Published online Aug 14, 2008. doi: 10.3748/wjg.14.4800
Revised: April 1, 2008
Accepted: April 8, 2008
Published online: August 14, 2008
AIM: To establish nude mouse human gastric cancer orthotopic transplantation models using OB glue paste technique.
METHODS: Using OB glue paste technique, orthotopic transplantation models were established by implanting SGC-7901 and MKN-45 human gastric cancer cell strains into the gastric wall of nude mice. Biological features, growth of the implanted tumors, the success rate of transplantation and the rate of auto-metastasis of the two models were observed.
RESULTS: The success rates of orthotopic transplan-tation of the two models were 94.20% and 96%. The rates of hepatic metastasis, pulmonary metastasis, peritoneal metastasis, lymphocytic metastasis and splenic metastasis were 42.13% and 94.20%, 48.43% and 57.97%, 30.83% and 36.96%, 67.30% and 84.06%, and 59.75% and 10.53%, respectively. The occurrence of ascites was 47.80% and 36.96%.
CONCLUSION: OB glue paste technique is easy to follow. The biological behaviors of the nude mouse human gastric cancer orthotopic transplantation models established with this technique are similar to the natural processes of growth and metastasis of human gastric cancer, and, therefore, can be used as an ideal model for experimental research of proliferative metastasis of tumors.
- Citation: Shi J, Wei PK, Zhang S, Qin ZF, Li J, Sun DZ, Xiao Y, Yu ZH, Lin HM, Zheng GJ, Su XM, Chen YL, Liu YF, Xu L. OB glue paste technique for establishing nude mouse human gastric cancer orthotopic transplantation models. World J Gastroenterol 2008; 14(30): 4800-4804
- URL: https://www.wjgnet.com/1007-9327/full/v14/i30/4800.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.4800
Establishment of human gastric cancer nude mouse transplantation models has undergone three representative stages[1-5]: subcutaneous transplantation of cell suspension[6], gastric wall seeding of cell suspension[7-12], and intra-gastric wall transplantation of histologically intact tissues[13-17], of which the fresh tissue orthotopic transplantation model has two types in method: the “suture method”[18] and the “gastric bursa method”[19]. Enlightened by surgical use of OB glue, the present study used OB glue paste technique to implant SGC-7901 and MKN-45 human gastric cancer cell strains to the gastric wall of nude mice in an attempt to examine the biological features of proliferative metastasis of gastric cancer cells.
The study included 314 6-week BALB/C nude mice of both sexes weighing 18-20 g provided by Shanghai Experimental Animal Center of the Chinese Academy of Sciences (qualification No.: SCXK, Shanghai, 2004-0003). In this study, 166 cases of models for SGC-7901 and 148 cases for MKN-45 were established. The animals were raised in the said center in SPF experimental animal rooms (approval No.: SYXK, Shanghai, 2004-0011) with free access to water.
SGC-7901 and MKN-45 cell strains were kind gifts from the laboratory of experimental pathology of Shanghai Institute of Tumor Research, CAS. Cancer cells were cultured in RPMI-1640 solution containing 10% bovine serum in a CO2 thermostatic incubator and passaged routinely.
In vitro cancer cells were collected to the content of 1 × 107/mL. Each nude mouse was injected with 0.2 mL cancer cells under the cervical skin. When the implanted tumor grew to about 1cm diameter, it was removed out of the mouse. The tumor was cut into 1 mm × 1 mm × 2 mm pieces after scraping off the surrounding fibrous capsule, and implanted directly into the cervical skin of the nude mice. Each inter-mouse passage used two mice. The third generation subcutaneously transplanted tumor was used as the source of orthotopic transplantation in this study.
