Published online Aug 14, 2008. doi: 10.3748/wjg.14.4739
Revised: April 16, 2008
Accepted: April 23, 2008
Published online: August 14, 2008
AIM: To detect the nuclear factor kappa B (NF-κB) condition in human stage IV gastric carcinoma patients and to explore the correlation between NF-κB activation and survival of these patients after chemotherapy.
METHODS: Expression of NF-κB-p65 was determined by immunohistochemical analysis. Activity of NF-κB DNA-binding in carcinoma tissue was detected by electrophoretic mobility shift assay. Kaplan-Meier survival analysis was performed to show the relation between NF-κB and progression-free survival (PFS) or overall survival (OS) of the patients.
RESULTS: The positive expression rate of NF-κB-p65 in 60 gastric cancer tissue samples was 76.7% (46/60). The expression of NF-κB-p65 was reduced in adjacent carcinoma and normal tissue samples. Electrophoretic mobility shift assay (EMSA) analysis showed a strong activation of NF-κB in cancer tissue samples. A survival difference was found in NF-κB-p65 positive and negative patients. NF-κB-p65 expression was negative in cancer tissue samples (n = 14). PFS was 191.40 ± 59.88 d and 152.93 ± 16.99 d, respectively, in patients with positive NF-κB-p65 expression (n = 46) (P = 0.4028). The survival time of patients with negative and positive NF-κB-p65 expression was 425.16 ± 61.61 d and 418.85 ± 42.98 d, respectively (P = 0.7303). Kaplan-Meier analysis showed no significant difference in PFS or OS. The 46 patient tissue which positive NF-κB-p65 expression was found in the tissue samples from the 46 patients whose PFS and OS were 564.89 ± 75.94 d and s 352.37 ± 41.32 d, respectively (P = 0.0165).
CONCLUSION: NF-κB is activated in gastric carcinoma tissue, which is related to the OS after chemotherapy.
- Citation: Ye S, Long YM, Rong J, Xie WR. Nuclear factor kappa B: A marker of chemotherapy for human stage IV gastric carcinoma. World J Gastroenterol 2008; 14(30): 4739-4744
- URL: https://www.wjgnet.com/1007-9327/full/v14/i30/4739.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.4739
| FOLOFOX group (n = 44) | PLF group (n = 16) | P | |
| Sex (male/female) | 29/15 | 11/5 | 0.547 |
| Age (yr) | 51.51 ± 13.10 | 49.06 ± 12.36 | 0.521 |
| General state of health ECOG patients | |||
| 0 score | 1 | 1 | 0.721 |
| 1 score | 32 | 12 | |
| 2 scores | 11 | 4 | |
| Primary location | |||
| Cardiac orifice | 11 | 2 | 0.431 |
| Stomach | 19 | 12 | |
| Gastric remnant | 4 | 2 | |
| Accumulative organs | |||
| 1 | 3 | 1 | 0.1642 |
| 2 | 23 | 3 | |
| 3 | 16 | 8 | |
| > 3 | 2 | 4 | |
| Pathologic classification | |||
| Moderately-differentiated | 9 | 4 | 0.691 |
| Poorly-differentiated | 33 | 12 | 0.336 |
| Undifferentiated | 2 | 0 | 0.132 |
Nuclear factor kappa B (NF-κB) contributes to cell differentiation, proliferation, and death, as well as plays a prominent role in the immune response of mammals[1]. It consists of homodimers or heterodimers of the Rel family members: NF-κB1 (p50), NF-κB2 (p52), c-Rel, RelA (p65), and RelB[2]. The ability of NF-κB to suppress apoptosis and regulate cell-cycle transition indicates that NF-κB may participate in oncogenesis[3].
There is evidence that NF-κB level is higher in gastric carcinoma cells than in normal adjacent epithelial cells, and activation of NF-κB in gastric carcinoma is related to lymphatic invasion[4]. It was reported that patients with high NF-κB activation in carcinoma tissue would not survive as long as those with low NF-κB activation[5], and p65, but not NF-κB, is a prognostic indicator of gastric carcinoma[5]. It was also reported that NF-κB is frequently activated in the early-stage gastric carcinoma, and negatively associated with lymphatic invasion, but significantly associated with a better prognosis[6].
