Published online Aug 14, 2008. doi: 10.3748/wjg.14.4745
Revised: June 16, 2008
Accepted: June 23, 2008
Published online: August 14, 2008
AIM: To re-evaluate the diagnostic criteria of insulin resistance hepatic iron overload based on clinical, biochemical and histopathological findings.
METHODS: We studied 81 patients with hepatic iron overload not explained by known genetic and acquired causes. The metabolic syndrome (MS) was defined according to ATPIII criteria. Iron overload was assessed by liver biopsy. Liver histology was evaluated by Ishak’s score and iron accumulation by Deugnier’s score; steatosis was diagnosed when present in ≥ 5% of hepatocytes.
RESULTS: According to transferrin saturation levels, we observed significant differences in the amount of hepatic iron overload and iron distribution, as well as the number of metabolic abnormalities. Using Receiving Operating Curve analysis, we found that the presence of two components of the MS differentiated two groups with a statistically significant different hepatic iron overload (P < 0.0001). Patients with ≥ 2 metabolic alterations and steatosis had lower amount of hepatic iron, lower transferrin saturation and higher sinusoidal iron than patients with < 2 MS components and absence of steatosis.
CONCLUSION: In our patients, the presence of ≥ 2 alterations of the MS and hepatic steatosis was associated with a moderate form of iron overload with a prevalent sinusoidal distribution and a normal transferrin saturation, suggesting the existence of a peculiar pathogenetic mechanism of iron accumulation. These patients may have the typical dysmetabolic iron overload syndrome. By contrast, patients with transferrin saturation ≥ 60% had more severe iron overload, few or no metabolic abnormalities and a hemochromatosis-like pattern of iron overload.
- Citation: Riva A, Trombini P, Mariani R, Salvioni A, Coletti S, Bonfadini S, Paolini V, Pozzi M, Facchetti R, Bovo G, Piperno A. Revaluation of clinical and histological criteria for diagnosis of dysmetabolic iron overload syndrome. World J Gastroenterol 2008; 14(30): 4745-4752
- URL: https://www.wjgnet.com/1007-9327/full/v14/i30/4745.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.4745
| HIO | ||||||
| HIO (n = 81) | P | HFE-HH (n = 57) | Men (n = 67) | P | Women (n = 14) | |
| Age (yr) | 52 (28-76) | > 0.05 | 50 (26-71) | 54 (28-76) | > 0.05 | 51 (29-70) |
| Transferrin saturation (%) | 39 (21-118) | < 0.0001 | 76 (29-100) | 38 (21-118) | 0.02 | 56 (28-99) |
| Serum ferritin (μg/L) | 982 (413-5050) | > 0.05 | 1100 (274-10 990) | 964 (413-5050) | > 0.05 | 1286 (520-3987) |
| AST (U/L) | 27 (12-174) | > 0.05 | 27 (17-87) | 27 (12-174) | > 0.05 | 28 (13-65) |
| ALT (U/L) | 38 (11-271) | > 0.05 | 43 (11-114) | 38 (14-271) | > 0.05 | 36 (11-118) |
| Total Iron Score | 19 (12-46) | 0.0071 | 24 (8-49) | 18 (12-40) | 0.0006 | 32 (12-46) |
| Hepatocytic iron score | 15 (6-27) | 0.001 | 18 (3-27) | 12 (6-27) | 0.0022 | 21 (9-27) |
| Sinusoidal iron score | 5 (3-8) | < 0.0001 | 3 (0-10) | 5 (3-7) | > 0.05 | 6 (3-8) |
| Portal iron score | 0 (0-12) | 0.0002 | 2 (0-12) | 0 (0-8) | < 0.0001 | 3.5 (0-12) |
| SIS/TIS | 0.25 (0.09-0.5) | < 0.0001 | 0.13 (0-0.63) | 0.25 (0.10-0.5) | 0.001 | 0.16 (0.09-0.29) |
| HHII | 0.38 (0.20-1.21) | 0.0012 | 0.53 (0.15-0.97) | 0.36 (0.20-1.08) | 0.004 | 0.64 (0.23-1.21) |
| Hepatic fibrosis (Ishak’s score) | 34 (42) | > 0.05 | 33 (57.8) | 26 (38.8) | > 0.05 | 8 (57.1) |
| Hepatic cirrhosis (Ishak’s score) | 7 (8.6) | 0.027 | 13 (22.8) | 3 (4.5) | 0.015 | 4 (28.6) |
| Patients with hepatic steatosis | 47 (58) | 0.015 | 20 (36.3) | 41 (61.2) | > 0.05 | 6 (42.9) |
| Patients with moderate-severe hepatic steatosis | 35 (43.2) | < 0.0001 | 6 (10.5) | 31 (46.3) | > 0.05 | 4 (28.6) |
| Metabolic syndrome | 20 (24.7) | > 0.05 | 91 (16.1) | 19 (28.4) | > 0.05 | 1 (7.1) |
| r | P | |
| Hepatocytic iron score | 0.94 | < 0.0001 |
| Sinusoidal iron score | 0.54 | < 0.0001 |
| Portal iron score | 0.58 | < 0.0001 |
| Serum ferritin (μg/L) | 0.53 | < 0.0001 |
| Transferrin saturation (%) | 0.43 | < 0.0001 |
| Age (yr) | 0.23 | 0.04 |
| Hepatic fibrosis | 0.28 | 0.012 |
| Body mass index (kg/m2) | -0.48 | < 0.0001 |