The mice were purchased two days ahead of the experiment for environmental adaptation. The animals were fasted 12 h before operation and anesthetized with 0.4% pentobarbital sodium (60 mg/kg) intraperitoneally. The skin was sterilized routinely and a 1 cm incision was made along the left paramedian line to expose the peritoneum and gastric wall meticulously. The serous layer of the greater curvature of stomach where there are abundant blood vessels, was carefully ruptured with an injection needle until bleeding was visible, into which the tumor tissue was implanted. One to 2 drops of medical OB glue (Cyanoacrylate, medical OB 508 series for anastomosis, Guangzhou Bai Yun Medical Glue Co., Ltd., Batch No. 030703) were applied to seal the rupture. After the glue coagulated for about 10 s, the peritoneum was closed with No. 3 suture and the skin was closed with No. 1 suture.
When the mice were seen developing failing signs such as leanness, limited activity and listlessness, they were sacrificed by cervical dislocation and anatomized for comprehensive exploration of the chest and abdominal cavities and macroscopic observation of any transplanted tumor with regard to local growth, ascites, adjacent lymph nodes and distal organ metastasis. The transplanted tumor, enlarged lymph nodes, liver, spleen, pancreas and lungs were excised, and the specimens were fixed, sliced and stained for histopathologic observation under light and electron microscope. The tumor was weighed with an analytical balance and recorded. Four mice which died during the study were treated in the same manner without including into statistics, but for reference and later causative analysis.
Part of the transplanted tumor tissues was sheared with aseptic technique and placed in serum-free 1640 medium for primary culture. When the cells grew vigorously and formed a single layer, the first generation passage was done, for which chromosomal specimens were prepared. The specimens were observed under an × 40 light microscope and photographed for chromosomal metakesis of one cell with a microscopic camera.
One mouse died from excessive bleeding during establishment of the SGC-7901 orthotopic transplantation model. The skin suture fell off at about day 5 and the wound healed completely in a week. At week 3-4, 4 mm nodules were palpable in the upper abdomen, which grew large gradually by week 5-6 and became markedly large by week 8-10. At week 10 some of the tumors were even visible through the wall and as large as 10-20 mm in diameter. The surfaces of these tumors were nodular and hard in consistency. From week 11 on, the animals began presenting failing signs such as leanness, limited activity, listlessness and hypoactivity. One animal died. At week 12 the failing signs were more evident and severe, and tumors in some mice subcutaneously projected out, or the abdomens were bulky looking like a frog abdomen. The animals were sacrificed by cervical dislocation.
One animal in the MKN-45 orthotopic trans-plantation model died on the second day because of suture failure due to mutual fierce biting of the animals. The situation of the remaining animals was much the same as that of the SGC-7901 model. The only difference in the MKN-45 model was that the tumors grew faster in fewer days. By week 2, hard nodules about 4mm were palpable in both right and left upper abdomen; the tumors became large gradually by week 3-4 and grew to 10-15 mm in diameter by week 5-6; 3 animals died by week 6-7; and the failing signs became worse by week 8 when giant tumors of 15-20 mm in diameter were palpable. The animals were sacrificed by cervical dislocation.
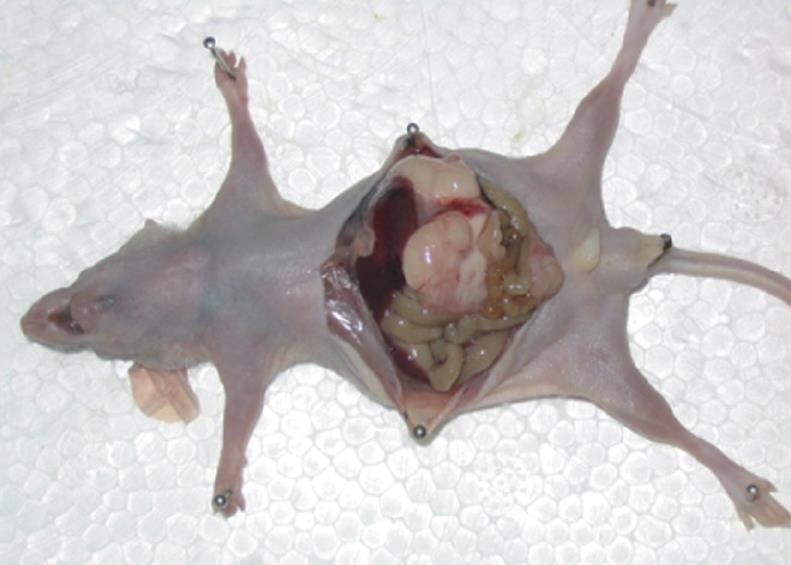
A total of 164 cases of models with SGC-7901 were established in this study, including 159 cases of SGC-7901 orthotopic transplantation with a success rate of 97% (159/164). Gross anatomy revealed: the body of stomach was enlarged and the fundus was dilated; grayish fish meat like tumors were seen on the gastric wall; the tumor tissue was parenchymatous with vague margins and round or oval in shape. There were nodular processes on the surface, which infiltrated into the surrounding tissues and adhered with the mesentery, liver, spleen and peritoneum in varying degrees. There was bloody ascites in some mice. The tumor sections were grayish, looking like fish meat and homogenous in consistency, on which there were abundant capillaries. In some large tumors, there were small necrotic patches in the center. The mean weight of the tumors was 2.31 ± 0.75 g.
