Published online Aug 14, 2008. doi: 10.3748/wjg.14.4841
Revised: June 23, 2008
Accepted: June 30, 2008
Published online: August 14, 2008
Pancreatic pseudocysts (PPs) are collections of pancreatic secretions that are lined by fibrous tissues and may contain necrotic debris or blood. The interventions including percutaneous, endoscopic or surgical approaches are based on the size, location, symptoms and complications of a pseudocyst. With the availability of advanced imaging systems and cameras, better hemostatic equipments and excellent laparoscopic techniques, most pseudocysts can be found and managed by laparoscopy. We describe a case of a 30-year-old male patient with a pancreatic pseudocyst amenable to laparoscopic cystogastrostomy. An incision was made through the anterior gastric wall to expose the posterior gastric wall in close contact with the pseudocyst using an ultrasonically activated scalpel. Then, another incision was made for cystogastrostomy to obtain complete and unobstructed drainage. The patient recovered well after operation and was symptom-free during a 6-mo follow-up, suggesting that laparoscopic cystogastrostomy is a safe and effective alternative to open cystogastrostomy for minimally invasive management of PPs.
- Citation: Sheng QS, Chen DZ, Lang R, Jin ZK, Han DD, Li LX, Yang YJ, Li P, Pan F, Zhang D, Qu ZW, He Q. Laparoscopic cystogastrostomy for the treatment of pancreatic pseudocysts: A case report. World J Gastroenterol 2008; 14(30): 4841-4843
- URL: https://www.wjgnet.com/1007-9327/full/v14/i30/4841.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.4841
Pancreatic pseudocysts (PPs), common sequelae of acute or chronic pancreatitis and trauma, are fluid collections arising in or adjacent to the pancreas enclosed by a wall of fibrous granulation tissue, but lacking a true epithelial lining. Interventions indicated for symptomatic, large (> 6 cm in diameter), complicated and persistent (> 6 wk) PPs[1], include percutaneous, endoscopic or surgical approaches[2]. With the advent of minimally invasive techniques such as cystogastrostomy, cystojejunostomy and cystoduodenostomy[3], laparoscopy plays a great role in the management of PPs. Moreover, laparoscopic cystogastrostomy has been described as a safe and efficacious alternative to open drainage of PPs in adults[1,2].
We report, in this paper, a case of a patient with a pancreatic pseudocyst caused by acute pancreatitis, who underwent intragastric laparoscopic cystogastrostomy.
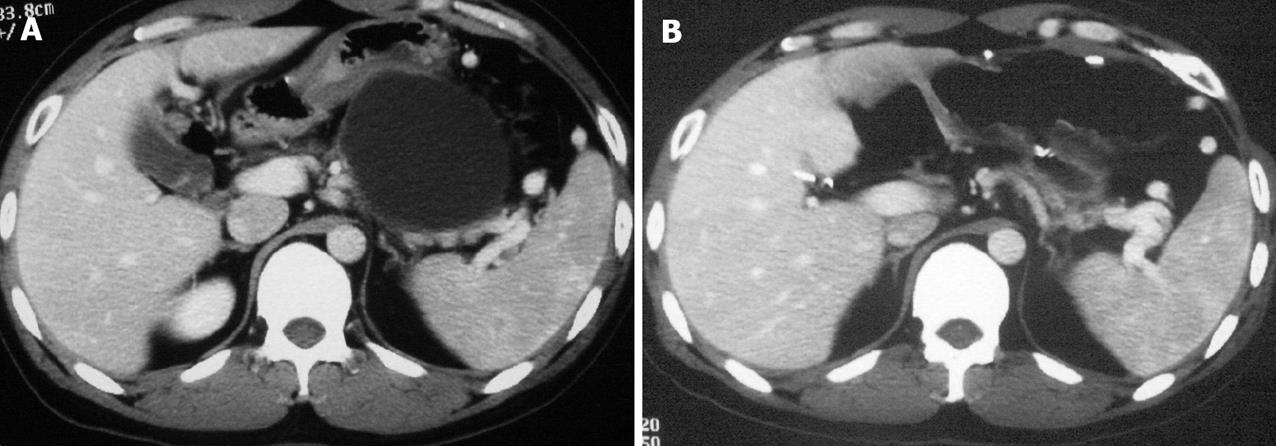
A 30-year-old male patient, complaining of epigastric pain and postprandial distension, was admitted to our hospital. He developed acute pancreatitis 10 mo ago. Regular B type ultrasound and computed tomography (CT) showed a pseudocyst at the body of the pancreas. A recent CT scan (Figure 1) demonstrated a 7.5 cm × 6.0 cm mass with its anterior wall closely contacted with the posterior wall of the stomach, as well as splenic vein compression and splenomegaly.
