INTRODUCTION
Lamivudine has been commonly used in the treatment of chronic hepatitis B as a first-line antiviral agent. Long-term lamivudine therapy can usually suppress hepatitis B virus (HBV) replication, however, prolonged monotherapy leads to the emergence of lamivudine-resistant HBV mutants[1–3]. The emergence of rtM204I/V (YMDD) mutation of HBV polymerase gene is associated with rebounds in serum HBV DNA and flares of transaminase level[4].
Adefovir dipivoxil is an effective rescue therapy for lamivudine-resistant HBV[56]. However, adefovir resistance occurs more frequently in second-line treatment of lamivudine-resistant patients than in naïve patients[7]. It is well known that sequential monotherapy with antiviral agents induces the sequential occurrence of viral mutations[8]. We here report the rapid redetection of YMDD mutants and hepatic decompensation in patients with prior lamivudine resistance during lamivudine retreatment.
CASE REPORTS
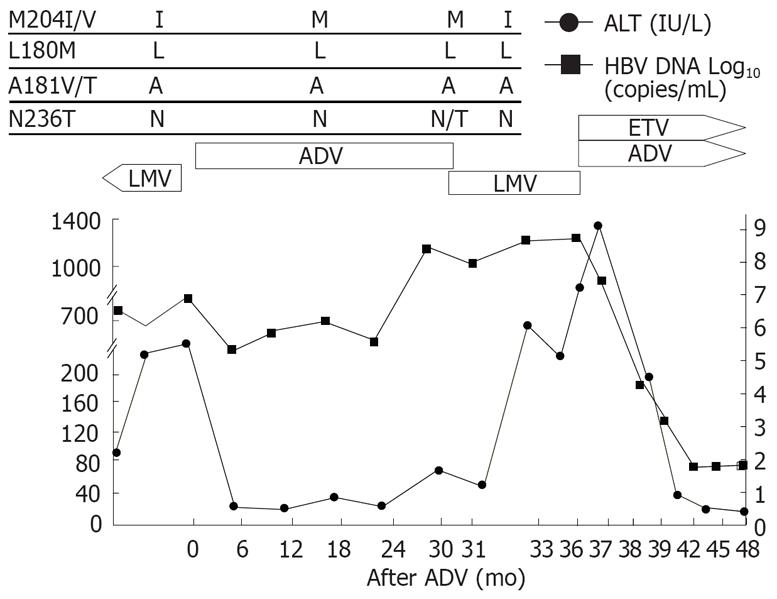
Patient 1 (Figure 1) was a 46-year-old man with HBeAg-positive chronic hepatitis B. Lamivudine was started initially and good virological and biochemical responses were observed in the patient. However, HBV-DNA increased and rtM204I mutation was detected by restriction fragment length polymorphism (RFMP) assay[9] after 2 years of lamivudine therapy. After lamivudine was switched to adefovir, HBV-DNA dropped by 2 logs with ALT normalization. Viral breakthrough (defined as a ≥ 1 log10 increase in HBV DNA from nadir after initial viral response) and rtN236T mutation developed after 30 mo of adefovir therapy. Lamivudine was restarted with discontinuation of adefovir. After lamivudine was restarted, the rtM204I mutation reemerged and rtN236T adefovir-resistant mutants disappeared 1 mo after the treatment. He showed persistently high levels of HBV-DNA, ALT and total bilirubin. Lamivudine was changed to adefovir and entecavir combination treatment as a rescue therapy. After the combination treatment, HBV-DNA and ALT dropped significantly.
Figure 1 Viral responses and clinical courses in a 46-year-old man with chronic hepatitis (patient 1).
The underline represents sequential evolution of lamivudine- or adeforvir- resistant genotypic mutations in HBV polymerase gene. LMV: Lamivudine; ADV: Adefovir; ETV: Entecavir; ALT: Alanine aminotransferase.
