Published online Aug 14, 2008. doi: 10.3748/wjg.14.4763
Revised: June 19, 2008
Accepted: June 26, 2008
Published online: August 14, 2008
AIM: To evaluate the effect of propolis administration on the healing of colon anastomosis with light and transmission electron microscopes.
METHODS: Forty-eight Wistar-Albino female rats were divided into two groups and had colon resection and anastomosis. In group I, rats were fed with standard rat chow pre- and postoperatively. The rats in group II were fed with standard rat chow and began receiving oral supplementation of propolis 100 mg/kg per day beginning 7 d before the operation and continued until they were sacrificed. Rats were sacrificed 1, 3, 7 and 14 d after operation, and anastomotic bursting pressures measured. After the resection of anastomotic segments, histopathological examination was performed with light and transmission electron microscopes by two blinded histologists and photographed.
RESULTS: The colonic bursting pressures of the propolis group were statistically significantly better than the control group. Ultrastructural histopathological analysis of the colon anastomosis revealed that propolis accelerated the phases of the healing process and stimulated mature granulation tissue formation and collagen synthesis of fibroblasts.
CONCLUSION: Bursting pressure measurements and ultra structural histopathological evaluation showed that administration of propolis accelerated the healing of colon anastomosis following surgical excision.
- Citation: Kilicoglu SS, Kilicoglu B, Erdemli E. Ultrastructural view of colon anastomosis under propolis effect by transmission electron microscopy. World J Gastroenterol 2008; 14(30): 4763-4770
- URL: https://www.wjgnet.com/1007-9327/full/v14/i30/4763.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.4763
| Time | Sham group | Control group | Propolis group | Sham vs Control | Sham vs Propolis | Control vs Propolis |
| 1st day | 214.7 ± 5.50 | 33.8 ± 2.99 | 43.3 ± 1.51 | P = 0.002 | P = 0.002 | P = 0.002 |
| 3rd day | 43.7 ± 1.86 | 52.7 ± 3.33 | P = 0.002 | P = 0.002 | P = 0.002 | |
| 7th day | 146.5 ± 6.53 | 179.8 ± 6.71 | P < 0.001 | P < 0.001 | P < 0.001 | |
| 14th day | 176.7 ± 4.41 | 200.8 ± 7.19 | P < 0.001 | P = 0.002 | P < 0.001 |
Despite the use of optimal surgical techniques and medical treatments, the integrity of intestinal anastomosis may be compromised, resulting in wound dehiscence[1]. The frequency of this complication, which carries a high morbidity and mortality rate, increases if surgery has to be performed in the presence of high-risk situations, such as surgeries performed in emergency settings, on infected and necrotic stumps, in unsafe anatomical areas (rectum, esophagus), or in patients with metabolic derangements[2,3]. If we consider these high-risk situations annihilated, there are several factors in the etiology of colon anastomotic leakage. These factors can be separated into two groups, as systemic (advanced age, malignancy, malnutrition, anemia, hypovolemia, hypoproteinemia) and local (colonic perfusion disorders, septic colonic contents, perfusion impairment at anastomotic line, anastomotic tension, haematoma, inadequate surgical technique, type of preparation prior to surgery and primary colonic pathology)[4,5]. Many studies focused on the detrimental effects of these local and systemic factors. However, in the last century researchers have drawn attention to the cellular mechanisms of the healing and suggested many agents to decrease anastomotic insufficiency and leakage. This experimental study was performed to investigate the cellular ultrastructure of the anastomotic healing process besides to examine the effects of propolis.
Propolis is a resinous material collected by bees from various plants. Bees use material actively secreted by plants or exuded from wounds. Once collected, this material is enriched with salivary and enzymatic secretions of bees. Propolis is used by bees to cover hive walls, fill cracks or gaps, and embalm killed invader insects[6]. It also found its way into a wide spectrum of applications from scolicidal efficacy to cosmetic products such as face creams, ointments, lotions, and solutions[7]. Propolis contains a variety of flavonoids, phenols, alcohols, terpenes, sterols, vitamins, and amino acids[8]. Chemical composition of propolis samples was detected by gas chromatography mass spectrometry (GC-MS) and high-performance liquid chromatography (HPLC)[9]. Antimicrobial properties of propolis seem attributable mainly to the flavonoids, pinocembrin, galangin, and pinobanksin. Pinocembrin also exhibits antifungal properties. Other active compounds are esters of coumaric and caffeic acids. Prenylated p-coumaric and diterpenic acids possess antibacterial and cytotoxic activities. Caffeoylquinic acid derivates show immunomodulatory and hepatoprotective actions and furofuran lignans inhibit the growth of some bacteria. It also has antioxidative, immunostimulative and regenerative properties[6].
According to these properties, we planned to use propolis for determining the effects on the healing of colonic anastomoses in the rats after colonic resection and anastomosis.
Forty-eight Wistar-Albino female rats, weighing 225 ± 25 g were included in this study. Twelve hours before anesthesia, animals were deprived of food, and had free access to water until two hours before anesthesia. No enteral or parenteral antibiotics were administered at all. Rats were housed under constant room temperature (21 ± 2°C) individually in wire cages with a 12-h light-dark cycle. The rats, which died during the experimental period, were excluded from the study and were not replaced with new rats. Additionally, 6 sham-operated rats (no surgery performed on colons) were used to measure basal bursting pressure. The procedures in this experimental study were performed in accordance with the National Guidelines for the Use and Care of Laboratory Animals and approved by the Animal Ethics Committee of Ankara Research and Training Hospital.
Rats were randomly divided into two groups each including 24 animals. Colon resection and anastomosis were performed on all animals. In group I, rats were fed with standard rat chow pre- and postoperatively. The rats in group II were fed with standard rat chow and began receiving oral supplementation of propolis 100 mg/kg per day beginning 7 d before the operation and continued until they were sacrificed. Propolis was given by nasogastric tube everyday at 8 am.