Although NF-κB is activated in human gastric carcinoma tissue, there is no evidence that NF-κB activation is associated with the clinicopathological features of cancer[6]. Our present study was to detect whether NF-κB is activated in human stage IV gastric carcinoma and explore the correlation between its activation with the survival of gastric cancer patients after chemotherapy.
The criteria for inclusion of patients in this study were (1) those with histologically or cytologically proved gastric cancer, (2) those with stage IV gastric cancer diagnosed according to the 2002 American Joint Committee on Cancer (AJCC) modified staging criteria, (3) those treated in our hospital with follow-up data, (4) those not receive chemotherapy prior to admission to our hospital, (5) those who received treatment with chemotherapeutics FOLFOX or paclitaxel/LV5Fu2 (PLF), (6) those with their therapeutic effect divided into complete remission (CR), partial remission (PR) and progression of disease (PD). The exclusion criteria for patients from this study were those with less than two cycles of treatment with FOLFOX or PLF, those with their therapeutic effect not estimate after two cycles of treatment with FOLFOX or PLF, those with stable effect of chemotherapeutics after several cycles of treatment, those having received two cycles of chemotherapy with FOLFOX and PLF. The response evaluation criteria used were the WHO and UICC criteria. We collected 60 gastric carcinoma samples from the First Affiliated Hospital of Sun Yat-Sen University in November 1999-June 2005. The data obtained from patients are shown in Table 1.
All samples were fixed with 100 mL/L formalin and embedded in paraffin. Each paraffin block was cut into 5-μm thick sections. Strept Actividin-Biotin Complex (SABC) Immunohistochemistry was performed according to the manufactures' instructions to detect the expression of NF-κB-p65. Briefly, the tissue sections were deparaffinized in xylene at 37°C for 20 min. Endogenous peroxide was blocked by incubating the sections with 30 mL/L H2O2 for 10 min at 37°C. The sections were incubated with primary antibodies to NF-κB-p65 at 4°C overnight. Staining was visualized with diaminobenzidine (DAB) for 10 min at room temperature. Finally, the sections were counterstained for nuclei with hematoxylin solution. Each section was observed microscopically in eight visual fields (× 400 magnification) and at least 100 cells were counted in each field. Positive staining for NF-κB-p65 appeared as buffy grains in the nuclei and cytoplasm. A semi-quantitative method was adopted to judge the results as previously described[7]. Immunohistochemistry score was calculated by combining an estimate of the percentage of immunoreactive cells (quantity score) with an estimate of the staining intensity (staining intensity score) as follows: no staining as 0, 1%-10% of cells stained as 1, 11%-50% cells stained as 2, 51%-80% cells stained as 3, and 81%-100% cells stained as 4. Staining intensity was rated on a scale of 0-3, with 0 = negative, 1 = weak, 2 = moderate, and 3 = strong. The staining was evaluated by color intensity and density of grade products as follows: 0-3 = negative (-), > 3 = positive (+). All specimens were evaluated by two pathologists without any knowledge about the clinical data obtained from the patients. Negative control was designed using PBS instead of primary antibody. Adjacent glandular epithelium served as an internal positive control of NF-κB-p65 protein expression.
Activity of NF-κB DNA-binding was detected by EMSA as previously descried[8] with certain modifications. Double-stranded oligonucleotide sequence of 5'-AGTTGAGGGGACTTTCCCAGGC-3', 5'-GCCTGGGAAAGTCCCCTCAACT-3', which corresponds to the NF-κB binding site, was end-labeled with [γ-32P] ATP by T4 polynucleotide kinase (Promega). Labeled probes were purified and detected with Whatman DE81(> 5000 r/min). Nuclear extract (15 μg) was mixed with 5 × 2 μL binding buffer at room temperature for 10 min, then labeled probe (0.0175 pmol) was incubated at room temperature for 20 min. The mixture was then subjected to electrophoresis on 4% polyacrylamide gel at 150 V in 0.5 × TBE buffer for 40 min at 4°C. After drying, the gel was exposed to an imaging system of FX P screen (Bio-Rad, America) for 24 h. The result was analyzed with software Bandscan 4.0 (denary logarithm of total gray value).