| Degree of hepatic steatosis | -0.53 | < 0.0001 |
| No. of components of MS | -0.33 | 0.0026 |
| HOMA index | -0.3 | 0.0125 |
| r | P | |
| Age (yr) | 0.3 | 0.0055 |
| Degree of hepatic steatosis (%) | 0.57 | < 0.0001 |
| HOMA index | 0.59 | < 0.0001 |
| Transferrin saturation (%) | -0.25 | 0.0293 |
| Total iron score | -0.33 | 0.0053 |
| Histological hepatic iron index | -0.53 | < 0.0001 |
| Sinusoidal iron score/Total iron score | 0.13 | > 0.05 |
| Number of MS components | Hepatic steatosis | |||||
| 0-1 (n = 34) | P | ≥2 (n = 47) | Absent (n = 34) | P | Present (n = 47) | |
| Transferrin saturation (%) | 44 (28-118) | 0.0058 | 36 (21-99) | 39 (23-118) | > 0.05 | 39 (21-76) |
| Total Iron Score | 24 (12-46) | 0.0008 | 17 (12-39) | 24.5 (12-46) | < 0.0001 | 16 (12-39) |
| HHII | 0.51 (0.3-1.21) | < 0.0001 | 0.33 (0.2-0.57) | 0.43 (0.23-1.1) | 0.0004 | 0.34 (0.19-1.2) |
| SIS/TIS | 0.21 (0.1-0.5) | 0.0185 | 0.25 (0.11-0.4) | 0.22 (0.1-0.5) | 0.032 | 0.25 (0.09-0.4) |
| MS components ≥ 2 | - | - | 11 (32) | < 0.0001 | 36 (77) | |
| Degree of hepatic steatosis (%) | 0 (0-80) | < 0.0001 | 40 (0-98) | - | - | |
Iron overload includes a wide spectrum of conditions from mild to marked tissue iron accumulation and iron-related organ dysfunctions[1-3]. Apart from the known hereditary and acquired conditions of iron overload[4], there are other conditions in which iron overload cannot be explained and the pathophysiology of iron accumulation is still obscure. Increased hepatic iron deposits have been frequently described in association with overweight and alterations of lipid or glucose metabolism. These abnormalities are part of the metabolic syndrome (MS) that is closely linked to insulin resistance, affecting a large number of adults in Western countries, and is associated with non-alcoholic fatty liver disease (NAFLD), now considered the hepatic manifestation of MS[5]. This form of iron overload has been variably named dysmetabolic or insulin resistance hepatic iron overload syndrome (IR-HIO)[6]. However, the etiopathogenetic mechanisms that link metabolic abnormalities, insulin resistance and/or NAFLD to iron accumulation are still undefined. The fact that only a proportion of subjects with metabolic abnormalities are at risk for iron overload[7] suggests that other factors may be involved. In addition, the original definition of IR-HIO[6] includes hyperferritinemia with normal or high transferrin saturation, presence or absence of NAFLD, and even a single metabolic alteration among the following: body mass index (BMI) > 25 kg/m2, dyslipidemia and abnormal glucose metabolism. These criteria are generous compared with those more recently established to define the presence of MS[8,9].
In the present study, we evaluated patients with alterations of serum iron indices and hepatic iron overload not explained by mutations in iron related genes or by acquired factors that can lead to iron overload through known pathogenetic mechanisms[3]. We defined this condition as hepatic iron overload (HIO) irrespective of the presence of metabolic alterations. Our aims were to obtain information in order to differentiate the variegated phenotypes in patients with HIO and to better characterize the dysmetabolic iron overload syndrome.
To this end, we carried out the following: (1) Analyzed iron status either at biochemical and histological levels. We hypothesized that the level of transferrin saturation and the cellular distribution of iron deposits in the liver might give insight into the mechanism leading to iron accumulation[3,10]; (2) Determined the frequency of abnormalities that are part of the MS (according to the more recent criteria), the frequency and severity of hepatic steatosis and their relation with iron indices. We hypothesized that the presence and the number of metabolic alterations might be associated with a peculiar iron phenotype; (3) Studied a control group of patients with HFE-related hemochromatosis (HFE-HH), taken as the model of primary iron overload, in order to compare hepatic iron distribution, the frequency of metabolic alterations and hepatic steatosis with those found in the group of patients with HIO.
