INTRODUCTION
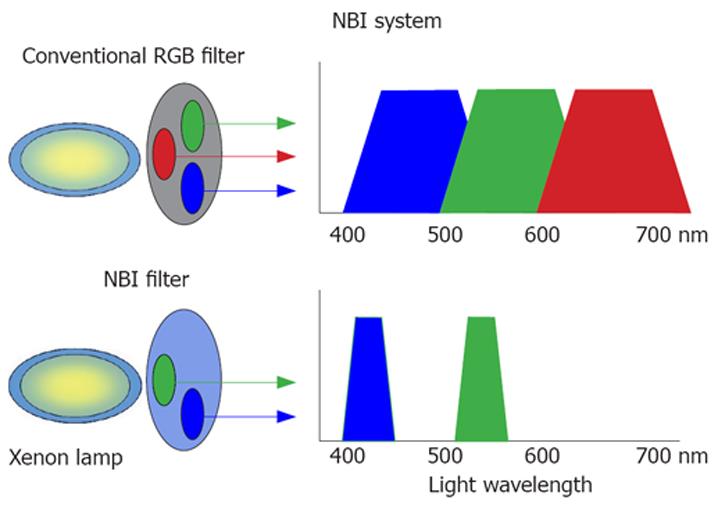
Figure 1 NBI system.
Different from the conventional RGB filter, the NBI filter consists of two narrow bands (415 ± 30 nm and 540 ± 30 nm, respectively) that make it possible to observe clearly superficial vascular patterns for clinical evaluation.
Figure 2 NBI colonoscopy image with non-sequential system.
A: Conventional view of an Is polyp, 12 mm in diameter located in the sigmoid colon; B: NBI view clearly showing the superficial meshed vascular pattern on the polyp’s surface indicating an adenomatous polyp.
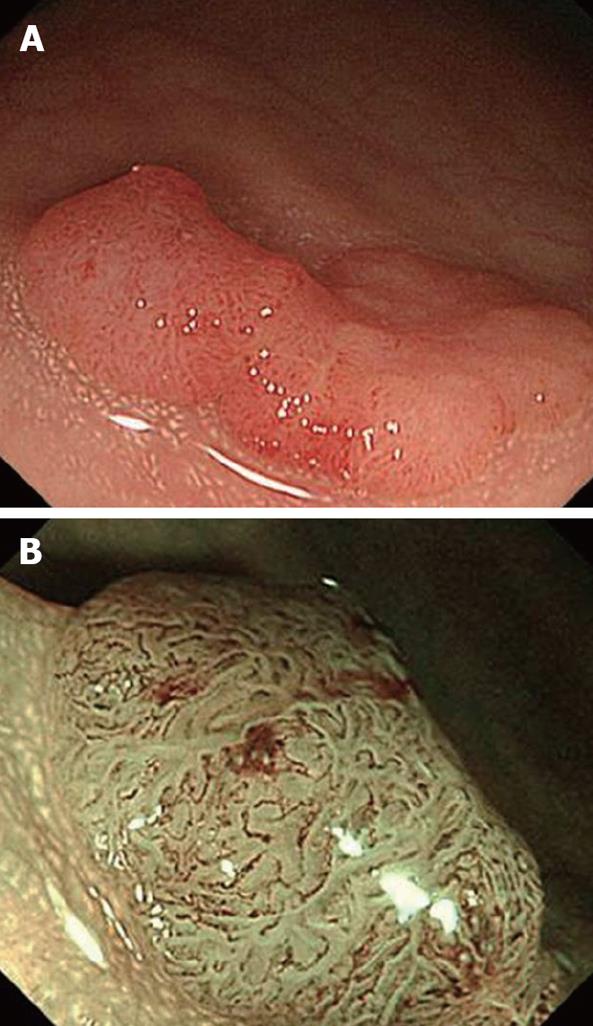
Figure 3 NBI colonoscopy image with sequential system.
A: Conventional view of an IIa polyp, 20 mm in diameter located in the rectum; B: Meshed capillary vessels are clearly seen using magnifying NBI as dark brown areas diagnosing an intramucosal cancer.