All patients were followed up in out-patient clinic, or by telephone or letters, two times each year, until June 4, 2006. We calculated progression-free survival (PFS) and overall survival (OS) rates from the day of treatment. Life-table methods were used to estimate PFS and OS[9]. For OS, deaths, irrespective of cause, were coded as events. For PFS, an event was defined as relapse, progression, or death during the period of active follow-up. Kaplan-Meier curves for PFS and OS were calculated for the subgroups with positive or negative NF-κB-p65 staining. In addition, other sets of Kaplan-Meier curves were derived to determine the PFS and OS of patients after FOLFOX and PLF therapy when NF-κB-p65 staining was positive. To assess variables influencing PFS and OS, univariate analysis using log-rank tests and proportional hazards models was performed. This study was approved by the local human investigation committee.
Statistical analysis was performed using SPSS 10.0 software. Significant differences were compared using one-way ANOVA test. Chi square test was performed for numerical data. Survival analysis was carried out using the Kaplan-Meier product-limit method, and survival curves were plotted. Differences were evaluated by the log rank test. P < 0.05 was considered statistically significant.
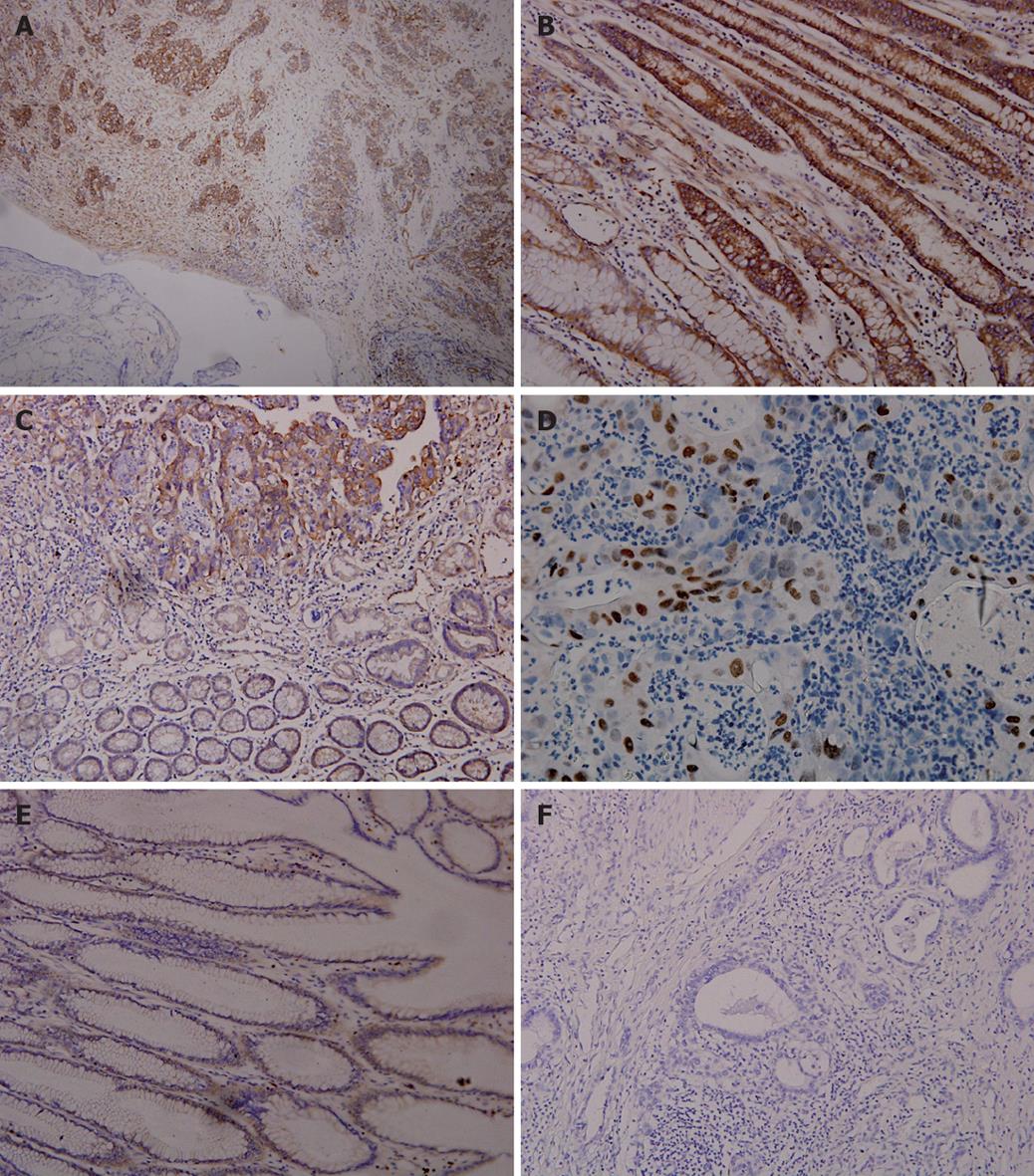
