Published online Aug 14, 2008. doi: 10.3748/wjg.14.4830
Revised: June 18, 2008
Accepted: June 25, 2008
Published online: August 14, 2008
We present 2 cases of hepatocyte nuclear factor 1α (HNF1α)-mutated adenomatosis, discovered for reasons unrelated to this disease, and identified using immunohistochemical methods. These new tools may further our understanding of the link between adenomas/adenomatosis subtypes and their complications, and their association with other abnormalities.
- Citation: Laumonier H, Rullier A, Saric J, Balabaud C, Bioulac-Sage P. Unexpected discovery of 2 cases of hepatocyte nuclear factor 1α-mutated infracentimetric adenomatosis. World J Gastroenterol 2008; 14(30): 4830-4833
- URL: https://www.wjgnet.com/1007-9327/full/v14/i30/4830.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.4830
Hepatocellular adenomas (HCAs) are found as unique or multiple entities. The term adenomatosis is used[1], if the number of HCAs is greater than ten; specific etiological factors, including glycogenosis or patients taking male hormones, were initially excluded from the definition[1], as well as women that previously were but are no longer taking oral contraceptives[2]. Adenomatosis is suspected if multiple nodules are observed on ultrasound performed in various circumstances: pain, discomfort, mass, shock related to bleeding, hepatocellular carcinoma (HCC), or by chance[3].
HCAs belong to various categories, as shown by molecular testing of hepatocyte nuclear factor 1α (HNF1α)[4-6], and β-catenin[5,6], and by the expression of members of the acute phase inflammatory response [serum amyloid A (SAA), and C-reactive protein (CRP)] at both the mRNA and protein levels[7]. We used genotype/phenotype classification to identify four groups of HCA: HNF1α-mutated, β-catenin-mutated, inflammatory HCA and HCA without known mutation. Some inflammatory adenomas are also β-catenin-mutated. This classification also applies to adenomatosis. However, the number of cases of adenomatosis studied in the series used for classification is less than the number of cases of single or multiple HCA.
We report two cases in which the diagnosis of adenomatosis was unexpected, based on radiological and clinical grounds, and for whom molecular and immunohistochemistry testing revealed HNF1α-mutations.
A 54-year-old woman was admitted to our surgical department in September, 2004, due to the discovery of hyperechoic infracentimetric nodules, within the context of malignancy. Her past history was remarkable: she had a haemangioma of the bulbar area for over 20 years, a cerebellar meningioma discovered later and surgically removed in June, 1999, and a choroidal melanoma treated with proton therapy in February, 2004. At the time of those diagnoses, liver nodules were not visible on ultrasound. She had 2 children and took the pill for a very short period of time (less than 1 year prior to consulting our clinic). She weighed 70 kg and was 163 cm high. Physical examination revealed a cerebellar syndrome. Several punctiform angiomas were present on the thorax and limbs. Liver function tests were normal, with the exception of 39 IU/L GGT (normal < 34 IU/L). Her blood glucose was 7.1 mmol/L (normal < 6.1 mmol/L). She had a family history of non-insulin-dependant diabetes (father and mother of the father). Magnetic resonance imaging confirmed the presence of several infra-centimetric liver nodules, but the results were inconclusive in relation to their nature. A liver biopsy was proposed under laparoscopic guidance to confirm the suspected diagnosis of melanoma metastasis. Multiple tan nodules were observed on the surface of the liver by the surgeon. A surgical biopsy (1.5 cm × 0.8 cm) containing two yellowish nodules (0.8 mm), and a histological examination of the tissue ruled out melanoma metastasis.