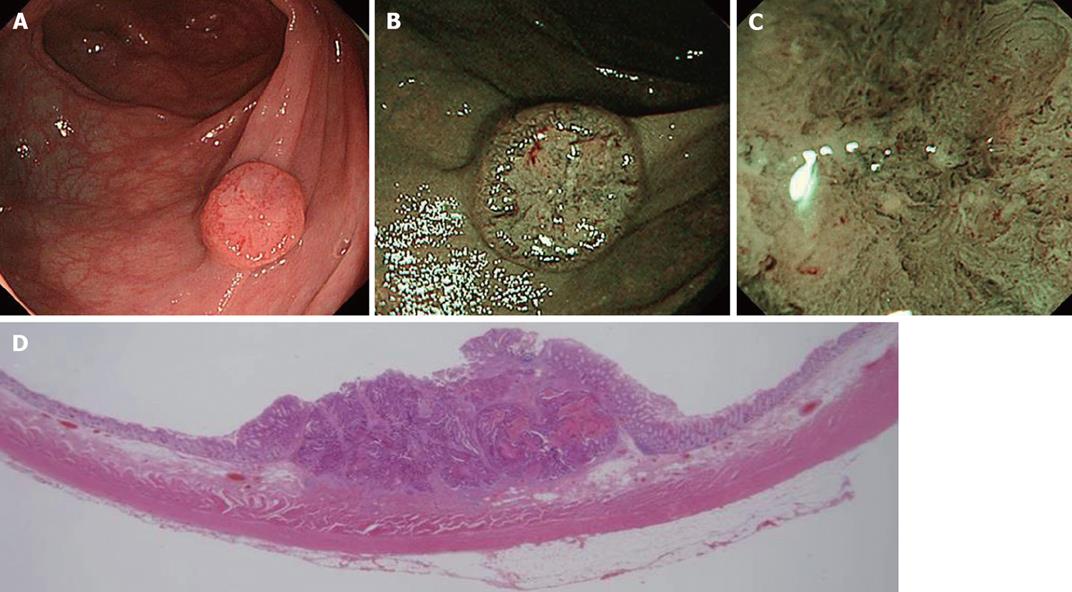
Figure 4 NBI image of colorectal cancer.
A: Conventional view of an IIa + IIc lesion, 12 mm in size, located in the transverse colon; B: NBI view shows a well demarcated area and meshed capillary vessels clearly visible characterized by thick diameter, branching and curtail irregularity; C: Magnifying NBI view additionally shows the presence of a nearly avascular or loose microvascular area due to histological desmoplastic changes in the stromal tissue, suggesting deep submucosal invasion; D: Histopathological analysis revealed an adenocarcinoma invading deeply into the submucosa (2500 μm) with lymphovascular invasion.
In 1971, Folkman proposed that all tumor growth was angiogenesis-dependent. This was the foundation for the development of angiogenic research and helped to stimulate investigation that is now being pursued by scientists in many different fields worldwide[1]. New blood vessel creation favors a transition from hyperplasia to neoplasia (i.e., the passage from a state of cellular multiplication to a state of uncontrolled proliferation characteristic of tumor cells)[2].
An in vivo means for visualizing angiogenesis or microvessel morphological changes in superficial neoplasms would constitute a promising method for the diagnosis of early gastrointestinal tumors. Narrow-band imaging (NBI) is an innovative optical technology developed in Japan that modifies the center wavelength and bandwidth of an endoscope’s light into a narrow-band illumination of 415 ± 30 nm. By utilizing this narrow spectrum, contrast in the capillary pattern of the superficial layer is markedly improved[3], thereby facilitating clearer visualization of vascular structures during gastrointestinal endoscopy[4].
The first clinical study of the NBI system for the diagnosis of gastrointestinal tumors was reported by Sano et al[5] in 2001. Their promising observations resulted in the first pilot colorectal study in which the NBI system demonstrated better vascular pattern visualization than conventional colonoscopy in the diagnosis of colorectal polyps[6]. These early studies opened the way for subsequently using NBI in the diagnosis of pre-malignant and malignant lesions of the hypo-pharynx, esophagus and stomach[4,7,8].