A total of 144 cases of models with MKN-45 were established, including 138 cases of orthotopic transplantation, with a success rate of 96% (138/144). Gross anatomic findings were much the same as those of the SGC-7901 model. The tumors were oval in shape, uneven and hard in consistency. There was a serous necrotic area in the center. The mean weight of the tumors was 2.53 ± 0.84 g (Figure 1).
Intraperitoneal lymph nodes were enlarged in most mice of the SGC-7901 model. Involvement of the tumors was various. Several small grayish miliary nodules were seen in the liver of most tumor-bearing mice, which went into the hepatic parenchyma and were difficult to separate. Pyloric obstruction and bloody ascites were seen in some tumor-bearing mice, and pulmonary, splenic and peritoneal metastases were seen in other animals. The metastasis rates of the liver, lungs, peritoneum, lymph node and spleen were 42.02% (67/159), 48.43% (77/159), 30.82% (49/159), 67.30% (107/159) and 59.75% (95/159), respectively. The prevalence of ascites was 47.80% (76/159).
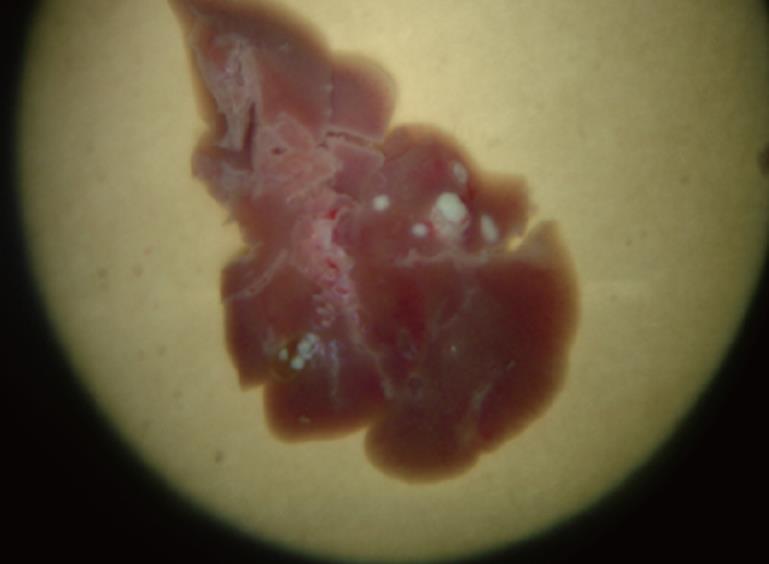
The situation in the MKN-45 model was similar to that of the SGC-7901 model, where the hepatic focus of metastasis was 2-3 mm (Figure 2). The lung surface was congested in some animals and transparent nodules about 1mm were visible. The metastasis rates of the liver, lungs, peritoneum, lymph node and spleen were 94.20% (130/138), 57.97% (80/138), 36.96% (51/138), 84.06% (116/138) and 10.86% (15/138), respectively. The prevalence of ascites was 31.88% (44/138).