Animals were anesthetized by intramuscular injection of 30 mg/kg ketamine hydrochloride (Ketalar®; Parke-Davis, Istanbul, Turkey) and 5 mg/kg xylazine (Rompun®; Bayer, Istanbul, Turkey). Under sterile conditions, midline laparotomy was performed. The left colon was transected 3 cm proximal to the peritoneal reflection and a 1 cm-long colon segment was resected. End-to-end anastomosis was performed by one-layer, inverted, interrupted sutures by using 6-0 polydioxanone (PDS, Ethicon, UK). The same surgeon, who was unaware of the grouping of each rat, performed all anastomoses. The fascia and skin layers of the abdomen were closed separately with continuous 3-0 silk sutures. Animals had free access to food after the operation. The rats were sacrificed on postoperative day 1, 3, 7, and 14 by high-dose diethyl ether inhalation. The abdominal cavity was inspected through a U-shaped incision.
The anastomotic segment was separated from the surrounding organs. The tissues that adhered to the anastomosis too tightly were not forced away. A colon segment including the anastomosis with 2.5 cm proximal and distal parts was removed. One end of the segment was tied by a 3-0 silk suture. The cannula was inserted into the proximal colonic segment. A catheter was inserted into the other end and tied by 3-0 silk suture to avoid air leaks; it was connected to an infusion pump and to the computer for pressure readings (Logger computer program). Air was pumped into the colon segment at a constant rate of 8 mL/min. The maximum pressure reading before the pressure declined suddenly was recorded as the bursting pressure.
The histopathological analysis of this study was carried out in the Histology and Embryology Department of Ankara University Faculty of Medicine. For light microscopic analyses, a colonic anastomosis-site segment was removed from each rat and the specimens were fixed in 10% neutral buffered formalin solution for 2 d. Tissues were washed in flowing water and dehydrated with increasing concentrations of ethanol (50%, 75%, 96%, and 100%). After dehydration, specimens were put into xylene to obtain transparency and then infiltrated with and embedded in paraffin. Embedded tissues were cut into sections of 5-μm thickness using a Leica RM 2125 RT. Systematically randomly selected sections were stained with hematoxylin and eosin and mallory-azan dyes. Histopathological examinations were performed by two histologists blinded to the study design and photographed with a Nikon eclipse E 600.
For the transmission electron microscope (TEM) analyses, samples were fixed with phosphate buffered (pH 7.3) 2.5% glutaraldehyde and a 2% PFA mixture solution for 2 h at room temperature. They were washed with phosphate-buffered saline solution (PBS, pH 7.3) and fixed with 1% osmium tetraoxide for 2 hours as the secondary fixative. After washing, they were embedded in Araldite 6005 and cut with a Leica EM FCS (Vienna-Austria) ultramicrotome. One micrometer semi-thin sections were stained by toluidine blue-Azur II to select the region of interest for the following procedures. Sixty-seventy nm thin sections were stained with uranil acetate and lead citrate. The sections were examined and photographed using a LEO 906 E TEM (80 kV, Oberkochen, Germany) microscope.