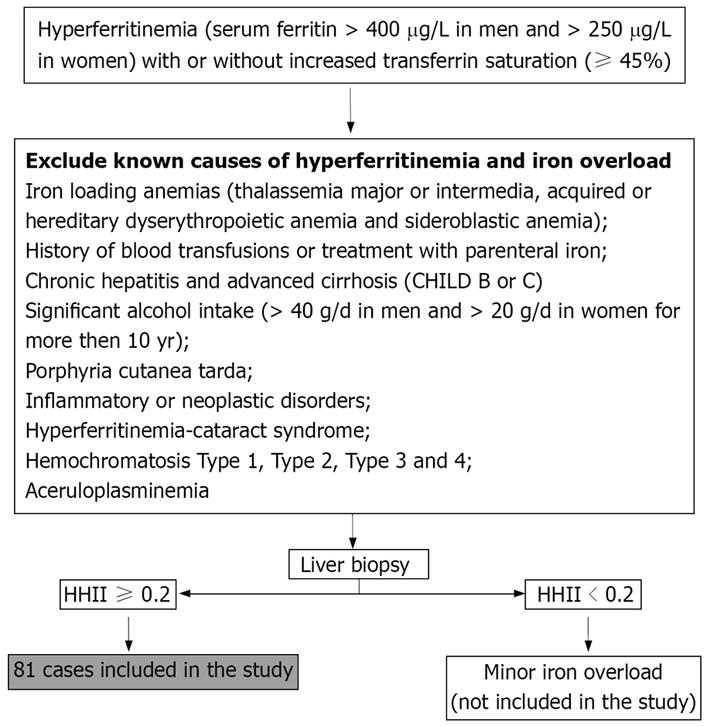
We studied 81 Italian unrelated adult patients (67 males and 14 females) with HIO. They were consecutively selected, since 1998, from outpatients who were referred to us for abnormalities of serum iron indices. Selection criteria are reported in Figure 1.
To compare patients’ data we also selected from our own database 57 patients affected by HFE-HH (51 were C282Y homozygotes, two were compound heterozygous for C282Y/E168X and four for C282Y/H63D). Criteria for selection of HFE controls were: availability of liver biopsy, semiquantitative iron grading and metabolic indices. All patients gave their written consent to liver biopsy, which was done for diagnostic and/or prognostic evaluation according to international guidelines[11].
All data were recorded at the time of diagnosis. All patients with HIO and HFE-HH underwent liver biopsy. Liver sections were stained with standard methods for histological evaluation and with Perl’s stain for iron grading and assessed by an independent observer (G.B.).
The following data were collected: glucose and insulin, total cholesterol, HDL, triglycerides, serum alanine and aspartate-aminotransferases, and γ-glutamyl transferase, which were measured using commercial kits, BMI, waist circumference and blood pressure. Overweight was defined by BMI > 25 kg/m2 and < 30 kg/m2 and obesity by BMI ≥ 30 kg/m2[12]. Diagnoses of arterial hypertension, diabetes mellitus and glucose intolerance were based on the ESH/ESC and American guidelines, respectively[13,14]. The presence of dyslipidemia and MS was based on NCEP-ATPIII criteria[8]. HOMA index was available in 68 patients (84%)[15]. A HOMA index of 2.77 was chosen to distinguish insulin resistant and insulin sensitive patients according to Bonora et al[16].
Serum iron indices were determined using standard methods. Transferrin saturation was calculated as follows: serum iron/(serum transferrin × 1.4) × 100[17]. A semi-quantitative evaluation of hepatic iron was done according to Deugnier et al[18]. Iron deposits were assessed according to size, cellular and lobular locations in Rappaport’s acinus, leading to three different scores: hepatocytic (HIS; range, 0-36), sinusoidal (SIS; range, 0 to 12) and portal iron scores (PIS; range, 0-12). The sum of these scores defined the total iron score (TIS; range, 0-60). Previous studies have shown that age-related histological hepatic iron index (the ratio of TIS to age, HHII) is an accurate means of predicting the genetic status of HH patients (heterozygotes vs homozygotes)[19]. Accordingly, all the patients with HIO and HFE-HH had a histological hepatic iron index in the range of homozygous HH.
Hepatic fibrosis was graded according to Ishak et al[20]. Hepatic steatosis was diagnosed when at least 5% of hepatocytes were involved and steatosis was graded as follows: absence (≤ 5% of hepatocytes), mild (> 5% ≤ 33%), moderate (> 33% and ≤ 66%), severe (> 66%)[21].
Data were expressed as median and range due to the non-Gaussian distribution of iron indices. The Student’s t test was used for quantitative variables with a normal distribution. All the comparisons involving hepatic iron distribution according to Deugnier et al[18] and variables with non-skewed distributions were performed on medians by non-parametric methods (Mann-Whitney or Kruskall-Wallis when more than two groups were involved). The Fisher’s exact test or the Chi-square test was used for qualitative data. Correlations between variables were evaluated by the Spearman’s test. The receiving operating curve (ROC) was used to evaluate if a threshold number of MS components was associated with different hepatic iron overload as defined by the HHII. We compared the Area under the curve (AUC) of the ROCs relative to 1, 2, 3, 4 and 5 components of the MS according to HHII. The influence of age, gender, alcohol intake, BMI, serum iron indices and hepatic iron distribution (HIS, SIS and PIS) on hepatic fibrosis, was evaluated by a logistic regression model. All tests were two-sided and with a significance level of P≤ 0.05.