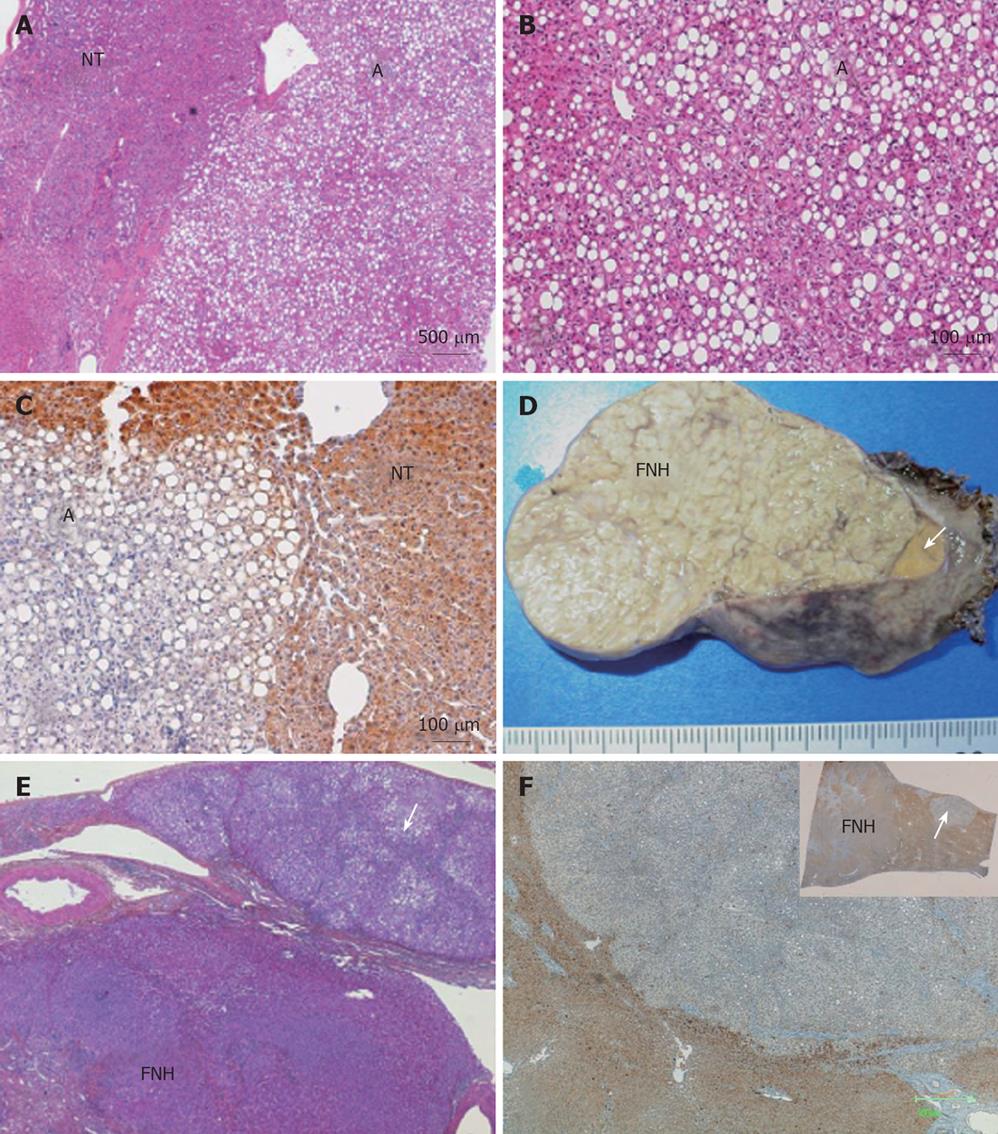
Both small liver nodules were non-encapsulated and showed a benign steatotic hepatocytic proliferation, intermingled with thin-walled isolated arteries and veins (Figure 1A and B). Cytokeratin (CK) 19 was negative and very few progenitor-like cells were visible on CK7 immunostaining (not shown). Therefore, the most probable diagnosis of these small nodules was HCA. Also, a complete absence of liver fatty acid-binding protein (LFABP), in contrast to normal expression detected in hepatocytes of the surrounding liver, favoured a diagnosis of HNF1α-mutated HCA (Figure 1C). The non-tumoral liver was limited to a thin band of tissue containing mildly enlarged fibrotic portal tracts.