Data analysis was performed using the SPSS (Statistical Package for Social Science, version 11.5) software package. Whether the bursting pressure measurements were normally distributed or not was determined by using the Shapiro Wilk test. Descriptive statistics were shown as mean ± standard deviation (SD). The differences among groups and days were evaluated by one-way ANOVA post-hoc Tukey test or Kruskal Wallis test, where appropriate. When the P-value from the Kruskal-Wallis test statistics was statistically significant, Bonferroni adjusted Mann Whitney U test was used to know which groups or days differ from which others. Bonferroni correction was applied for all possible within group or day comparisons. A P value less than 0.05 was considered statistically significant.
The rats were sacrificed on postoperative day 1, 3, 7, and 14. One rat from each group died in the early postoperative period, probably due to anesthesia. On gross evaluation, no signs of anastomotic leakage, intraabdominal abscess, peritonitis, or ileus were detected.
Anastomotic wound healing was evaluated by means of bursting pressure and histopathological assessment. The biggest P value among the P values as P < 0.001 was found as 5.6E-6. To obtain a standardized projection the notation was prefered as P < 0.001 for most of the P values. The colonic bursting pressures of the propolis group were significantly better than the control group (Table 1).
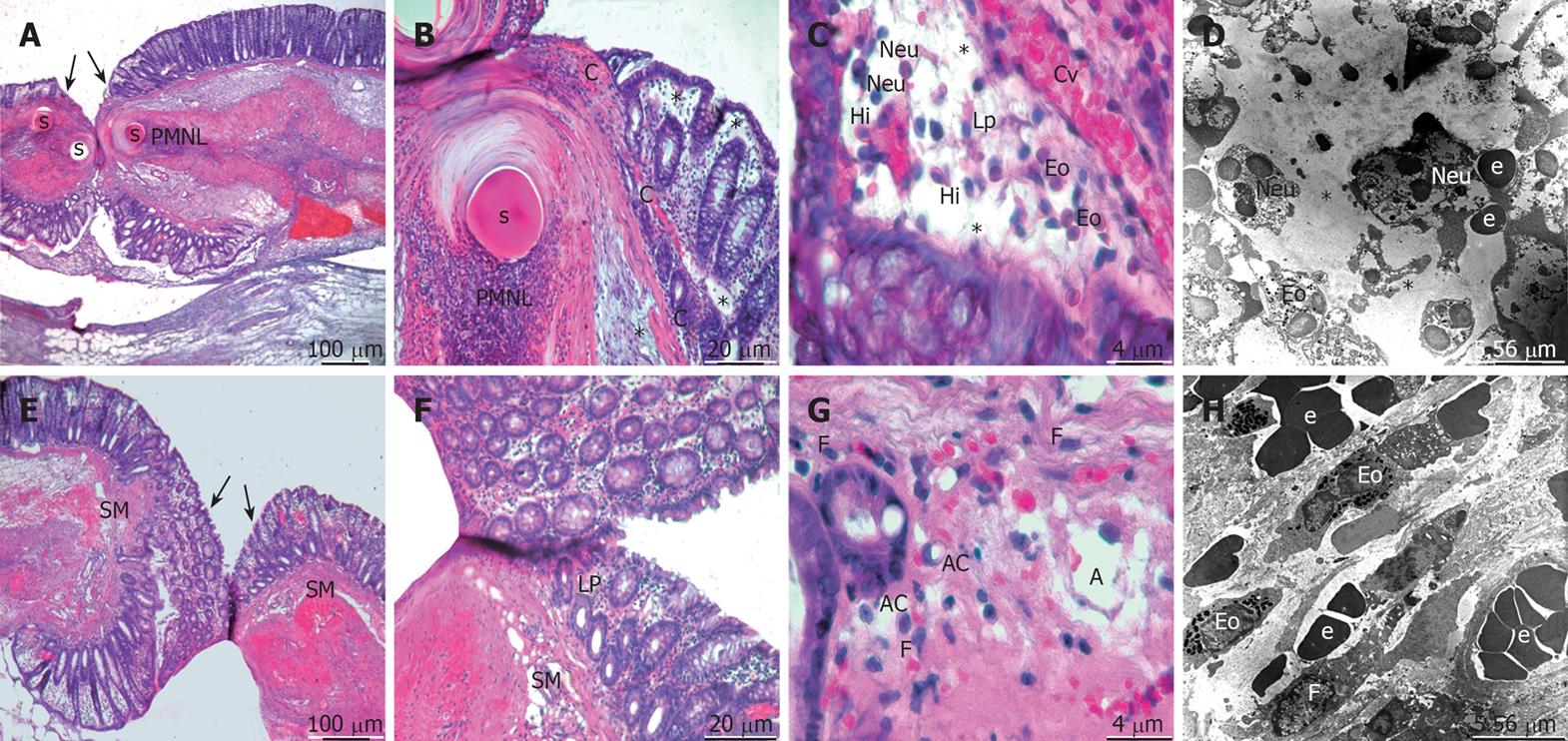
On the first postoperative day of the control group, healing began with a strong influx of polymorphonuclear leucocytes (PMNL), most prominent in the anastomotic line. There was a massive infiltration of neutrophils, eosinophils, and histiocytes in the lamina propria, submucosa, and the area surrounding the suture material. The subepithelial region of the mucosal layer and the submucosa were highly congested and edematous. The subepithelial edema was expanded to the wide adjacent area of the anastomotic line (Figure 1A-D). In the propolis group, the subepithelial edema was not striking and not expanded very far from the anastomotic line. Congestion and infiltration of PMNL was also present; additionally the fibroblasts’ migration became apparent in the wound edges and around the suture material. The thing to note was that angiogenesis became visible in focal areas of the submucosa (Figure 1E-H).
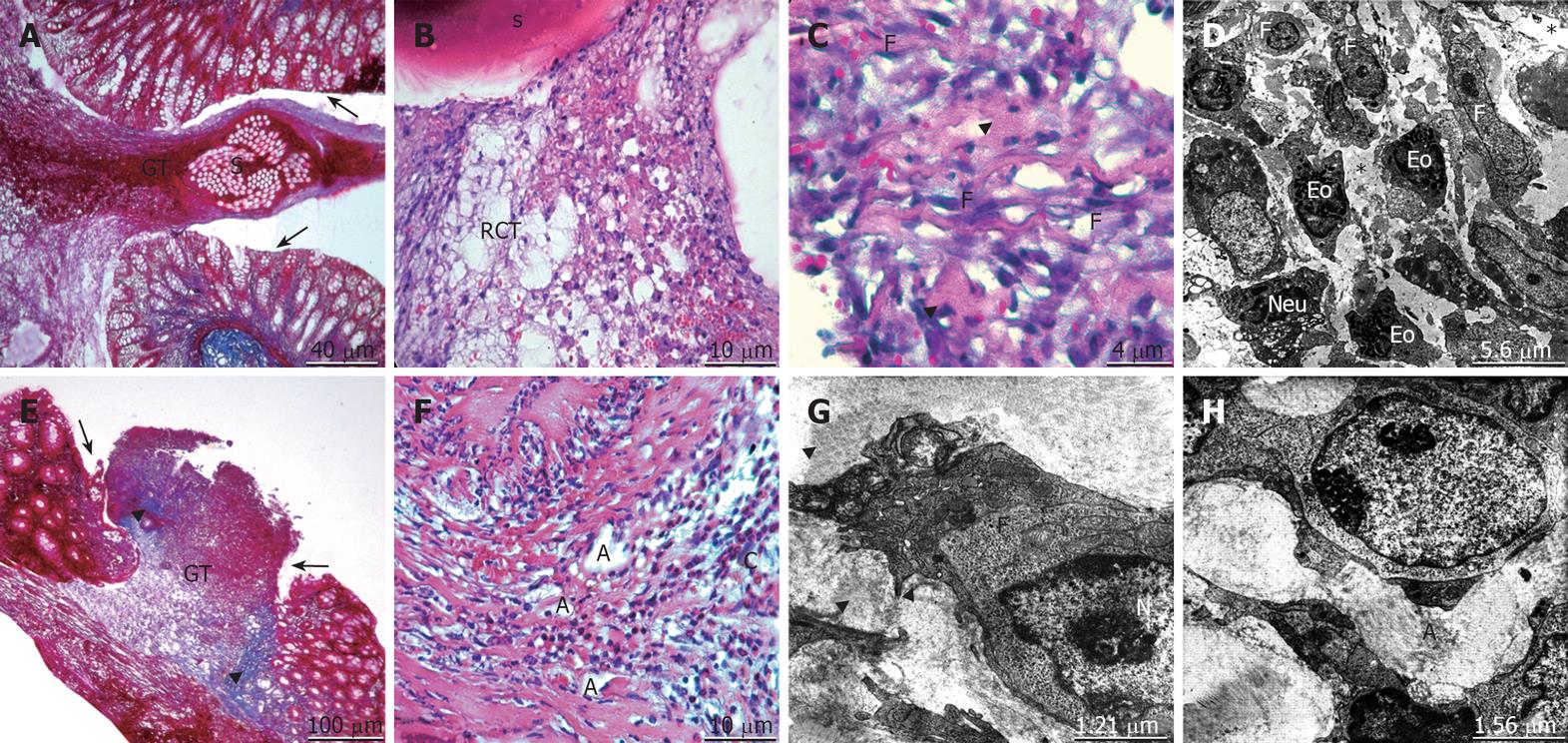
On the third postoperative day, edema of the control group was persistent. The existence of PMNL and a slight increase in the lymphocyte infiltration was seen. The hemorrhage, inflammation and the intensity of eosinophils in the lamina propria were recognizable. The granulation tissue formation and the resorption of the suture material were observed. The reticular connective tissue formation by fibroblasts and the wrapping of this tissue around the suture line were evident (Figure 2A-D). In the propolis group, the edema and the swelling of the tissue ends regressed. The fibroblastic activity and the synthesis of new collagen were observed. Angiogenesis, a component of granulation tissue formation, was prominent. The spatial arrangement of collagen fibers in the submucosal layer and inclining towards the anastomotic line to form bridging of the gap was recognizable (Figure 2E-H).