This review focuses on the current advantages and limitations of using the NBI system in the diagnosis of colorectal lesions.
SCIENTIFIC BASIS FOR NBI
Video endoscopes use white light from a xenon source for illumination. In order to understand the reflectance spectrum of any tissue, both the scattering process and absorption must be taken into account. Based on the Monte Carlo simulation, several investigations into the mechanism of scattering from tissue structures have determined that the penetration depth of the light depends on the wavelength. The depth of penetration into the gastrointestinal tract mucosa is superficial for the blue band, intermediate for the green band and deep for the red band (penetration depth range: 0.15 to 0.30 mm). As a result, NBI systems use optical filters for green and blue sequential illumination and narrow the bandwidth of spectral transmittance[9,10] (Figure 1).
The scientific basis for the NBI system is that light with a short wavelength falls within the hemoglobin absorption band, so that blood vessels may be more clearly seen due to sufficient contrast[6].
IMAGE RECONSTRUCTION FROM REFLECTED LIGHT
Two different types of NBI systems are used to reconstruct images from the reflected light. The non-sequential system (Exera II), also referred to as the “color chip system”, uses a color charge coupled device (CCD) in which pixels are selectively assigned to specific wavelength ranges. The CCD captures the full range of the white light and transfers it in a single step to the processor in order to reconstruct natural color on the video monitor (Figure 2).
In contrast, the sequential system (Lucera Spectrum) uses a monochrome CCD in which pixels are not selectively attributed to specific colors, but transferred sequentially in the RGB bands to the processor. A rotating RGB interference filter is interposed after the white light source and the mucosa is illu-minated alternately in each of the three RGB bands[11] (Figure 3).
Although the concept and basic design is the same for both the NBI sequential and non-sequential systems, a difference in color images exists due to differences in the color spectral characteristics of the RGB rotary filters used in the Lucera Spectrum and the color CCD used in the Exera II. There is considerable potential for further development, however, by improving NBI technology in the non-sequential endoscopic video system.
ARE YIELDS OF SMALL AND FLAT ADENOMAS HIGHER WITH NBI?
An interesting Japanese study involving 48 patients in which conventional white light colonoscopy was first performed followed later by blind NBI colonoscopy on the same patients found that the total number of neoplastic lesions detected by NBI was significantly higher than the total number of neoplastic lesions detected using conventional colonoscopy (P = 0.02). Based on macroscopic appearance, location and tumor size, flat lesions < 5 mm located in the right colon in particular were more frequently diagnosed using NBI[12].
Although no Western study has as yet validated those Japanese results, a recent report indicated that adenomas were detected more frequently in the NBI group (23%) than in the control group (17%), but the difference was not statistically significant (P = 0.129)[13]. In contrast, it has also been recently reported that NBI did not result in better detection of adenomas. In that particular study, a colonoscopist with a known high detection rate using white light colonoscopy conducted patient examinations with high-definition colonoscopes using either white light or NBI[14].
The fact that differences still exist between Japan and Western countries demonstrates that prospective studies are needed to determine which of these early reports are valid.
NBI FOR NON-NEOPLASTIC AND NEOPLASTIC LESIONS
For lesions < 10 mm, it is generally accepted that hyperplastic polyps and other non-neoplastic colorectal lesions do not require endoscopic treatment because they are benign and have no malignant potential[15,16]. In contrast, adenomatous polyps should be removed to prevent progression of the adenoma-carcinoma sequence[17].
Magnified chromocolonoscopy (MCC) has been presented as the best means for in vivo selective management of colorectal polyps[18,19] and it is suggested that colorectal polyps should not be treated only on the basis of polyp size, but also with respect to the underlying histological characteristics observed during MCC[20]. The NBI system has been proposed for optical image-enhanced endoscopy because it features a simple one-touch button for changing from white light to NBI and does not require indigo carmine dye spraying.