A 34-year-old woman visited our surgical clinic in February, 2004, for consultation. Two liver nodules were discovered by chance (one in segment III: 40 mm × 35 mm and one in segment VI: 20 mm × 20 mm) and there were at least two additional infracentimetric nodules. The two larger nodules were described as characteristic focal nodular hyperplasia (FNH), but no final diagnosis of the smaller nodules was made. The patient had been taking oral contraceptives for 12 years. However, she was no longer taking them in the last 2 years prior to consultation at our clinic. Her family history was complex (two maternal uncles had a cerebral vascular accident at a young age, with at least one of their children having an aneurysm resulting in a death, another uncle died after cardiac surgery, her mother and two maternal ants also had breast cancer). Blood tests, including liver function tests, were normal. An operation was ruled out, and instead the patient was monitored. She came back 6 mo later complaining of epigastric pain. The size of the largest nodule increased (60 mm × 40 mm). Segmentectomy III and VI were performed under coelioscope. The surgeon observed multiple tan nodules on the surface of the liver. The two large nodules were macro and microscopically typical FNH. Three other small nodules were also clearly visible on the resected specimen, one of which was adjacent to the FNH (Figure 1D).
Routine examination of the nodules (cases 1 and 2) consisted of HE, Masson’s trichrome, and reticulin stains, as well as CK7, CK19, and a smooth muscle actin (SMA), LFABP, serum amyloid A, glutamine synthase (GS) and β-catenin immunostains[7].
All the three small nodules were steatotic, with mild ductular reaction and inflammation, as well as a few entrapped portal tracts at the periphery. It was, thus, not possible to distinguish clearly between small, incomplete, so-called FNH-like or pre-FNH[8,9] and adenomas (Figure 1E). In case 1, the complete absence of LFABP in the nodule favoured a diagnosis of HNF1α-mutated HCA, whereas the LFABP immunostaining was normal both in non-tumoral livers and in adjacent FNH (Figure 1F).
SAA, GS and β-catenin immunostaining were negative in both cases (data not shown).
The above two cases lead to several comments.
Such cases of adenomatosis would have been totally ignored, if for specific reasons (fear of metastasis in case 1, and surgery for a growing and painful FNH in case 2) the surface of the liver had not been observed. This is indeed true for any benign lesion without clinical or biological manifestations, including FNH, HCA, haemangioma, etc. Thus, the true prevalence of adenomatosis is impossible to calculate, as long as we do not have the tools to recognize clinically-, biologically- and radiologically-silent HCA, particularly microadenomas. We usually ignore the outcome of such cases, but it is likely that such small nodules will remain silent, particularly in patients that are no longer on oral contraception, which was the case in our two patients.
One can argue that the presence of multiple nodules on surface of the liver does not definitely prove that all nodules are microadenomas, this is particularly true for case 2, a case associated with FNH. The possibility of multiple FNH associated with occasional adenomas cannot be excluded. However, in our experience we have never observed such a case, whereas the presence of FNH is not a rare event in adenomatosis[10].
As a result of advances made in molecular biology and immunohistochemistry, there has been much progress in the field of HCA. In particular with genotype/phenotype correlations, it is now possible, on routine pathological examinations, to identify typical cases of HNF1α-mutated or inflammatory adenomas with a greater certainty. HNF1α HCA is characterized by their fat distribution[6].
We can easily and rapidly classify 80% of adenomas with the use of immunohistochemitry, including SAA, LFABP, GS and β-catenin, the results of which correlate well with molecular studies[6]. The genotype/phenotype classification of HCA using immunohistochemical methods, particularly β-catenin immunostaining coupled to GS, is particularly important for detecting patients at risk of developing HCC, as recently reported[4,6,7,11]. Immunostains are also important for identifying atypical nodules and small sized-resected nodules and have also been particularly used to differentiate micro adenomas from other types of nodules, especially pre-FNH[8,10,12] if fibrosis or some bile ductules are present.
The next step is to validate immunohistochemical methods on liver biopsy. To better interpret the results, it is mandatory to compare the data obtained in the non tumoral liver. Non tumoral livers express LFABP, but not SAA. GS is expressed only around hepatic veins.