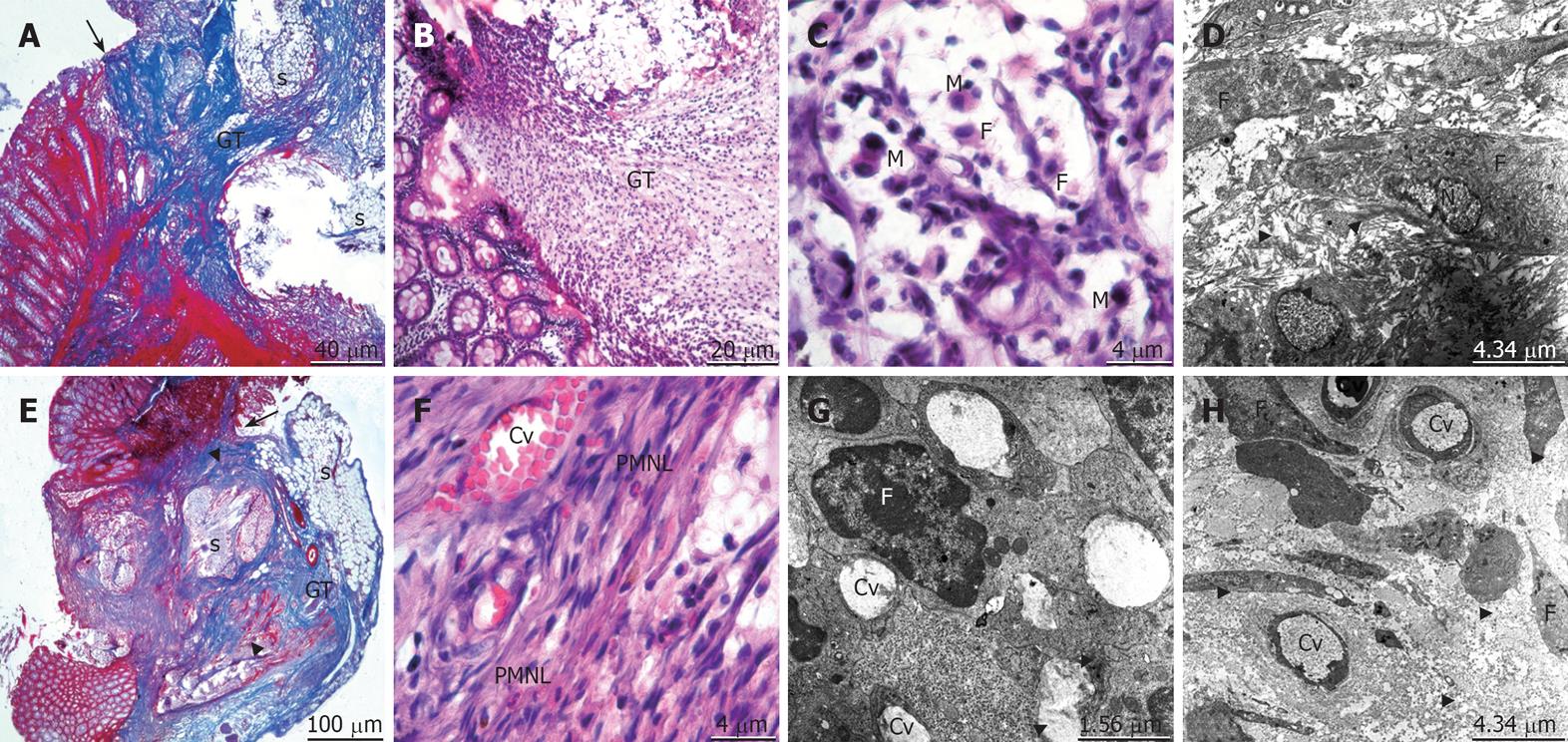
On the seventh postoperative day, edema of the control group had almost disappeared. The granulation tissue was well organized and wrapped around the suture material particles. The collagen formation with increased fibroblastic activity was present but irregular. The macrophage infiltration removing the debris was observed (Figure 3A-D). In the propolis group, a very small tissue defect was still visible in the mucosal layer. Many bands of collagen fibers and well-developed capillary vessels were distinguished. The collagen bundles were well arranged to form the bridging between mucosal and submucosal layers. The macrophage cells clearing off the debris and the mature cells of the connective tissue as fibrocytes were easily viewed (Figure 3E-H).