The positive expression rate of NF-κB-p65 in 60 gastric cancer tissue samples was 76.7% (46/60). Most positive expression of NF-κB-p65 showed brown-stained signals in cell cytoplasm or membrane (Figure 1A-C). Only a small amount of expression was found in the nuclei (Figure 1D). The immunohistochemistry score was 7.18 ± 2.72. The expression of NF-κB-p65 was reduced in the carcinoma tissue samples and the expression rate was 26.7% (16/60, Figure 1C). Weak expression of NF-κB-p65 was observed in normal tissue samples (Figure 1E). The immunohistochemistry score was 1.85 ± 1.29 and 0.17 ± 0.38, for carcinoma and normal tissue samples, respectively (P < 0.00001). No stained signal was detectable in negative control (Figure 1F).
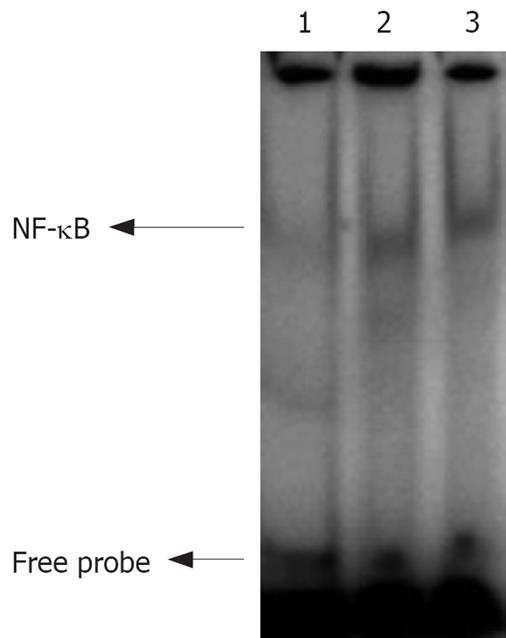
Nuclear extracts from normal, moderately- and poorly-differentiated gastric carcinoma tissue samples were analyzed by EMSA, which showed a strong activation of NF-κB in cancer tissue samples (Figure 2).
We analyzed PFS and OS using the Kaplan-Meier method. The results showed that the expression of NF-κB-p65 was negative in cancer tissue samples (immunohistochemistry score ranged from 1 to 3 scores, n = 14) and PFS was 191.40 ± 59.88 d (95% CI = 74.04 - 308.76), while it was positive in adjacent and normal tissue samples (immunohistochemistry score > 3, n = 46) and PFS was 152.93 ± 16.99 d (95% CI = 119.62 - 186.24). The survival time of patients with negative and positive expression of NF-κB-p65 was 425.16 ± 61.61 d (95% CI = 304.41 - 545.92) and 418.85 ± 42.98 d (95% CI = 334.62 - 503.08) , respectively.