An early study of an NBI prototype used for differentiating non-neoplastic from neoplastic lesions in 34 patients with 43 lesions reported better visualization of the mucosal vascular network and lesion compared to conventional endoscopy. Chromocolonoscopy and NBI both had a sensitivity of 100% and a specificity of 75%[6]. Thereafter, the effectiveness of conventional colonoscopy, chromoendoscopy and the NBI system in distinguishing between non-neoplastic and neoplastic colonic polyps was assessed in 78 patients with 110 lesions. No significant difference existed between the NBI system and chromoendoscopy, but the sensitivity, specificity and accuracy of conventional colonoscopy were significantly lower (82.9%, 80.0% and 81.8%, respectively) compared to both chromoendoscopy and the NBI system (95.7%, 87.5% and 92.7%, respectively)[21].
More recently, a classification of colorectal polyps based on the presence or absence of superficial meshed capillary vessels and their diameter, observed under NBI (CP type I-III) was proposed in Japan by Sano et al[22]. Although a promising and exciting alternative to differentiate the nature of colorectal polyps, Western prospective studies, however, are needed for its standardization worldwide.
NBI FOR INVASIVE AND NON-INVASIVE COLORECTAL CANCER
There is growing evidence to support the theory that lesions with submucosal (sm) invasion < 1000 μm (sm1) without lympho-vascular invasion or a poorly differentiated component do not involve lymph node metastases[23]. In Japan, analysis of the pit pattern types proposed by Kudo et al[24] has been proven effective in predicting the level of sm invasion. In practice, however, limitations have been reported using the VI pit pattern to discriminate between mucosal (m), slight submucosal (sm1) and, deep submucosal (sm2) or deeper invasion[25]. The invasive pattern proposed by Fujii et al[26-28] (distorted and irregular crypts and a demarcated area) has also been reported to be effective in predicting sm2.
One promising area for NBI is in the accurate estimation of invasive depth for early colorectal cancers. Hirata et al[29] analyzed 148 colorectal lesions and recently reported a high degree of correspondence between pit pattern analysis by NBI and chromoendoscopy although the correspondence between MCC and NBI in evaluating the VI pit pattern of 48 early carcinomas was only 78%. Diagnosis using the type V pit pattern was possible by also evaluating various capillary features including vessel diameter, irregularity and the capillary network observed during NBI and not by relying solely on the pit pattern.
Two other promising studies on predicting the depth of invasion of early colorectal cancer by analyzing the microvascular architecture were published recently. Using NBI with magnification, Fukuzawa et al[30] observed several microvascular architecture characteristics in 61 early colorectal lesions (m-sm1: 37; sm2-3: 24). Univariate analysis showed that wide caliber, irregular caliber, tortuousity, irregularity, short length and non-dense arrangement were significantly more frequent in sm2-3 lesions compared to m-sm1 lesions (P < 0.001). Multivariate analysis, however, revealed that irregularity and non-dense arrangement were the remaining independent factors[30] (Figure 4). Horimatsu et al[31] analyzed the presence of “meshed brown capillary vessels” in 27 colorectal lesions (m-sm1: 12; sm2: 15) also using NBI colonoscopy with magnification. The overall diagnostic accuracy, sensitivity, and specificity of microvessel density and the lack of uniformity in microvessel diameters for distinguishing between sm1 and sm2 lesions was 82.4% (14/17), 93.3% (14/15) and 75.0% (9/12), respectively[31] (Figure 4).
DETECTION OF DYSPLASTIC AREAS IN ULCERATIVE COLITIS
Although patients with longstanding ulcerative colitis are at increased risk of developing colorectal cancer, endoscopic detection of early neoplasia is difficult because these lesions can be subtle and even macroscopically invisible at times. A laborious protocol has been proposed involving not only target biopsies from suspicious lesions, but also two to four random biopsies taken every 10 cm of the colon[32]. MCC has emerged as the best method currently available for identifying dysplastic lesions in an inflammatory bowel disease setting[33,34].