Seven patients (8.6%) were heterozygous the C282Y and 27 (33.3%) the H63D mutation. Table 1 shows the main clinical, biochemical and histological data of patients with HIO (as a whole and according to gender) and with HFE-HH.
Thirty patients (37%) had transferrin saturation repeatedly ≥ 45% and 39 patients (48%) had serum ferritin >1000 μg/L. All patients showed homogeneous hemosiderin deposits in the hepatic lobules and a decreasing hepatocytic iron gradient from Rappaport Ito III. In this series, females had more iron overload and a higher frequency of cirrhosis, lower BMI (P = 0.0007) and a number of MS components more than males. TIS correlated with age in males (r = 0.4, P = 0.0007), but not in females. TIS significantly correlated with several indices, as reported in Table 2, showing an inverse correlation with metabolic indices. AT linear regression analysis, gender (P = 0.0005), grade of fibrosis (P = 0.0005), transferrin saturation (P < 0.0001) and severity of steatosis (P < 0.0001) retained statistical significance with TIS.
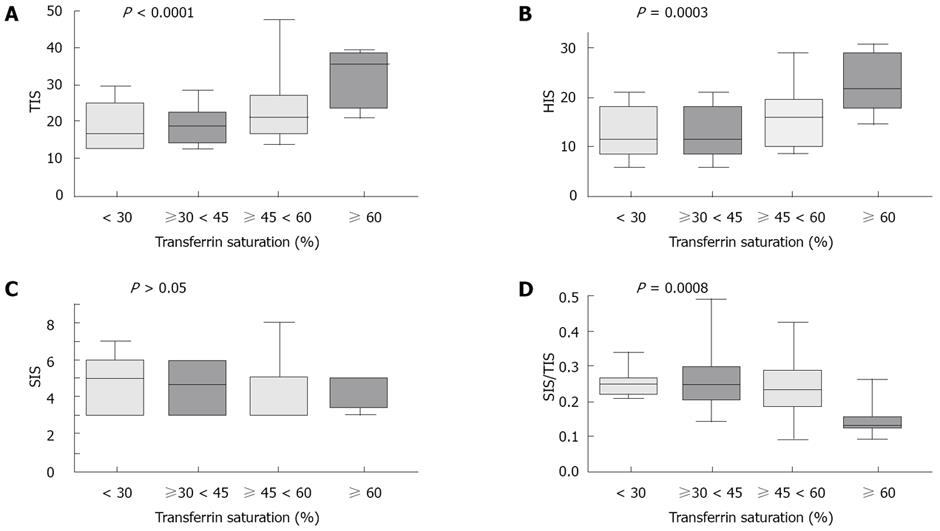
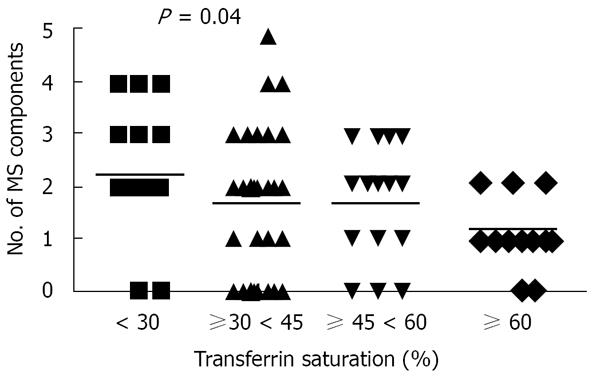
Dividing HIO patients according to transferrin saturation, we observed that TIS, HIS and PIS significantly increased with the increasing transferrin saturation. By contrast, SIS did not change, whereas the SIS to TIS ratio significantly decreased (Figure 2). We observed that the higher the transferrin saturation level, the lower was the number of MS components (Figure 3). Patients with transferrin saturation < 45% (n = 51) had lower amount of iron overload in hepatocytic (P = 0.0004) and portal (P = 0.0007) compartments than patients with increased transferrin saturation but with a proportionally greater amount of iron in sinusoidal compartment (SIS/TIS: 0.25 ± 0.07 vs 0.2 ± 0.08, P = 0.003). These patients also had a lower prevalence of fibrosis (Ishak’s score ≥ 1: 16/51 vs 18/30, P = 0.019). Patients with the highest levels of transferrin saturation (≥ 60%) had a SIS to TIS ratio (median, 0.15; range, 0.1-0.25) not different than that observed in HFE-HH (median, 0.13; range, 0-0.63). They also had a prevalence of hepatic steatosis and hepatic fibrosis comparable to that observed in patients with HFE-HH (data not shown) but without MS.
One patient (1.2%) had all five components of MS, five (6.2%) had four components, 14 (17.2%) had three, 27 (33.3%) had two, 17 (20.9%) had one and 17 (20.9%) had none. The frequency and severity of hepatic steatosis and MS prevalence in HIO are reported in Table 1 and are compared with those observed in HFE-HH patients. Thirty-one patients with HIO (45.6%) were insulin resistant. Steatosis was more common in Rappaport zones 2 and 3 and more prevalent in HIO patients with MS than in those without (90% vs 47.5%, P = 0.0007). The number of MS components correlated positively with age, severity of steatosis and HOMA index, and inversely with transferrin saturation and hepatic iron indices, but not with serum ferritin (Table 3).