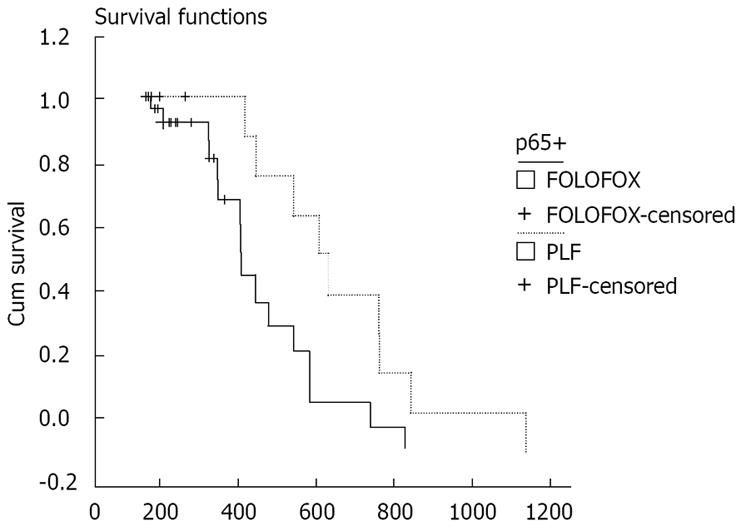
Kaplan-Meier analysis showed that the PFS time of patients after treatment with PLF and FOLFOX was 135.79 ± 15.09 d (95% CI = 106.22 - 165.35) and 183.08 ± 30.97 d (95% CI = 122.37 - 243.78), respectively. The OS time of patients after treatment with PLF and FOLFOX was 539.51 ± 69.82 d (95% CI = 402.66 - 676.35) and 363.12 ± 35.70 d (95% CI = 293.15 - 433.09), respectively. There was no significant difference in PFS or OS time of patients after treatment with FOLFOX and PLF (P = 0.2931 and P = 0.0548, respectively). The NF-κB-p65 expression was positive in the tissue samples from 46 patients. PFS time of patients with positive p65 expression was 132.60 ± 16.62 d (95% CI = 100.03 - 165.17) and 163.64 ± 23.99 d (95% CI = 116.62 - 210.65), respectively, after treatment with PLF and FOLFOX (P = 0.2407). However, the OS time of patients with negative p65 expression was 564.89 ± 75.94 d (95% CI = 416.04 - 713.74) and 352.37 ± 41.32 d (95% CI = 271.38 - 433.36), respectively, after treatment with PLF and FOLFOX (P = 0.0165). Their survival cure is shown in Figure 3.
NF-κB plays a role in oncogenic transformation. v-Rel, a highly oncogenic retroviral homologue of c-Rel, causes carcinogenesis in avian lymphoid cells[3,10]. Inhibition of NF-κB, by over expression of a degradation-resistant IκB, delays the development of T-cell lymphomas and prolongs the survival of v-Rel transgenic mice[3,10]. Chromosomal alterations in NF-κB family genes provide additional evidence for the role of NF-κB in oncogenesis. It has been demonstrated that genes encoding c-Rel, NF-κB2 (p100/p52), p65/RelA, and Bcl-3 proteins are all located within breakpoint regions of the genome involving oncogenic rearrangements or amplifications[3,11]. Indeed, increased NF-κB activity is evident in a number of human cancers, including breast cancer, non-small cell lung carcinoma, thyroid cancer, T- or B- lymphocyte leukemia, melanoma, colon cancer, bladder cancer, and several virus-induced tumors[12].