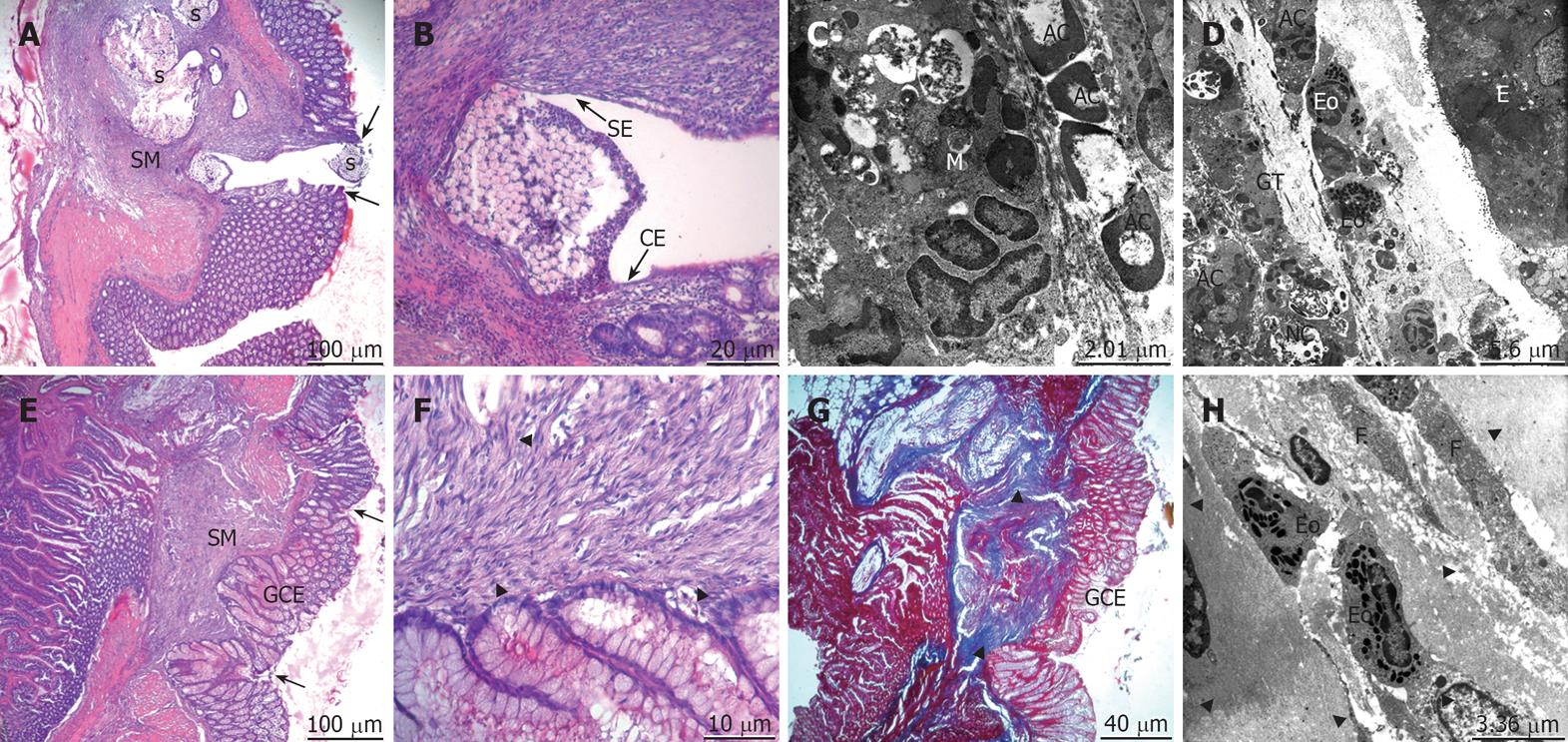
On the fourteenth postoperative day for the control group, the mucosal integrity was still not gained. There was a very small (< 1 mm) tissue defect between the anastomotic ends. The regeneration of the epithelium and the mucosa only partially covered by simple squamous and cuboidal epithelium was observed. The apoptotic cells and the macrophages clearing the debris were still remarkable. The granulation tissue and the epithelium of the anastomotic ends were very close (Figure 4A-D). In the propolis group, the mucosa had completely healed. The regeneration of epithelium was with glandular columnar epithelium. The lamina propria of the anastomotic line under the restored epithelium was cleaned out from the debris. There was a dense collagen deposition in the submucosal layer. The collagen bundles were regular and harmoniously organized (Figure 4E-H).
Leakage of anastomoses is a very serious and severe complication in gastrointestinal surgery. Morbidity and mortality rates are still high despite advances in operative techniques and suture materials. Therefore, basic research on the mechanisms of intestinal healing and the factors affecting it is very crucial[10]. Even though wound healing is a complex and dynamic process, it can be divided into three distinct, but at the same time overlapping phases of (1) hemostasis and inflammation, (2) proliferation, and (3) maturation and remodeling[2]. Intestinal anastomotic healing differs slightly from the general healing process, as it is a controlled full-thickness injury, followed by reconstitution of luminal integrity with artificial sutures. During this period, an organized and complex cascade of cellular and biochemical events take place, and many disturbances in any phase of this healing process may result in complications[11].
The first phase of the healing, described as the inflammatory phase is seen on the first 3 d of the healing process. As Steed and Portera et al reported, this is the essential phase in which the polymorphonuclear leucocytes (PMNL) migrate from the circulation to the wound[12,13]. It is really an essential phase because of the large microorganism content of the colonic lumen. During the first three-day period, we found the regressive effect of propolis on edema might be by decreasing the level of IL-5 as Leticia et al reported[14]. The cellular content on the first day were supporting the regression of edema with the rare number of eosinophils. Marcucci and later Krol et al studied the antiinflammatory activity of propolis and 19 phenolic compounds[15,16]. Also supporting these observations, we found in our study that, while in the control group the PMNLs were leading on the first and third postoperative days, in the propolis group proliferation of fibroblasts began immediately following the decrease in the amount of PMNLs.
Extensive bacterial invasion was well known to impair healing at anastomotic sites. This is most likely secondary to increased PMNLs invasion and activation by microorganisms. Granulocyte collagenase activity of PMNLs’ has been implicated at increasing collagen breakdown and delaying the anastomotic healing process. The potential protective effect of propolis may be suggested by our findings of early decreased number of PMNLs and less inflammatory reaction at the anastomotic line and further away during first and third day[17,18].