SGC-7901 gastric cancer tissue was adenocarcinoma of low differentiation. The tumor cells present as oval shape with large malformed nuclei, most of which were of pathologic mitosis with clear and multiple nucleoli. They were deranged like a nestle with rich sinusoids, in which filtration of lymphocytes was seen.
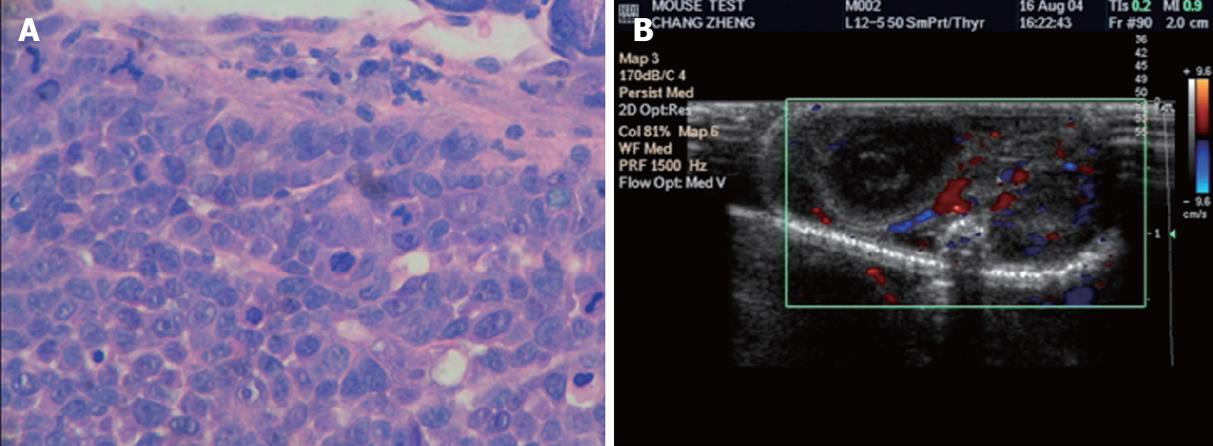
Histological sections of MKN-45 tumor body was also characterized by poorly differentiated adenocarcinoma, where cells were in patchy, nestle or streaky arrangement and shaped round, oval or irregular. Cell differentiation was poor, with large deep stained nuclei and a large nuclear-cytoplasmic ratio, where karyokinetic phase was seen. Fibrous connective tissue was seen in the mesenchyma, and infiltrative growth of the tumor tissue was seen in the gastric wall (Figure 3A).
The histological structure of the tumor metastatic foci in the liver and lungs was consistent with that of the orthotopic gastric tumor, mostly distributing around blood vessels of the lung parenchyma and hepatic sinusoid and growing infiltratively to the surrounding tissues. Large numbers of cancer cells were seen in the enlarged lymph nodes; they arranged closely, destroying almost all lymphatic structure. Cytohistological morphology of ascites smear was similar to that of the orthotopic tumor.
Under × 40 light microscope, no single chromosome in the metaphase was seen as a telocentric chromosome, indicating that it was a human chromosome. Distribution of the chromosomes was more than 46, indicating that they were malignant tumor cells.
B-ultrasound detected low echo masses in the upper abdomen of the nude mice, which grew into the abdominal cavity (Figure 3B). Most blood flow of the tumors was in the periphery, or mixed blood flow was seen both inside and around the tumor. The course of vessels was irregular and the vessels were various in size and deranged, forming winding, fork-like and net-like blood flows. Blood flow was more abundant and faster in the periphery than in the center of the tumor (Figure 3B).