In terms of NBI research, the limitations of the first NBI prototype were recently shown in a prospective randomized crossover study of 42 patients with longstanding ulcerative colitis. In that study, the sensitivity of NBI for the detection of neoplasia was merely comparable to conventional colonoscopy although a larger number of suspicious lesions were found during NBI colonoscopy[35]. A more positive report on the effectiveness of a third generation NBI prototype plus magnification indicated that just as NBI reveals fine superficial blood vessels whose diameters and densities are increased in neoplastic lesions compared with normal mucosa, dysplastic lesions observed using NBI also have a darker capillary vascular pattern compared with normal mucosa[36].
WILL CONVENTIONAL CHROMOCOLONOSCOPY BE REPLACED BY NBI?
It is still too early to answer this question. In Japan, chromocolonoscopy has demonstrated its effectiveness in the differentiation between adenomatous and hyperplastic polyps and is a promising method for distinguishing superficial from deep submucosal cancers, but it is regarded as an inconvenient and difficult procedure in Western countries[37]. Indigo carmine dye spraying is inexpensive and differs in practice from the NBI system in that it does not target superficial vascular patterns, but instead accentuates lesion contours and highlights the pit pattern of colonic crypts[25]. It is interesting to note that indigo carmine dye spraying is not recommended before an NBI examination because it might obscure blood vessel visualization.
In contrast, NBI even without magnification when using the non-sequential system provides accurate definition of vascular vessels throughout the entire colonic mucosa and more clearly defines the borders of a lesion without the necessity of using dye spraying. The recently developed NBI system requires an expensive new processor, however, so the cost-benefit issue requires further analysis[38]. In addition, the diagnostic accuracy of NBI is affected by the learning curve associated with this new methodology and extra time may be needed to perform the examination.
USELESS TECHNOLOGY IN UNQUALIFIED HANDS
The acquisition and use of NBI technology is increasing in many countries, but it should be emphasized that accurate analysis of the pit pattern types and familiarity with MCC are basic fundamentals necessary to become proficient in NBI diagnosis of colorectal lesions. Modern optical technology without proper image interpretation wastes valuable resources, can cause confusion for inadequately trained endoscopists and may result in the delay of inter-institutional validation studies. Training general endoscopists in the principles and applications of optical image-enhanced endoscopy as practiced in Japan (i.e., stereomicroscopy, conventional chromoendoscopy, magnifying endoscopy and pit pattern analysis)[20,24-26] in approved centers by qualified experts will be required to narrow and, hopefully, close the existing gap between the latest advancements in optical technology and their clinical usefulness.
CONCLUSION
Several studies have previously reported on the advantages and limitations of NBI optical image-enhanced colonoscopy in the diagnosis of colorectal diseases. One difficulty in evaluating the results, however, has been non-standardization of the NBI systems and prototypes used in the research. Despite this shortcoming, there seems to be considerable potential for further development by improving NBI technology in the non-sequential endoscopic video system by modifying the characteristics of the interference filters.
In practice, the latest technological advancements incorporated into third generation NBI prototypes appear to offer a clear advantage over conventional chromocolonoscopy. Additional validation studies are needed, however, to confirm the effectiveness of NBI for screening colonoscopy, identification of adenomatous polyps, determining depth of invasion of early colorectal cancers, evaluating free margins after endoscopic resection and detection of dysplastic lesion in an inflammatory bowel disease setting.
A number of other questions remain unsolved that deserve additional examinations including whether NBI is less time-consuming, its cost effectiveness, whether magnification is absolutely required and whether the NBI system should completely replace chromocolonoscopy. Further studies assessing these issues are ongoing at various medical centers worldwide.
At the present time, NBI constitutes an effective and reliable alternative to chromocolonoscopy for in vivo visualization of vascular structures. Due to widespread incorporation of NBI technology outside Japan, however, there is an increasing need to train general endoscopists in the basic principles and applications of advanced optical image-enhanced endoscopy.
Peer reviewer: Peter L Lakatos, MD, PhD, Assistant Professor, 1st Department of Medicine, Semmelweis University, Koranyi S 2A, Budapest H1083, Hungary
S- Editor Li DL L- Editor Kumar M E- Editor Yin DH