Propolis has a constructive antibacterial effect besides antiinflammatory properties on bacterial invasion, which plays a crucial role in the healing process. There are a number of studies documenting the antimicrobial functions of propolis, its extracts, and constituents. This is a broad-spectrum activity against Gram-positive and Gram-negative rods and cocci, and viruses[19]. Park et al tested antimicrobial activity on oral microorganisms[20]; and Sosa et al observed that propolis samples inhibited the growth of some gram-positive bacteria and Candida albicans[21]. Kujumgiev et al further examined the antibacterial, antifungal, and antiviral activity of propolis from different geographical origins. All the propolis samples were active against the fungal and gram-positive bacterial test strains, and most showed antiviral activity. The antibacterial activities of these samples were very similar, despite differences in their chemical composition[22]. We had also shown its protective effect on ileal mucosa and reduced bacterial translocation in an experimental obstructive jaundice model in our previous researches[23]. Although the chemical composition of propolis varies depending on the site of its collection, antimicrobial properties seem attributable mainly to the flavonoids pinocembrin, galangin, and pinobanksin[24]. Other active compounds are esters of coumaric acid and caffeic acids. Phenylated p-coumaric and diterpenic acids possess antibacterial and cytotoxic activities. Caffeoylquinic acid derivatives show immunomodulatory and hepatoprotective actions and furofuran lignans inhibit the growth of some bacteria[25].
Propolis has been used in the treatment of cutaneous lesions such as burns, wounds, and ulcers. Morales et al[26] used a hypoallergic formula of propolis and obtained a very satisfactory evolution and cicatrisation in cases of wounds with and without infection. A fast cure, shorter treatment period, and less septic complications were also obtained. The cicatrisation was evident between the 4th and 5th day by the early formation of granulation tissue. The antimicrobial capacity was evident with a fast regression of the septic component of the suppurated wound. Claus et al reported that, propolis also exhibits immunostimulatory and immunomodulatory effects in vitro on macrophages[27], while Kimoto et al reported it increases the ratio of CD4/CD8 T cells in vivo in mice[28]. An intact cellular immune response, particularly of T lymphocytes, is not necessary for the initial phase of wound healing but seems to be essential for a normal outcome of tissue repair. In our study, the propolis group indicated that lymphocytes appear earlier in contrast with the control group. Propolis exerts a wound healing effect by minimizing acute inflammatory exudate and stimulating macrophage and CD4 T lymphocyte activity.
In this study, angiogenesis was remarkable in the propolis group beginning on the third postoperative day; unfortunately, the capillaries were poor in the control group. We know that oxygen is critical for anastomotic wound healing[2]. The exact cellular and molecular mechanisms caused by hypoxia, which results in impaired anastomotic healing, are not well understood. While hypoxia may act as an early stimulus for neovascularization, it is unlikely to sustain it. The process of angiogenesis, whereby new capillaries are formed from pre-existing blood vessels, is essential for wound healing[29]. At the molecular level of angiogenesis, vascular endothelial growth factor (VEFG) and nitric oxide (NO) are the two key factors that are relevant[30]. Tan-no et al reported the anti-inflammatory effect of propolis through inhibition of nitric oxide production[31] and also Attard et al reported the expression of both VEGF and inducible nitric oxide synthase (iNOS) significantly increased at the anastomotic site exposed to a hypoxic environment but the healing was not enhanced[32]. These two hypotheses above warrant further evaluation. The molecular mechanisms of angiogenesis and the reflection of it to the healing process are not clearly understood yet; however, we definitely observed new capillary formation in the early period of the propolis group.
Propolis mostly contains flavonoids and phenolic compounds, which have been reported to have antioxidative properties. Due to its antioxidative effects, propolis may protect humans from deleterious oxidative processes. Several groups of authors thus studied the antioxidative properties of propolis and their active constituents[33-35]. Propolis may also act as a scavenger against oxygen radicals. Recent studies indicate that propolis is able to inhibit the formation of superoxide anion, which is produced during autooxidation of β-mercaptoethanol[25].
In the healing process, the first phase of inflammation is followed by the second phase, fibroblastic proliferation. Fibroblasts are normally found in the later phases of normal wound healing. They are responsible for the production of collagen and for establishing the structural extracellular matrix. Although the healing process can be divided into phases as we mentioned before, they are overlapping. The maturation and remodeling phase following the fibroblastic proliferation are very close to each other. Because remodeling of tissue requires the deposition of adequate amounts of extracellular matrix components, particularly collagen fibers, in the wound area. As comprehended, fibroblasts have a key position. Graham et al reported, in contrast to dermal tissue, both smooth muscle cells and fibroblasts manufacture collagen in the gastrointestinal tract[36]. We know that the bulk of collagen within the intestinal tract is contained within the submucosa since intestinal smooth muscle cells in the lamina propria produce and maintain intestinal collagen. As a result, most of the strength of the intestine is found in the submucosal layer. Furthermore, it is responsible for anchoring the sutures that hold anastomosed bowel ends together[11].
Simpson and Ross reported that, as early as 3 d after wounding, immature fibroblasts could be identified within the wound exudate[37]. We observed the fibroblasts on the third postoperative day of the control group, but interestingly, they were present on the first postoperative day of the propolis group. On the first day, possibly they were immature fibroblasts, but on the third day as these cells matured, they acquired the characteristic ultrastructural features of actively synthetic fibroblasts. By day 5, small collagen fibers were observable by electron microscopy within the extracellular spaces; the collagen fibers were distinguishable on the third postoperative day of the propolis group. The collagen fibers were in a spatial arrangement and inclining towards the anastomotic line.