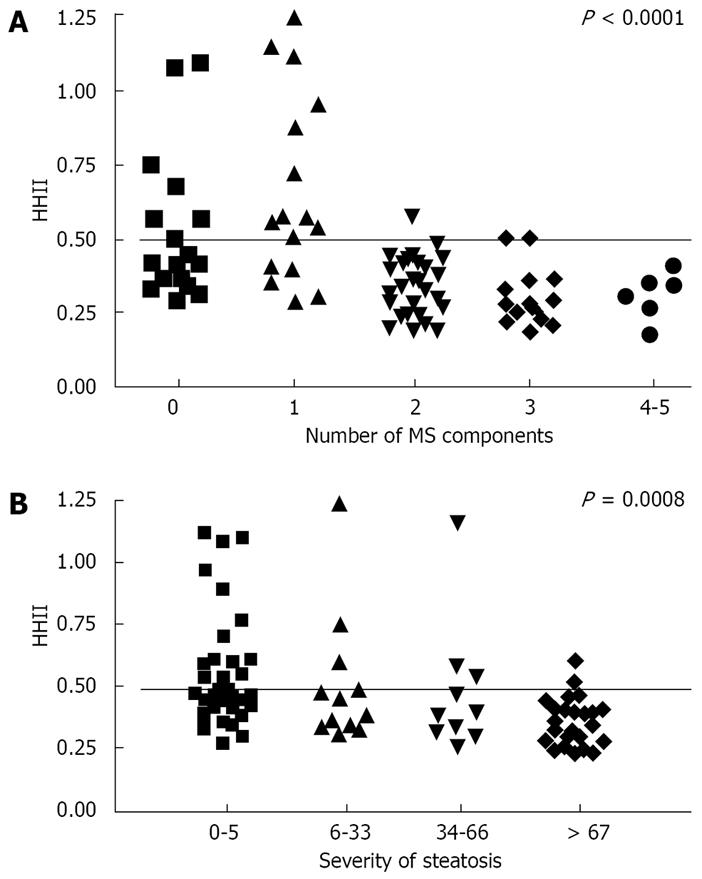
By the ROC analysis (Figure 4), we found that the presence of two MS alterations best differentiates HIO patients according to HHII (P < 0.0001). Patients with 0-1 MS components showed a wide range of HHII from low to very high, whereas in patients with two or more MS features the HHII was ≤ 0.50 in all but one (Figure 5A). Similar findings were observed when patients were divided according to the presence of steatosis (Figure 5B). In addition, the percentage of hepatocytes involved by steatosis inversely correlated with HIS (r = -0.52, P < 0.0001) and with the amount of iron removed by phlebotomies (r = -0.45, P = 0.0019).
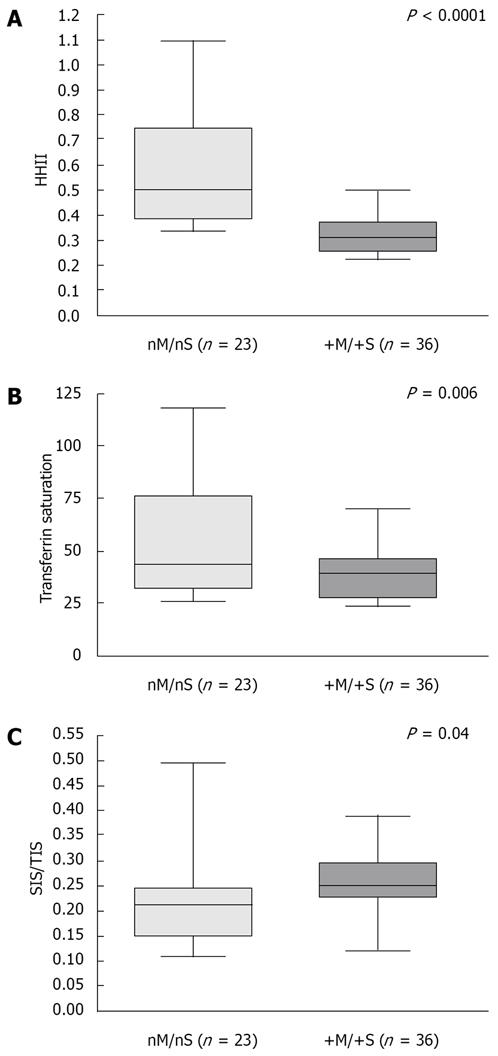
Table 4 compares iron indices, in patients according to the presence of ≥ 2 components of MS or steatosis. Figure 6 compares the same data in two subgroups: patients with a “metabolic phenotype” (+M/+S, patients with ≥ 2 MS alterations and hepatic steatosis) and patients without (nM/nS, patients with 0-1 MS components and absence of hepatic steatosis).