Human gastric cancer fresh tissue orthotopic transplan-tation is the main means of model establishment. The site of transplantation is usually between the serous layer and seromuscular layer of the greater curvature of stomach. The “suture method”[20] or the “gastric bursa method”[21-22] has many practical difficulties and disadvantages in establishing the model of transplantation, for example, the tumor tissue is easy to fall off postoperatively; manipulation is complex and needs relatively high skills; and there may be great blood loss during suturing, and mortality is relatively high. OB glue is biologic glue and has been used widely in surgery owing to its secure wound adhesion. We have had two-years experience with the use of “OB glue paste technique” to establish the human gastric cancer nude mouse orthotopic transplantation model since 2003, when the technique was first attempted in our department[23-30]. The present study used OB glue paste technique to establish two tumor strain orthotopic transplantation models. Observations showed that although different tumor strains grew at different rates, infiltrative growth and multi-organ metastases are common features of the two models, and these features are similar to the clinical presentation of invasive metastasis. Chromosomal identification also demonstrated that both orthotopic and metastatic tumors came from the human gastric cancer implanted. This technique is an ideal means of model establishment owing to its easier manipulation, shorter operating time, less blood loss, quicker postoperative recovery, and higher survival of experimental animals and avoidance of tumor falling off.
The success rate was not 100% in both cases. Anatomy of the mice that failed to bear tumors showed that there was local organ adhesion arising from manipulation, and part of the transplanted tumor tissues stop growing. With our experience, the following points are worthy of mention with regard to factors affecting the success rate: (1) The amount of glue should be appropriate, 1-2 drops are enough, too much glue would envelope the implant, halting its growth; (2) It is best to wait for 10 seconds or so before closing the abdomen so that the glue can coagulate sufficiently; or it may cause extensive adhesion of the surrounding tissues; (3) It is preferable to use the seromuscular layer near the antrum of the greater curvature, because rich blood flow there facilitates tumor growth and metastasis; (4) Rupture of the seromuscular layer should not be too superficial, and bleeding is the hallmark. In addition, the tumor tissue to be implanted should be placed into the ruptured site before dropping OB glue. Smooth forceps can be used to push the rupture inward to form a denture before implanting the tumor tissues, if necessary; and (5) It is not preferable to leave too much suture outside the abdomen to avoid suturing failure due to fierce biting between animals.
Establishment of human gastric cancer nude mouse transplantation models has undergone three representative stages: subcutaneous transplantation of cell suspension, gastric wall seeding of cell suspension, and intra-gastric wall transplantation of histologically intact tissues. The fresh tissue orthotopic transplantation model has two types in method: the “suture method” and the “gastric bursa method”.
Human gastric cancer fresh tissue orthotopic transplantation is the main means of model establishment. But the “suture method” or the “gastric bursa method” transplantation has many practical difficulties and disadvantages in establishing the model. The “OB glue paste technique” was attempted to establish the human gastric cancer nude mouse orthotopic transplantation model.
Observations showed that the “OB glue paste technique” is an ideal means of model establishment owing to its easier manipulation, shorter operating time, less blood loss, quicker postoperative recovery, higher survival of experimental animals and avoidance of tumor falling off.
OB glue paste technique is easy to follow and can be used as an ideal model for experimental research of proliferative metastasis of tumors.
Shi et al established the nude mouse human gastric cancer orthopedic transplantation model using OB glue paste technique. This is an interesting paper. However, several points should be addressed. If possible, the success rate of orthotopic transplantation of SGC-7901 and MKN-45 models should be indicated without using OB glue. If additional effect of OB glue was clarified, this paper would be more convincing.