The term adenomatosis has frequently been used in cases with more than 10 nodules[1]. If nodules are visible on surface of the liver, the diagnosis is usually easy, irrespective of the number of larger nodules detected by imaging techniques. Adenomatosis may also be suspected, if microadenomas are present on the resected specimen containing one or several HCAs from the non-tumoral liver[13]. In our opinion, the term adenomatosis has been used incorrectly, if defined on the basis of there being more than 3 lesions on imaging[13], without the presence of nodules visible on the liver surface or microadenomas discovered by the pathologist on the resected specimen from the non-tumoral liver. In our experience, adenomatosis is associated with HNF1α-mutations (90% being somatic) in most cases[7].
Patients with adenomas/adenomatosis may have other organ abnormalities or diseases that may or may not be related to the pathogenesis of adenomas. In 1989, Wanless et al[14] noticed that patients with diseases, including meningioma, astrocytoma, telangiectasia of the brain, berry aneurysm, had what he called “telangiectatic FNH” more often (TFNH). Genotype/phenotype analysis[4,6,7] has revealed that TFNH are indeed inflammatory/telangiectatic adenomas[15,16]. Curiously enough in case 1, meningioma and telangiectasia of the brain were not associated with inflammatory/telangiectatic adenomatosis, but with HNF1α-mutated adenomatosis.
With this new HCA classification at hand and the knowledge that adenomas/adenomatosis can be associated with various factors, including FNH[10], diabetes with or without familial form of adenomas/adenomatosis[17-20], polycystic ovaries[21], and obesity/NASH[2,7,22], it is of interest to collect clinical, biological and radiological data, which should not only be related to the liver but also be related to other organs (such as the brain, skin, thyroid, ovaries, kidneys, pancreas etc) to understand if there is a link or not between these various types of abnormalities.
Peer reviewers: Xiao-Ping Chen, Professor, Institute of Hepatopancreatobiliary Surgery, Tongji Hospital, 1095# Jiefang Dadao, Wuhan 430030, Hubei Province, China; Jian Wu, Associate Professor of Medicine, Internal Medicine/Transplant Research Program, University of California, Davis Medical Center, 4635 2nd Ave. Suite 1001, Sacramento CA 95817, United States
S- Editor Li DL L- Editor Wang XL E- Editor Zhang WB
| 1. | Flejou JF, Barge J, Menu Y, Degott C, Bismuth H, Potet F, Benhamou JP. Liver adenomatosis. An entity distinct from liver adenoma? Gastroenterology. 1985;89:1132-1138. |
| 2. | Brunt EM, Wolverson MK, Di Bisceglie AM. Benign hepatocellular tumors (adenomatosis) in nonalcoholic steatohepatitis: a case report. Semin Liver Dis. 2005;25:230-236. |
| 3. | Bioulac-Sage P, Balabaud C, Bedossa P, Scoazec JY, Chiche L, Dhillon AP, Ferrell L, Paradis V, Roskams T, Vilgrain V. Pathological diagnosis of liver cell adenoma and focal nodular hyperplasia: Bordeaux update. J Hepatol. 2007;46:521-527. |
| 4. | Rebouissou S, Bioulac-Sage P, Zucman-Rossi J. Molecular pathogenesis of focal nodular hyperplasia and hepatocellular adenoma. J Hepatol. 2008;48:163-170. |
| 5. | Bluteau O, Jeannot E, Bioulac-Sage P, Marques JM, Blanc JF, Bui H, Beaudoin JC, Franco D, Balabaud C, Laurent-Puig P. Bi-allelic inactivation of TCF1 in hepatic adenomas. Nat Genet. 2002;32:312-315. |
| 6. | Zucman-Rossi J, Jeannot E, Nhieu JT, Scoazec JY, Guettier C, Rebouissou S, Bacq Y, Leteurtre E, Paradis V, Michalak S. Genotype-phenotype correlation in hepatocellular adenoma: new classification and relationship with HCC. Hepatology. 2006;43:515-524. |