Data on hepatic damage in HIO patients are summarized in Table 1. TIS correlated with fibrosis (r = 0.28, P = 0.01), but PIS showed the highest correlation (r = 0.51, P < 0.0001) and SIS none. Logistic regression modelling indicated that PIS, age, HOMA index and presence of steatosis independently correlated with hepatic fibrosis (P < 0.0001, P = 0.004, P < 0.0001 and P = 0.04, respectively).
In the present study, we evaluated 81 patients with hepatic iron overload not explained by known genetic or acquired disorders. HIO as expected, patients showed a wide range of variability in the biochemical iron status, in hepatic iron deposits and lobular iron distribution, and had a variable number of metabolic alterations that are part of the MS, with or without NAFLD. There was an inverse relationship between transferrin saturation and sinusoidal iron. Patients with transferrin saturation < 45% had a lower amount of iron overload in the hepatocytic and portal compartments than patients with increased transferrin saturation, but have a proportionally greater amount of iron in sinusoidal compartment as shown by the higher SIS to TIS ratio. Accordingly, when compared to HFE-controls, patients with transferrin saturation < 45% had higher SIS and SIS to TIS ratios (data not shown). These findings indicate that, in HIO patients with normal transferrin saturation, the mechanism of hepatic iron accumulation is different than that of the classical HH[1,2] and suggest the existence of macrophage iron retention and an iron recycling defect. By contrast, in patients with increased transferrin saturation, hepatic iron distribution was comparable to that observed in HFE-HH, thus supporting the idea that the pathogenetic mechanism of iron overload was due to increased iron absorption[22]. The strongest identity with HFE-HH was observed in patients with the highest transferrin saturation (≥ 60%), suggesting that between 45% and 60% there is a grey zone that includes mixed conditions. In addition, patients with transferrin saturation ≥ 60% had the same prevalence of hepatic steatosis and of organ damage as HFE-HH, however, none had MS. Thus, transferrin saturation can distinguish, among HIO patients, two main subgroups with likely different pathogenetic mechanisms of iron overload.
Another part of the study concerns the metabolic aspects and their relation with serum and hepatic iron indices. First, in agreement with the theory that NAFLD is the hepatic manifestation of the MS, we found that the number of MS components correlated with the severity of hepatic steatosis and hyperinsulinemia and that 90% of the patients with the MS had NAFLD[5]. Second, we found that the presence of metabolic abnormalities and/or NAFLD was associated with a peculiar iron phenotype characterised by a lower hepatic iron overload, a more prevalent iron in the sinusoidal compartment and a lower transferrin saturation that corresponds to the more typical features of IR-HIO[6]. We demonstrated that this phenotype was common in patients presenting at least two of the MS features and in the subgroup with ≥ 2 MS abnormalities and NAFLD.
An inverse relation between steatosis and hepatic iron has been previously observed and variably explained[23-25]. The significant inverse correlation that we found between the degree of steatosis and the amount of iron removed by phlebotomies provides the strongest evidence that the higher the percentage of hepatocytes involved by steatosis, the lower the amount of iron accumulation. Recent studies in severely obese patients[26] and in an animal model of insulin resistance[27] suggest that obesity or hyperinsulinemia favour the development of iron deficiency. Increased adipocyte hepcidin production has been reported in morbid obesity that could result in reduced intestinal iron absorption, macrophage iron release and eventually in low iron stores[26]. Although these findings need to be further elucidated and confirmed, they support the hypothesis that metabolic abnormalities associated with insulin resistance and MS might protect from iron overload. Accordingly, in C282Y homozygous patients, obesity (in females)[28] and hepatic steatosis[29] were associated with a decreased transferrin saturation[28] and hepatic iron concentration[28,29]. It was suggested that the overweight-related chronic inflammatory state may increase hepcidin production (likely at extrahepatic sites) and partially compensates for the defect of hepcidin synthesis due to the HFE mutation[7,28]. We recently found that urinary hepcidin levels, although inappropriate for the iron overload, were indeed significantly higher in patients with dysmetabolic iron overload than in controls[30]. Overall, these findings seem to contradict the hypothesis that iron overload and metabolic abnormalities are linked by a common pathogenetic mechanism in IR-HIO, unless a hepcidin-resistance state is hypothesized. Another possible explanation is that IR-HIO is a multifactorial disease that results from the association of a mild-moderate, maybe polygenic, defect of hepcidin production and insulin resistance or MS. This may explain some of the features of the disease: late-onset and mild-moderate iron overload tending to a plateau[31]. As observed in subjects carrying the low expressing C282Y/H63D HFE genotype[32], these patients may retain some ability to increase hepcidin production in response to iron load that possibly explains their slight phenotype. In patients with dysmetabolic iron overload, NAFLD and MS might foster hepcidin production leading to the typical IR-HIO phenotype. Further studies are needed to clarify this issue and the role of dysmetabolisms in dysregulating iron regulatory pathways. This is not unimportant considering that the dysmetabolic iron overload syndrome is currently the most prevalent iron overload disorder in the adult population.