It was reported that NF-κB is constitutively activated in gastric carcinoma tissue[4-6,13,14]. The expression rate of NF-κB-p65 in gastric carcinoma is 18%-78.3%, which is higher than in normal adjacent epithelial cells. Sasaki et al[4] hold that increased NF-κB expression is correlated to the clinicopathological features of tumor aggression (especially its invasive ability) of gastric carcinoma. In the present study, NF-κB-p65 expression was mainly observed in gastric carcinoma cytoplasm with an expression rate of 77.6% (46/60), which is consistent with the previously reported data[4,14]. Some representative tumor specimens showed that p65 staining was increased in nuclei (Figure 1D), suggesting that subuint of NF-κB is translocated from cytoplasm to nuclei. We also determined p65 expression in samples from normal gastric glands and intestinal metaplasia except in cancer tissue samples. Weaker staining was found in such tissue samples, suggesting that NF-κB activation is associated with cell proliferation. EMSA showed that the increased nuclear translocation of NF-κB-p65 showed a higher NF-κB DNA-binding activity in differently differentiated tumor tissue samples than in adjacent normal tissue samples (Figure 2). The NF-κB-p65 expression rate is not consistent the previously reported rate[6]. The discrepancy might be due to the following reasons. (1) The classification reference for positively-stained cells might have decreased the expression rate of NF-κB-p65. Lee et al[6] reported that cells showing p65 nuclear staining irrespective of cytoplasmic staining display constitutive NF-κB activation. (2) Carcinoma stage was different. In our study, the 60 patients had stage IV gastric cancer compared with 15.5% reported in the study of Lee et al[6], who found that NF-κB activation is more prominent in early-stage pTNM tumors than in late-stage tumors.
Sasaki et al[4] analyzed the relation between NF-κB activation and traditional clinicopathological parameters, showing that NF-κB activation is correlated with tumor size and lymphatic invasion. However, Lee et al[6] showed that NF-κB activation, as a prognostic factor, is not correlated with the prognosis of patients after curative resection of their tumors, but significantly associated with a better prognosis of early-stage gastric carcinoma patients, suggesting that NF-κB activity is less reliable than TNM criteria as a prognostic biomarker of gastric cancer[6]. In the present study, we selected specimens from stage IV gastric cancer patients to discriminate p65 positive and negative expression. Sixty samples were divided into positive p65 expression group (n = 46) and negative p65 expression group (n = 14). When no significant difference in age, sex, accumulative organs and pathologic classification was found between the two groups, we analyzed the PFS and OS time of the patients in the two groups. The PFS time and OS time were longer in the group with positive p65 expression than in the group with negative p65 expression (P > 0.05), which is not consistent with previous reports[5,6]. We speculate that this discrepancy is caused by (1) the different carcinoma stages, (2) different treatment modalities, and (3) the number of tumor cases not taken into account.
In the present study, the 60 patients were treated with PLF (n = 16) and FOLFOX (n = 44), respectively (Table 1). Their PFS time and OS time had no statistical significance (P > 0.05). However, when we divided the 46 patients into a PLF treatment group (n = 12) and a FOLFOX treatment group (n = 12) with positive p65 expression (n = 34), the OS time of the patients in the two groups was 564.89 ± 75.94 d (95% CI = 416.04 - 713.74) and 352.37 ± 41.32 d (95% CI = 271.38 - 433.36), respectively (P = 0.0165), suggesting that NF-κB activation is associated with the prognosis of gastric cancer patients.
In conclusion, NF-κB-p65 is constitutively activated in late-stage gastric carcinoma patients and NF-κB activation is correlated to the survival time of gastric cancer patients after chemotherapy.
Nuclear factor kappa B (NF-κB) contributes to cell differentiation, proliferation, and death. There is experimental evidence that NF-κB plays a major role in the development and progression of various human cancers including gastric carcinoma. The effect of anti-cancer drugs is related to NF-κB in cancer cells.
As it is known, there is evidence that NF-κB plays a major role in the development and progression of various human cancers including gastric carcinoma. On the other hand, NF-κB can suppress tumor growth by promoting apoptotic signals in response to certain cancer therapeutic agents. However, the molecular mechanism of NF-κB underlying cancer development remains to be elucidated.
NF-κB was found to be a biomarker of gastric cancer. Further study is needed to prove it.