| 7. | Bioulac-Sage P, Rebouissou S, Thomas C, Blanc JF, Saric J, Sa Cunha A, Rullier A, Cubel G, Couchy G, Imbeaud S. Hepatocellular adenoma subtype classification using molecular markers and immunohistochemistry. Hepatology. 2007;46:740-748. |
| 8. | Lepreux S, Laurent C, Balabaud C, Bioulac-Sage P. FNH-like nodules: Possible precursor lesions in patients with focal nodular hyperplasia (FNH). Comp Hepatol. 2003;2:7. |
| 9. | Bioulac-Sage P, Balabaud C, Wanless IR. Diagnosis of focal nodular hyperplasia: not so easy. Am J Surg Pathol. 2001;25:1322-1325. |
| 10. | Laurent C, Trillaud H, Lepreux S, Balabaud C, Bioulac-Sage P. Association of adenoma and focal nodular hyperplasia: experience of a single French academic center. Comp Hepatol. 2003;2:6. |
| 11. | Zucman-Rossi , Blanc JF, Sa Cunha A, Jeannot E, Couchy G, Rullier A, Cubel G, Laumonnier H, Capdepont , Le Bail B. b-catenin mutated hepatocellular adenomas (HCA). Hepatology. 2006;44:156. |
| 12. | Lepreux S, Laurent C, Blanc JF, Trillaud H, Le Bail B, Trouette H, Saric J, Zucman-Rossi J, Balabaud C, Bioulac-Sage P. The identification of small nodules in liver adenomatosis. J Hepatol. 2003;39:77-85. |
| 13. | Ribeiro A, Burgart LJ, Nagorney DM, Gores GJ. Management of liver adenomatosis: results with a conservative surgical approach. Liver Transpl Surg. 1998;4:388-398. |
| 14. | Wanless IR, Albrecht S, Bilbao J, Frei JV, Heathcote EJ, Roberts EA, Chiasson D. Multiple focal nodular hyperplasia of the liver associated with vascular malformations of various organs and neoplasia of the brain: a new syndrome. Mod Pathol. 1989;2:456-462. |
| 15. | Paradis V, Benzekri A, Dargere D, Bieche I, Laurendeau I, Vilgrain V, Belghiti J, Vidaud M, Degott C, Bedossa P. Telangiectatic focal nodular hyperplasia: a variant of hepatocellular adenoma. Gastroenterology. 2004;126:1323-1329. |
| 16. | Bioulac-Sage P, Rebouissou S, Sa Cunha A, Jeannot E, Lepreux S, Blanc JF, Blanche H, Le Bail B, Saric J, Laurent-Puig P. Clinical, morphologic, and molecular features defining so-called telangiectatic focal nodular hyperplasias of the liver. Gastroenterology. 2005;128:1211-1218. |
| 17. | Foster JH, Donohue TA, Berman MM. Familial liver-cell adenomas and diabetes mellitus. N Engl J Med. 1978;299:239-241. |
| 18. | Bacq Y, Jacquemin E, Balabaud C, Jeannot E, Scotto B, Branchereau S, Laurent C, Bourlier P, Pariente D, de Muret A. Familial liver adenomatosis associated with hepatocyte nuclear factor 1alpha inactivation. Gastroenterology. 2003;125:1470-1475. |
| 19. | Reznik Y, Dao T, Coutant R, Chiche L, Jeannot E, Clauin S, Rousselot P Fabre M, Oberti F, Fatome A, Zucman-Rossi J. Hepatocyte nuclear factor-1 alpha gene inactivation: cosegregation between liver adenomatosis and diabetes phenotypes in two maturity-onset diabetes of the young (MODY)3 families. J Clin Endocrinol Metab. 2004;89:1476-1480. |
| 20. | Bambha K, Nagorney D, Sanderson S, Gores GJ. Hepatic adenomatosis in a young woman with glucose intolerance. Nat Clin Pract Gastroenterol Hepatol. 2006;3:526-531; quiz (following 531). |
| 21. | Toso C, Rubbia-Brandt L, Negro F, Morel P, Mentha G. Hepatocellular adenoma and polycystic ovary syndrome. Liver Int. 2003;23:35-37. |
| 22. | Paradis V, Champault A, Ronot M, Deschamps L, Valla DC, Vidaud D, Vilgrain V, Belghiti J, Bedossa P. Telangiectatic adenoma: an entity associated with increased body mass index and inflammation. Hepatology. 2007;46:140-146. |