The patient underwent laparoscopic cystogastro-stomy. In brief, after general anesthesia, a 10-mm port was placed subumbilically for laparoscopy, two 5-mm working ports were placed in the left subcostal area and a 5-mm port was placed in the subxiphoid. Laparoscopic ultrasound showed that a pseudocyst was firmly adhered to the posterior wall of the stomach. An ultrasonically activated scalpel was used to create a 5 cm anterior gastrostomy at the maximal displacement site of the stomach. A laparoscopic needle was introduced to confirm the location of the pseudocyst and to sample fluid. Then, the scalpel was used to create a cystogastrostomy opening approximately 4 cm in size between the adherent posterior and anterior gastric walls of the pseudocyst. After the needle entered the pseudocyst, more than 500 mL of fluid contents was aspirated. Electrocautery diathermy and titanium clips were used to achieve hemostasis at the bleeding sites in the cystogastrostomy. The anterior gastrostomy was closed using a linear stapler. A nasojejunal tube was left in place at the end of the procedure which took 150 min. Histologic analysis revealed a typical pancreatic pseudocyst.
After the operation, the patient was given parenteral nutrition and enteral nutrition via a nasojejunal tube, and recovered well without complications. The nasojejunal tube was removed on the 8th postoperative day and the patient was discharged on the 11th postoperative day. A repeat CT scan (Figure 1B) before discharge revealed effective drainage of the pseudocyst and dramatic relief of splenic vein compression. The patient was free of symptoms and signs of recurrent pseudocyst duering a 6-mo follow-up.
Pancreatic pseudocysts occur in 2%-10% of patients after acute pancreatitis and in about 10%-30% of patients after chronic pancreatitis[4], and can be treated with different procedures. In general, spontaneous regression of small asymptomatic PPs may be observed in 30%-60% of acute pancreatitis patients[5]. Conservative management with bowel rest and parenteral nutrition increases the likelihood of spontaneous regression[6]. However, a large number of patients with PPs need interventions. Factors determining the route and time of intervention include (1) location, size and persistence of the cyst, (2) maturity of the cyst wall when the patient presents with symptoms, (3) presence or absence of complications, (4) availability of local expertise and experience[2]. Generally, indications for intervention of PPs include > 6 cm in diameter, > 6 wk in persistance, symptoms (including epigastric pain, nausea, vomiting, biliary obstruction, and duodenal obstruction), complications (including infection, hemorrhage, rupture) and matured wall[1,2,7].
Intervention options for treatment include percutaneous, endoscopic, and surgical procedures. Percutaneous drainage of PPs is a procedure of choice for infected and obstructed pseudocysts with an immature wall. It is also used in situations where definitive internal drainage could not be done[2]. However, this procedure seems to have a high risk of recurrence or development of pancreatic percutaneous fistula[8]. Furthermore, percutaneous drainage is inadequate in many cases because of thick viscous contents, which may cause luminal obstruction of drainage catheters[9]. Endoscopic drainage in the presence of endoscopic ultrasound (EUS) is an important procedure in the management of pseudocysts, especially cysts indenting the stomach or duodenum and in the absence of necrotic tissue[2]. However, endoscopic drainage is associated with a high rate of technical failure, cyst recurrence, infection, bleeding, stent blockage, and inadequate drainage. Aljarabah et al[1] hold that endoscopic drainage is more suitable for chronic PPs within the head and body of the gland, whereas acute PPs, particularly those that complicate necrotizing pancreatitis, are best managed with laparoscopic surgery where expertise is available. Laparoscopic drainage of mature PPs is minimally invasive and offers definitive drainage. Over the past years, laparoscopic surgery of the pancreas has increasingly emerged as a procedure in the treatment of PPs[3]. Laparoscopic procedures for pancreatic pseudocysts include pancreatic cystogastrostomy, cystoduodenostomy, and cystojejunostomy. Barragan et al[10] compared the laparoscopic procedures by analyzing their advantages and disadvantages, and concluded that when the pancreatic cyst is located in close contact with the posterior wall of the stomach, it is best drained with the anterior procedure.