Although healing in the gastrointestinal tract essentially follows the same phases as skin wound healing, our understanding of these events is much more limited. Just as with dermal reepithelialization, if the mucosa is the only injured layer, it can be reformed by migration and proliferation. Full-thickness injury provokes a fibroblastic response resulting in scar formation[38]. During the first few postoperative days, anastomotic strength is low, as collagen is degraded secondary to collagenase activity at the site of the injury. Early anastomotic strength is therefore dependent on the suture-holding capacity of existing collagen until large amounts of new collagen can be synthesized by both fibroblasts and smooth muscle cells. The final phase of healing involves maturation of newly formed anastomosis by the transformation of collagen into thick bundles and contractile units[39]. In the propolis group, the proliferation, activation, and synthesis capacity of fibroblasts were much better than the control group. As expected, the bridging of the gap between the anastomotic edges as the means of remodeling was completed.
All of the bee products, especially honey, have had a valued place in traditional medicine for centuries. In the last century, researchers rediscovered this old remedy and honey has been of proven value in many pathologies and also in the surgical wound. Honey is a supersaturated sugar solution which the half of it’s content is fructose and glucose with other chemical compounds in small quantities like flavonoids and phenolics. Consequently as a bee product, propolis (100 mg/kg per day) is recommended in this study because it is more effective than the same doses of honey (10 g/kg per day)[7,40].
In conclusion, the results of this study clearly demonstrate that, according to the histopathological parameters, administration of propolis, as an additional strategy to surgical excision, accelerated the healing of colon anastomosis. Propolis, a beehive product, possesses a broad spectrum of biological activities including hepatoprotective, antitumour, antioxidative, antimicrobial, and antiinflammatory effects. Current opinion is that the use of standardized preparations of propolis is safe and less toxic than many synthetic products. Our results suggest that propolis enhances not only the early phase of colon anastomotic healing by inhibiting the inflammatory response, but also stimulates the collagen synthesis of fibroblasts. The exact mechanisms of improved anastomotic wound healing by propolis administration are not known, although increase of neoangiogenesis, mononuclear cell infiltration, and the stimulation of collagen synthesis are all involved. These findings may provide insight into potential therapeutic approaches to improve and promote healing of colonic anastomosis and to minimize the morbidity and mortality associated with anastomotic leaks. We conclude that propolis should be further investigated for its capacity to enhance anastomotic healing and to reduce the incidence of the anastomotic dehiscence.
Inadequate healing of colon anastomosis may still be a cause of postoperative morbidity and mortality despite the use of optimal surgical techniques.
This study was to evaluate the effect of propolis administration on the healing of colon anastomosis with light and transmission electron microscopes.
The achievement of this article is to show the stages of anatomotic healing with clear light and electron micrographs without any doubt and draw attention to the cellular mechanisms of the healing. The breakthrough of our experience from the present study is that: propolis which is a natural, cheap and easily obtained material can be used safely in colonic anastomosis.
Administration of propolis, as an additional strategy to surgical excision, might be used to accelerate the healing of colon anastomosis and to prevent the anastomotic leakage, in clinical settings.
Our results suggest that propolis enhances not only the early inflammatory phase of colon anastomotic healing but also stimulates the collagen synthesis of fibroblasts. These findings may provide a new insight into potential therapeutic approaches to improve and promote healing of colonic anastomosis.
Peer reviewer: Marc Basson, MD, PhD, MBA, Chief of Surgery, John D. Dingell VA Medical Center, 4646 John R. Street, Detroit, MI 48301, United States
S- Editor Zhong XY L- Editor Alpini GD E- Editor Lin YP
| 1. | Fielding LP, Stewart-Brown S, Blesovsky L, Kearney G. Anastomotic integrity after operations for large-bowel cancer: a multicentre study. Br Med J. 1980;281:411-414. |
| 2. | Thornton FJ, Barbul A. Healing in the gastrointestinal tract. Surg Clin North Am. 1997;77:549-573. |
| 3. | Nursal TZ, Anarat R, Bircan S, Yildirim S, Tarim A, Haberal M. The effect of tissue adhesive, octyl-cyanoacrylate, on the healing of experimental high-risk and normal colonic anastomoses. Am J Surg. 2004;187:28-32. |
| 4. | Akgun A, Kuru S, Uraldi C, Tekin O, Karip B, Tug T, Ongoren AU. Early effects of fibrin sealant on colonic anastomosis in rats: an experimental and case-control study. Tech Coloproctol. 2006;10:208-214. |
| 5. | Golub R, Golub RW, Cantu R Jr, Stein HD. A multivariate analysis of factors contributing to leakage of intestinal anastomoses. J Am Coll Surg. 1997;184:364-372. |
| 6. | Dimov V, Ivanovska N, Manolova N, Bankova V, Nikolov N, Popov S: Immunomodulatory action of propolis, influence on anti-infectious protection and macrophage function. Apidologie. 1991;22:55-162. |
| 7. | Kismet K, Kilicoglu B, Koru O, Tanyuksel M, Oruc MT, Sorkun K, Salih B, Akkus MA. Evaluation on scolicidal efficacy of propolis. Eur Surg Res. 2006;38:476-481. |