Based on our findings and in the absence of a clear pathogenetic explanation of IR-HIO, we suggest a more strict clinical definition of this syndrome characterised by the presence of two or more components of the MS, hepatic steatosis and normal transferrin saturation. Liver biopsy should confirm the presence of steatosis and iron overload with typical involvement of sinusoidal compartment. In fact, in agreement with other studies[23,24,33], we showed that serum ferritin is not a reliable index of iron overload in patients with metabolic abnormalities and might overestimate the true amount of hepatic iron overload due to NAFLD-related hepatocellular damage, local cytokine activation or higher mesenchymal iron retention[7]. Noninvasive methodologies, such as magnetic resonance (MR) for iron quantification, can be an alternative option when liver biopsy is not feasible, provided the MR apparatus is calibrated and validated[34].
Finally, we showed that a number of HIO patients presented a classical hemochromatosis phenotype (high transferrin saturation and prevalent hepatocytic iron overload). Some of them, including a few young women, had a severe iron overload that lead to clinical complications. We had no etiopathogenetic explanation for this form of iron overload, which may represent the severe boundary of a complex trait due to gene-gene and gene-environmental interactions, or the result of a still undefined major locus defect. Studies aimed to clarify the genetic background and the complex pathways causing these forms of unexplained iron overload are needed to improve the diagnostic setting of iron overload disorders.
Hepatic iron overload includes a wide spectrum of conditions from mild to marked tissue iron accumulation. In some cases the pathophysiology of iron accumulation is still obscure. The association of hepatic iron overload and metabolic alterations and/or non-alcoholic fatty liver is common in Western population. A new syndrome, named Dysmetabolic Hepatic Iron Overload or Insulin Resistance Hepatic Iron Overload was described in 1999, based on the presence of hepatic iron overload in association with even a single metabolic abnormality. These criteria were generous compared to those more recently established for the metabolic syndrome. In addition, the pathogenetic mechanisms that link insulin resistance and/or steatosis to iron overload are still undefined.
To characterize clinical, biochemical and histological features of hepatic iron overload not explained by known genetic or acquired disorders, including the form associated to metabolic alterations. To understand the pathogenetic mechanisms of hepatic iron accumulation. To characterize the phenotype of the dysmetabolic iron overload syndrome, according to the more recent criteria defyining the metabolic syndrome, and the presence of fatty liver disease.
We demonstrated that patients with hepatic iron overload, not explained by known genetic or acquired disorders, need a systematic diagnostic approach, including the evaluation of hepatic iron overload and cellular iron distribution. We suggested the existence of two main different pathogenetic mechanisms leading to forms of unexplained iron overload: an iron recycling defect and an increased intestinal iron absorption. We suggested a new, updated definition of the dysmetabolic iron overload syndrome. We speculated on the pathogenesis of the iron overload syndrome based on our results and current knowledge of iron metabolism.
This study may improve the diagnostic approach in patients with unexplained forms of iron overload including the dysmetabolic iron overload syndrome. This is not trivial considering that the latter disorder is currently the most prevalent iron overload disease in adult population. In addition, our findings indicate simple and valid criteria to better classify these forms of iron overload. This will be useful: (a) to better understand the etiopathogenesis of these disorders and the dysregulation of iron homeostasis in future studies; (b) to better address therapeutical strategies, e.g. iron depletion vs treatment of metabolic abnormalities and hepatic steatosis.
In this manuscript authors give a different angle to a problem that has interested the scientific world recently. The study is well conducted.
Peer reviewer: Dr. Philip Abraham, Professor, Consultant Gastroenterologist & Hepatologist, P. D. Hinduja National Hospital & Medical Research Centre, Veer Savarkar Marg, Mahim, Mumbai 400016, India
S- Editor Zhong XY L- Editor Lutze M E- Editor Zhang WB
| 1. | Pietrangelo A. Hereditary hemochromatosis--a new look at an old disease. N Engl J Med. 2004;350:2383-2397. |
| 2. | Camaschella C. Understanding iron homeostasis through genetic analysis of hemochromatosis and related disorders. Blood. 2005;106:3710-3717. |
| 3. | Piperno A. Classification and diagnosis of iron overload. Haematologica. 1998;83:447-455. |
| 4. | Pietrangelo A. Hereditary hemochromatosis. Biochim Biophys Acta. 2006;1763:700-710. |