According to the history of acute pancreatitis and the location, size, and persistence of the pseudocyst in our case, percutaneous procedure and EUS-guided drainage were not suitable, and laparoscopic cystogastrostomy was performed via the anterior procedure. Laparoscopy was carried out using a 4-port technique. Because the pseudocyst shared a common wall, a cystogastrostomy, 4 cm in size, was created directly using an ultrasonical scalpel. The superior visualization provided by laparoscopy afforded the ability to obtain hemostasis efficiently, and the adequate incision helped establish effective drainage, thus preventing recurrence of the pseudocyst. The patient recovered well after the operation and was free of symptoms after 6 mo of follow-up.
Laparoscopic drainage, a minimally invasive technique, can definitively drain mature PPs. Our patient was treated with laparoscopic procedures without any untoward effects. Although our experience is limited, our minimally invasive procedure appears to be safe and effective. Laparoscopic cystogastrostomy should be considered a choice of treatment in the management of PPs.
Peer reviewer: Dr. Massimo Raimondo, Division of Gastroenterology and Hepatology, Mayo Clinic, 4500 San Pablo Road, Jacksonville, FL 32224, United States
S- Editor Zhong XY L- Editor Wang XL E- Editor Yin DH
| 1. | Aljarabah M, Ammori BJ. Laparoscopic and endoscopic approaches for drainage of pancreatic pseudocysts: a systematic review of published series. Surg Endosc. 2007;21:1936-1944. |
| 2. | Palanivelu C, Senthilkumar K, Madhankumar MV, Rajan PS, Shetty AR, Jani K, Rangarajan M, Maheshkumaar GS. Management of pancreatic pseudocyst in the era of laparoscopic surgery--experience from a tertiary centre. Surg Endosc. 2007;21:2262-2267. |
| 3. | Mori T, Abe N, Sugiyama M, Atomi Y. Laparoscopic pancreatic surgery. J Hepatobiliary Pancreat Surg. 2005;12:451-455. |
| 4. | Yang CC, Shin JS, Liu YT, Yueh SK, Chou DA. Management of pancreatic pseudocysts by endoscopic cystogastrostomy. J Formos Med Assoc. 1999;98:283-286. |
| 5. | Mehta R, Suvarna D, Sadasivan S, John A, Raj V, Nair P, Balakrishnan V. Natural course of asymptomatic pancreatic pseudocyst: a prospective study. Indian J Gastroenterol. 2004;23:140-142. |
| 6. | Saad DF, Gow KW, Cabbabe S, Heiss KF, Wulkan ML. Laparoscopic cystogastrostomy for the treatment of pancreatic pseudocysts in children. J Pediatr Surg. 2005;40:e13-e17. |
| 7. | Matsutani T, Sasajima K, Miyamoto M, Yokoyama T, Hiroi M, Maruyama H, Suzuki S, Tajiri T. Pancreatic cyst associated with pancreas divisum treated by laparoscopy-assisted cystgastrostomy in the intragastric approach: a case report and a review of the literature. J Laparoendosc Adv Surg Tech A. 2007;17:317-320. |
| 8. | Breckon V, Thomson SR, Hadley GP. Internal drainage of pancreatic pseudocysts in children using an endoscopically-placed stent. Pediatr Surg Int. 2001;17:621-623. |
| 9. | Haluszka O, Campbell A, Horvath K. Endoscopic management of pancreatic pseudocyst in children. Gastrointest Endosc. 2002;55:128-131. |
| 10. | Barragan B, Love L, Wachtel M, Griswold JA, Frezza EE. A comparison of anterior and posterior approaches for the surgical treatment of pancreatic pseudocyst using laparoscopic cystogastrostomy. J Laparoendosc Adv Surg Tech A. 2005;15:596-600. |