Peer reviewer: Toru Hiyama, MD, Department of Health Service Center, Hiroshima University, 1-7-1 Kagamiyama, Hiagshihiroshima 7398521, Japan
S- Editor Zhong XY L- Editor Ma JY E- Editor Zhang WB
| 1. | Zhu H, Xian LP, Zhang YM, Li XD, Zou DF. Human Cancer of the Stomach Transplanting Tumour Changes the Model of Nude Mouse Research General Situation. Kexue Jishu Yu Gongcheng. 2007;7:6031-6034. |
| 2. | Takao S, Shimazu H, Maenohara S, Hokita S, Aikou T. Tumorigenicity, invasion, and metastasis of human gastric cancer in nude mice. J Cancer Res Clin Oncol. 1991;117:533-538. |
| 3. | Fidler IJ. Critical factors in the biology of human cancer metastasis: twenty-eighth G.H.A. Clowes memorial award lecture. Cancer Res. 1990;50:6130-6138. |
| 4. | Rygaard J, Povsen CO. Heterotransplantation of a human malignant tumour to "nude" mice. 1969. APMIS. 2007;115:604-606; discussion 607-608. |
| 5. | Illert B, Otto C, Braendlein S, Thiede A, Timmermann W. Optimization of a metastasizing human gastric cancer model in nude mice. Microsurgery. 2003;23:508-512. |
| 6. | Katz MH, Takimoto S, Spivack D, Moossa AR, Hoffman RM, Bouvet M. An imageable highly metastatic orthotopic red fluorescent protein model of pancreatic cancer. Clin Exp Metastasis. 2004;21:7-12. |
| 7. | Yashiro M, Chung YS, Nishimura S, Inoue T, Sowa M. Peritoneal metastatic model for human scirrhous gastric carcinoma in nude mice. Clin Exp Metastasis. 1996;14:43-54. |
| 8. | Yamaguchi K, Ura H, Yasoshima T, Shishido T, Denno R, Hirata K. Liver metastatic model for human gastric cancer established by orthotopic tumor cell implantation. World J Surg. 2001;25:131-137. |
| 9. | Nakanishi H, Mochizuki Y, Kodera Y, Ito S, Yamamura Y, Ito K, Akiyama S, Nakao A, Tatematsu M. Chemosensitivity of peritoneal micrometastases as evaluated using a green fluorescence protein (GFP)-tagged human gastric cancer cell line. Cancer Sci. 2003;94:112-118. |
| 10. | Morikawa K, Walker SM, Nakajima M, Pathak S, Jessup JM, Fidler IJ. Influence of organ environment on the growth, selection, and metastasis of human colon carcinoma cells in nude mice. Cancer Res. 1988;48:6863-6871. |
| 11. | Luo MH, Tang WP, Huang PG, Cai QZ, Li FH, Mo MY. Biological behavior of orthotopic implantation model of human gastric carcinoma in nude mice. Shijie Huaren Xiaohua Zazhi. 1998;6:887-890. |
| 12. | Matsuoka T, Yashiro M, Sawada T, Ishikawa T, Ohira M, Hirakawa K, Chung YS. Effect of a matrix metalloproteinase inhibitor on a lymph node metastatic model of gastric cancer cells passaged by orthotopic implantation. J Exp Clin Cancer Res. 2001;20:213-218. |
| 13. | Furukawa T, Fu X, Kubota T, Watanabe M, Kitajima M, Hoffman RM. Nude mouse metastatic models of human stomach cancer constructed using orthotopic implantation of histologically intact tissue. Cancer Res. 1993;53:1204-1208. |
| 14. | Lin Q, Zhou SF. Establishment of Three Orthotopic Implant and M etastatic Models of Human Stomach Cancer in Nude mice. Jiangsu Daxue Xuebao (medicine). 2004;14:397-399. |
| 15. | Liu P, Zhao ZQ, Zhang XY, Pan SY, Yang GP, Ding XJ. Establishment of Two-orthotopic Implant-metastatic Models of Human Stomach Cancer in Nude Mice. Nanjing Yike Daxue Xuebao (Natural Science). 2003;23:103-105. |
| 16. | Liu P, Zhao ZQ, Zhang XY, Pan SY, Yang GP, Ding XJ. Establishment of Orthotopic Implant/Metastatic Model of Haman Stomach Cancer using Intact Tumor Tissue in Nude Mice. Zhongliu Fangzhi Yanjiu. 2001;28:15-16. |