| 5. | Kotronen A, Yki-Jarvinen H. Fatty liver: a novel component of the metabolic syndrome. Arterioscler Thromb Vasc Biol. 2008;28:27-38. |
| 6. | Mendler MH, Turlin B, Moirand R, Jouanolle AM, Sapey T, Guyader D, Le Gall JY, Brissot P, David V, Deugnier Y. Insulin resistance-associated hepatic iron overload. Gastroenterology. 1999;117:1155-1163. |
| 7. | Trombini P, Piperno A. Ferritin, metabolic syndrome and NAFLD: elective attractions and dangerous liaisons. J Hepatol. 2007;46:549-552. |
| 8. | Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001;285:2486-2497. |
| 9. | Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26 Suppl 1:S5-S20. |
| 10. | Pietrangelo A. Non-HFE hemochromatosis. Semin Liver Dis. 2005;25:450-460. |
| 11. | Adams P, Brissot P, Powell LW. EASL International Consensus Conference on Haemochromatosis. J Hepatol. 2000;33:485-504. |
| 12. | Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults--The Evidence Report. National Institutes of Health. Obes Res. 1998;6 Suppl 2:51S-209S. |
| 13. | 2003 European Society of Hypertension-European Society of Cardiology guidelines for the management of arterial hypertension. J Hypertens. 2003;21:1011-1053. |
| 14. | Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183-1197. |
| 15. | Haffner SM, Miettinen H, Stern MP. The homeostasis model in the San Antonio Heart Study. Diabetes Care. 1997;20:1087-1092. |
| 16. | Bonora E, Kiechl S, Willeit J, Oberhollenzer F, Egger G, Targher G, Alberiche M, Bonadonna RC, Muggeo M. Prevalence of insulin resistance in metabolic disorders: the Bruneck Study. Diabetes. 1998;47:1643-1639. |
| 17. | Trombini P, Coliva T, Nemeth E, Mariani R, Ganz T, Biondi A, Piperno A. Effects of plasma transfusion on hepcidin production in human congenital hypotransferrinemia. Haematologica. 2007;92:1407-1410. |
| 18. | Deugnier YM, Loreal O, Turlin B, Guyader D, Jouanolle H, Moirand R, Jacquelinet C, Brissot P. Liver pathology in genetic hemochromatosis: a review of 135 homozygous cases and their bioclinical correlations. Gastroenterology. 1992;102:2050-2059. |
| 19. | Deugnier YM, Turlin B, Powell LW, Summers KM, Moirand R, Fletcher L, Loreal O, Brissot P, Halliday JW. Differentiation between heterozygotes and homozygotes in genetic hemochromatosis by means of a histological hepatic iron index: a study of 192 cases. Hepatology. 1993;17:30-34. |
| 20. | Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696-699. |
| 21. | Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313-1321. |
| 22. | Deugnier Y, Turlin B. Pathology of hepatic iron overload. World J Gastroenterol. 2007;13:4755-4760. |
| 23. | George DK, Goldwurm S, MacDonald GA, Cowley LL, Walker NI, Ward PJ, Jazwinska EC, Powell LW. Increased hepatic iron concentration in nonalcoholic steatohepatitis is associated with increased fibrosis. Gastroenterology. 1998;114:311-318. |
| 24. | Bugianesi E, Manzini P, D'Antico S, Vanni E, Longo F, Leone N, Massarenti P, Piga A, Marchesini G, Rizzetto M. Relative contribution of iron burden, HFE mutations, and insulin resistance to fibrosis in nonalcoholic fatty liver. Hepatology. 2004;39:179-187. |
| 26. | Bekri S, Gual P, Anty R, Luciani N, Dahman M, Ramesh B, Iannelli A, Staccini-Myx A, Casanova D, Ben Amor I. Increased adipose tissue expression of hepcidin in severe obesity is independent from diabetes and NASH. Gastroenterology. 2006;131:788-796. |
| 27. | Le Guenno G, Chanseaume E, Ruivard M, Morio B, Mazur A. Study of iron metabolism disturbances in an animal model of insulin resistance. Diabetes Res Clin Pract. 2007;77:363-370. |
| 28. | Laine F, Jouannolle AM, Morcet J, Brigand A, Pouchard M, Lafraise B, Mosser J, David V, Deugnier Y. Phenotypic expression in detected C282Y homozygous women depends on body mass index. J Hepatol. 2005;43:1055-1059. |
| 29. | Powell EE, Ali A, Clouston AD, Dixon JL, Lincoln DJ, Purdie DM, Fletcher LM, Powell LW, Jonsson JR. Steatosis is a cofactor in liver injury in hemochromatosis. Gastroenterology. 2005;129:1937-1943. |
| 30. | Barisani D, Pelucchi S, Mariani R, Galimberti S, Trombini P, Fumagalli D, Meneveri R, Nemeth E, Ganz T, Piperno A. Hepcidin and iron-related gene expression in subjects with Dysmetabolic Hepatic Iron Overload. J Hepatol. 2008;49:123-133. |
| 31. | Piperno A, Vergani A, Salvioni A, Trombini P, Vigano M, Riva A, Zoppo A, Boari G, Mancia G. Effects of venesections and restricted diet in patients with the insulin-resistance hepatic iron overload syndrome. Liver Int. 2004;24:471-476. |
| 32. | Piperno A, Girelli D, Nemeth E, Trombini P, Bozzini C, Poggiali E, Phung Y, Ganz T, Camaschella C. Blunted hepcidin response to oral iron challenge in HFE-related hemochromatosis. Blood. 2007;110:4096-4100. |
| 33. | Fargion S, Mattioli M, Fracanzani AL, Sampietro M, Tavazzi D, Fociani P, Taioli E, Valenti L, Fiorelli G. Hyperferritinemia, iron overload, and multiple metabolic alterations identify patients at risk for nonalcoholic steatohepatitis. Am J Gastroenterol. 2001;96:2448-2455. |