicholson DW. Hsp60 accelerates the maturation of pro-caspase-3 by upstream activator proteases during apoptosis. EMBO J. 1999;18:2049-2056. |
| 29. | Vykhodtseva N, McDannold N, Hynynen K. Induction of apoptosis in vivo in the rabbit brain with focused ultrasound and Optison. Ultrasound Med Biol. 2006;32:1923-1929. |