| 17. | Wang X, Fu X, Brown PD, Crimmin MJ, Hoffman RM. Matrix metalloproteinase inhibitor BB-94 (batimastat) inhibits human colon tumor growth and spread in a patient-like orthotopic model in nude mice. Cancer Res. 1994;54:4726-4728. |
| 18. | Mori T, Fujiwara Y, Yano M, Tamura S, Yasuda T, Takiguchi S, Monden M. Prevention of peritoneal metastasis of human gastric cancer cells in nude mice by S-1, a novel oral derivative of 5-Fluorouracil. Oncology. 2003;64:176-182. |
| 19. | Nishimori H, Yasoshima T, Denno R, Shishido T, Hata F, Okada Y, Ura H, Yamaguchi K, Isomura H, Sato N. A novel experimental mouse model of peritoneal dissemination of human gastric cancer cells: different mechanisms in peritoneal dissemination and hematogenous metastasis. Jpn J Cancer Res. 2000;91:715-722. |
| 20. | Wang FY, Zhu RM, Wang L, Sun JF, Tian XY, Pan ZH. Establishment of orthotopic transplantation of human gastric tumor metastasis model in nude mice. Ai Zheng. 1997;16:343-347. |
| 21. | Liu QZ, Tuo CW, Zhang N, Zhang D, Ming CR. The high metastastic models of human gastric carcinoma established in nude mice by orthotopic transplantation. Zhongguo Xiaohua Waike Zazhi. 2002;1:89-92. |
| 22. | Bai F, Guo X, Yang L, Wang J, Shi Y, Zhang F, Zhai H, Lu Y, Xie H, Wu K. Establishment and characterization of a high metastatic potential in the peritoneum for human gastric cancer by orthotopic tumor cell implantation. Dig Dis Sci. 2007;52:1571-1578. |
| 23. | Xu L, Chen YL, Su XM, Wei PK. Study On Nude Mouse Model of Human Gastric Carcinoma Constructed by Using Orthotopic Transplantation and Their Biological Properties. Zhonghua Zhongliu Fangzhi Zazhi. 2003;10:476-478. |
| 24. | Su XM, Xu L, Chen YL, Zhang T, Wei D, Cheng P, Liu JH. Development of tumor metastasis model by implanting human stomach tumor in situ in nude rats. Di'er Junyi Daxue Xuebao. 2004;25:1042-1043. |
| 25. | Yu ZH, Wei PK, Xu L, Qin ZF, Shi J. Anticancer effect of jinlongshe granules on in situ-transplanted human MKN-45 gastric cancer in nude mice and xenografted sarcoma 180 in Kunming mice and its mechanism. World J Gastroenterol. 2006;12:2890-2894. |
| 26. | Yu ZH, Wei PK, Xu L, Qin ZF, Shi J, Xiao Y, Lin HM. [Effects of jinlongshe granules on apoptosis of MKN-45 human gastric cancer cells orthotopically transplanted in nude mice]. Zhong Xi Yi Jie He Xue Bao. 2006;4:275-280. |
| 27. | Xiao Y, Wei PK, Li J, Shi J, Yu ZH, Lin HM. [Effects of Rhizoma kaempferiae volatile oil on tumor growth and cell cycle of MKN-45 human gastric cancer cells orthotopically transplanted in nude mice]. Zhong Xi Yi Jie He Xue Bao. 2006;4:384-387. |
| 28. | Sun YQ, Zang H, Wang Q, Sun X, Xiao ZB. Establishment and improvement of orthotopic transplantation of human gastric tumor metastasis model in nude mice. Nantong Daxue Xuebao (Medical Sciences). 2005;25:169-170, 173. |
| 29. | Wang N, Wang B, Wang YJ. [Effect of vascular endothelial growth factor antibody Avastin on angiogenesis of human gastric cancer growing orthotopically in nude mice]. Ai Zheng. 2006;25:1076-1081. |
| 30. | Li YJ, He Yan, Wang QM, Liu M, Jin XM. Establishment of Orthotopic Implant M odel of Human Gastric Cancer in Nude Mice Using Glue Paste Technique. Shoulei Xuebao. 2007;15:369-371. |