NF-κB activation is correlated to the survival of gastric cancer patients after chemotherapy and may affect treatment of choice for late human gastric carcinoma.
In the present work, the authors revealed the differential expression of NF-κB p65 in normal and gastric carcinoma tissue samples, and the potential importance of p65 up-regulations was underscored in chemotherapy for human gastric carcinoma. Their findings support the theory that p65 functions as a tumor promoter in human gastric carcinoma. Therefore, identification of the functional and phenotypic characteristics of p65 is essential for designing a rational anti-cancer therapy, as suggested by the authors. The study is well designed, and the data are reliable.
Peer reviewer: Dr. Fan-Yin Meng, Department of Internal Medicine, Ohio State University, Room 514A Medical Research Facility, 420 West 12th Avenue, Columbus, OH 43210, United States
S- Editor Zhong XY L- Editor Wang XL E- Editor Zhang WB
| 1. | Baldwin AS Jr. The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649-683. |
| 2. | Siebenlist U, Franzoso G, Brown K. Structure, regulation and function of NF-kappa B. Annu Rev Cell Biol. 1994;10:405-455. |
| 3. | Chen F, Castranova V, Shi X. New insights into the role of nuclear factor-kappaB in cell growth regulation. Am J Pathol. 2001;159:387-397. |
| 4. | Sasaki N, Morisaki T, Hashizume K, Yao T, Tsuneyoshi M, Noshiro H, Nakamura K, Yamanaka T, Uchiyama A, Tanaka M. Nuclear factor-kappaB p65 (RelA) transcription factor is constitutively activated in human gastric carcinoma tissue. Clin Cancer Res. 2001;7:4136-4142. |
| 5. | Yamanaka N, Sasaki N, Tasaki A, Nakashima H, Kubo M, Morisaki T, Noshiro H, Yao T, Tsuneyoshi M, Tanaka M. Nuclear factor-kappaB p65 is a prognostic indicator in gastric carcinoma. Anticancer Res. 2004;24:1071-1075. |
| 6. | Lee BL, Lee HS, Jung J, Cho SJ, Chung HY, Kim WH, Jin YW, Kim CS, Nam SY. Nuclear factor-kappaB activation correlates with better prognosis and Akt activation in human gastric cancer. Clin Cancer Res. 2005;11:2518-2525. |
| 7. | Soslow RA, Dannenberg AJ, Rush D, Woerner BM, Khan KN, Masferrer J, Koki AT. COX-2 is expressed in human pulmonary, colonic, and mammary tumors. Cancer. 2000;89:2637-2645. |
| 8. | Molloy PL. Electrophoretic mobility shift assays. Methods Mol Biol. 2000;130:235-246. |
| 9. | Dinse GE, Lagakos SW. Nonparametric estimation of lifetime and disease onset distributions from incomplete observations. Biometrics. 1982;38:921-932. |
| 10. | Carrasco D, Rizzo CA, Dorfman K, Bravo R. The v-rel oncogene promotes malignant T-cell leukemia/lymphoma in transgenic mice. EMBO J. 1996;15:3640-3650. |
| 11. | Liptay S, Seriu T, Bartram CR, Schmid RM. Germline configuration of nfkb2, c-rel and bcl3 in childhood acute lymphoblastic leukemia (ALL). Leukemia. 1997;11:1364-1366. |
| 12. | Calzado MA, Bacher S, Schmitz ML. NF-kappaB inhibitors for the treatment of inflammatory diseases and cancer. Curr Med Chem. 2007;14:367-376. |
| 13. | Yang GF, Deng CS, Xiong YY, Luo J, Wang BC, Tian SF, Xu K. [Expression of NFkappaB p65 and its target genes in gastric cancer and precancerous lesions.]. Zhonghua Zhongliu Zazhi. 2004;26:551-553. |
| 14. | Wang W, Luo HS, Yu BP. Expression of NF-kappaB and human telomerase reverse transcriptase in gastric cancer and precancerous lesions. World J Gastroenterol. 2004;10:177-181. |