| 8. | Silici S, Kutluca S. Chemical composition and antibacterial activity of propolis collected by three different races of honeybees in the same region. J Ethnopharmacol. 2005;99:69-73. |
| 9. | Bruschi ML, Franco SL. Gremiao MPD: Application of an HPLC method for analysis of propolis extract. J Liq Chromatogr. 2003;26:2399-2240. |
| 10. | Verhofstad MH, Lange WP, van der Laak JA, Verhofstad AA, Hendriks T. Microscopic analysis of anastomotic healing in the intestine of normal and diabetic rats. Dis Colon Rectum. 2001;44:423-431. |
| 11. | Kuzu MA, Kuzu I, Koksoy C, Akyol FH, Uzal D, Kale IT, Orhan D, Terzi C. Histological evaluation of colonic anastomotic healing in the rat following preoperative 5-fluorouracil, fractionated irradiation, and combined treatment. Int J Colorectal Dis. 1998;13:235-240. |
| 12. | Portera CA, Love EJ, Memore L, Zhang L, Muller A, Browder W, Williams DL. Effect of macrophage stimulation on collagen biosynthesis in the healing wound. Am Surg. 1997;63:125-131. |
| 13. | Steed DL. The role of growth factors in wound healing. Surg Clin North Am. 1997;77:575-586. |
| 14. | Sy LB, Wu YL, Chiang BL, Wang YH, Wu WM. Propolis extracts exhibit an immunoregulatory activity in an OVA-sensitized airway inflammatory animal model. Int Immunopharmacol. 2006;6:1053-1060. |
| 15. | Marcucci MC. Propolis: chemical composition, biological properties and therapeutic activity. Apidologie. 1995;26:83-99. |
| 16. | Krol W, Scheller S, Czuba Z, Matsuno T, Zydowicz G, Shani J, Mos M. Inhibition of neutrophils' chemiluminescence by ethanol extract of propolis (EEP) and its phenolic components. J Ethnopharmacol. 1996;55:19-25. |
| 17. | Egger B, Tolmos J, Procaccino F, Sarosi I, Friess H, Buchler MW, Stamos M, Eysselein VE. Keratinocyte growth factor promotes healing of left-sided colon anastomoses. Am J Surg. 1998;176:18-24. |
| 18. | Hendriks T, Vereecken TH, Hesp WL, Schillings PH, de Boer HH. Loss of collagen from experimental intestinal anastomoses: early events. Exp Mol Pathol. 1985;42:411-418. |
| 19. | Banskota AH, Tezuka Y, Kadota S. Recent progress in pharmacological research of propolis. Phytother Res. 2001;15:561-571. |
| 20. | Park YK, Koo MH, Abreu JA, Ikegaki M, Cury JA, Rosalen PL. Antimicrobial activity of propolis on oral microorganisms. Curr Microbiol. 1998;36:24-28. |
| 21. | Sosa S, Bornancin A, Tubaro A, Loggia RD. Topical antiinflammatory activity of an innovative aqueous formulation of actichelated propolis vs two commercial propolis formulations. Phytother Res. 2007;21:823-826. |
| 22. | Kujumgiev A, Tsvetkova I, Serkedjieva Y, Bankova V, Christov R, Popov S. Antibacterial, antifungal and antiviral activity of propolis of different geographic origin. J Ethnopharmacol. 1999;64:235-240. |
| 23. | Sabuncuoglu MZ, Kismet K, Kilicoglu SS, Kilicoglu B, Erel S, Muratoglu S, Sunay AE, Erdemli E, Akkus MA. Propolis reduces bacterial translocation and intestinal villus atrophy in experimental obstructive jaundice. World J Gastroenterol. 2007;13:5226-5231. |
| 24. | Burdock GA. Review of the biological properties and toxicity of bee propolis (propolis). Food Chem Toxicol. 1998;36:347-363. |
| 25. | Castaldo S, Capasso F. Propolis, an old remedy used in modern medicine. Fitoterapia. 2002;73 Suppl 1:S1-S6. |
| 26. | Morales WF, Garbarino JL. Clinical evaluation of a new hypoallergic formula of propolis in dressings. Bee products: Properties, Application and Apitherapy. New York: Plenum Press 1997; 101-105. |
| 27. | Claus R, Kinscherf R, Gehrke C, Bonaterra G, Basnet P, Metz J, Deigner HP. Antiapoptotic effects of propolis extract and propol on human macrophages exposed to minimally modified low density lipoprotein. Arzneimittelforschung. 2000;50:373-379. |
| 28. | Kimoto T, Arai S, Kohguchi M, Aga M, Nomura Y, Micallef MJ, Kurimoto M, Mito K. Apoptosis and suppression of tumor growth by artepillin C extracted from Brazilian propolis. Cancer Detect Prev. 1998;22:506-515. |
| 29. | Gelaw B, Levin S. Wound-induced angiogenesis and its pharmacologic inhibition in a murine model. Surgery. 2001;130:497-501. |
| 30. | Li J, Zhang YP, Kirsner RS. Angiogenesis in wound repair: angiogenic growth factors and the extracellular matrix. Microsc Res Tech. 2003;60:107-114. |
| 31. | Tan-No K, Nakajima T, Shoji T, Nakagawasai O, Niijima F, Ishikawa M, Endo Y, Sato T, Satoh S, Tadano T. Anti-inflammatory effect of propolis through inhibition of nitric oxide production on carrageenin-induced mouse paw edema. Biol Pharm Bull. 2006;29:96-99. |
| 32. | Attard JA, Raval MJ, Martin GR, Kolb J, Afrouzian M, Buie WD, Sigalet DL. The effects of systemic hypoxia on colon anastomotic healing: an animal model. Dis Colon Rectum. 2005;48:1460-1470. |
| 33. | Katalinic V, Radic S, Ropac D, Mulic R, Katalinic A. [Antioxidative activity of propolis from Dalmatia (Croatia)]. Acta Med Croatica. 2004;58:373-376. |
| 34. | Marquele FD, Stracieri KM, Fonseca MJ, Freitas LA. Spray-dried propolis extract. I: physicochemical and antioxidant properties. Pharmazie. 2006;61:325-330. |
| 35. | Sun F, Hayami S, Haruna S, Ogiri Y, Tanaka K, Yamada Y, Ikeda K, Yamada H, Sugimoto H, Kawai N. In vivo antioxidative activity of propolis evaluated by the interaction with vitamins C and E and the level of lipid hydroperoxides in rats. J Agric Food Chem. 2000;48:1462-1465. |
| 36. | Graham MF, Drucker DE, Diegelmann RF, Elson CO. Collagen synthesis by human intestinal smooth muscle cells in culture. Gastroenterology. 1987;92:400-405. |
| 37. | Simpson DM, Ross R. The neutrophilic leukocyte in wound repair a study with antineutrophil serum. J Clin Invest. 1972;51:2009-2023. |
| 38. | Witte MB, Barbul A. Repair of full-thickness bowel injury. Crit Care Med. 2003;31:S538-S546. |
| 39. | Thompson SK, Chang EY, Jobe BA. Clinical review: Healing in gastrointestinal anastomoses, part I. Microsurgery. 2006;26:131-136. |