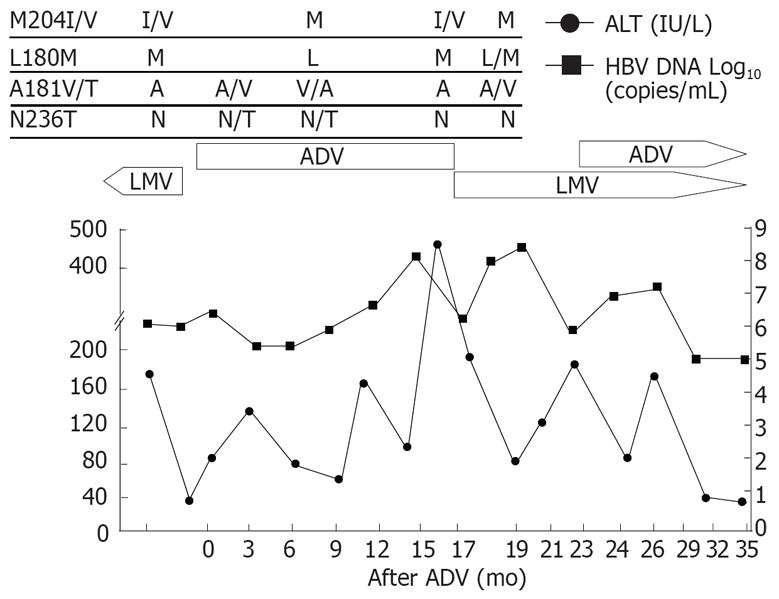
Patient 2 (Figure 2), a 49-year-old man with cirrhosis, showed the similar response to lamivudine retreatment as shown in patient 1. Viral breakthrough with YMDD mutation developed after 15 mo of the initial lamivudine treatment. Lamivudine was switched to adefovir, however, there was no decrease in HBV-DNA level but ALT elevation. After reintroduction of lamivudine for adefovir resistance, ALT level decreased without a significant drop in HBV-DNA level initially. Adefovir-resistant mutant was replaced by wild type, however, reemergence of the rtM204I/V mutation followed by HBV-DNA rebound and ALT fluctuation were observed. Adefovir was added to the ongoing lamivudine therapy.
Figure 2 Viral responses and clinical courses in a 49-year-old man with compensated cirrhosis (patient 2).
The underline represents sequential evolution of lamivudine- or adeforvir- resistant genotypic mutations in HBV polymerase gene. LMV: Lamivudine; ADV: Adefovir; ALT: Alanine aminotransferase.
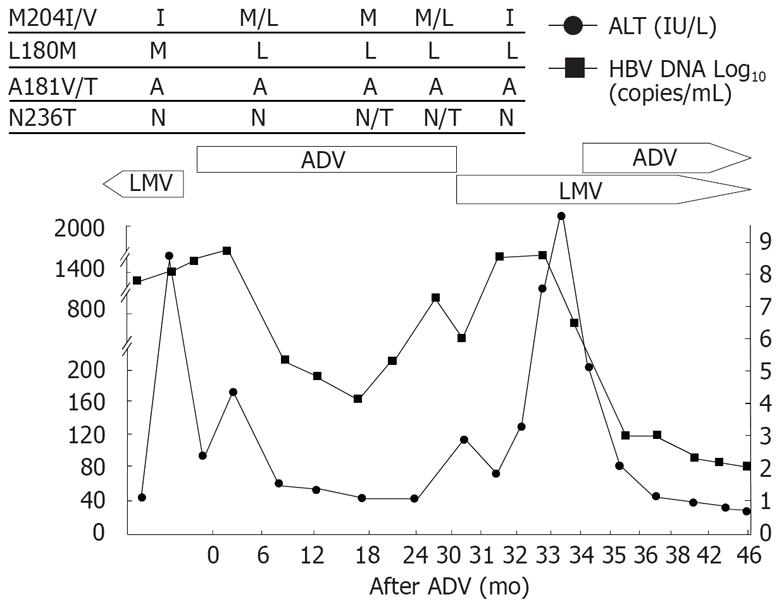
Patient 3 (Figure 3), a 50-year-old woman, had cirrhosis and received lamivudine as an initial treatment. After 16 mo of lamivudine therapy, she had elevated HBV-DNA followed by hepatic decompensation. Mutation profile showed rtM204I and rtL180M at the time of viral breakthrough. Lamivudine was switched to adefovir, and HBV-DNA decreased and her liver function was restored. Viral breakthrough occurred after 27 mo of adefovir treatment and rtN236T mutation was detected at this time. Lamivudine monotherapy was reintroduced consecutively. Rapid increase in HBV-DNA with the rtM204I mutants 2 mo after the lamivudine retreatment and ALT flare with jaundice and ascites occurred subsequently. The rtN236T mutation changed to wild type after the LMV treatment. Adefovir was added to lamivudine as a rescue therapy, and a rapid drop in HBV-DNA and ALT level was observed. The bilirubin decreased from the peak level of 18.7 to 1.6 mg/dL 6 mo after the combination treatment.
Figure 3 Viral responses and clinical courses in a 50-year-old woman with decompensated cirrhosis (patient 3).
The underline represents sequential evolution of lamivudine- or adeforvir- resistant genotypic mutations in HBV polymerase gene. LMV: Lamivudine; ADV: Adefovir; ALT: Alanine aminotransferase.
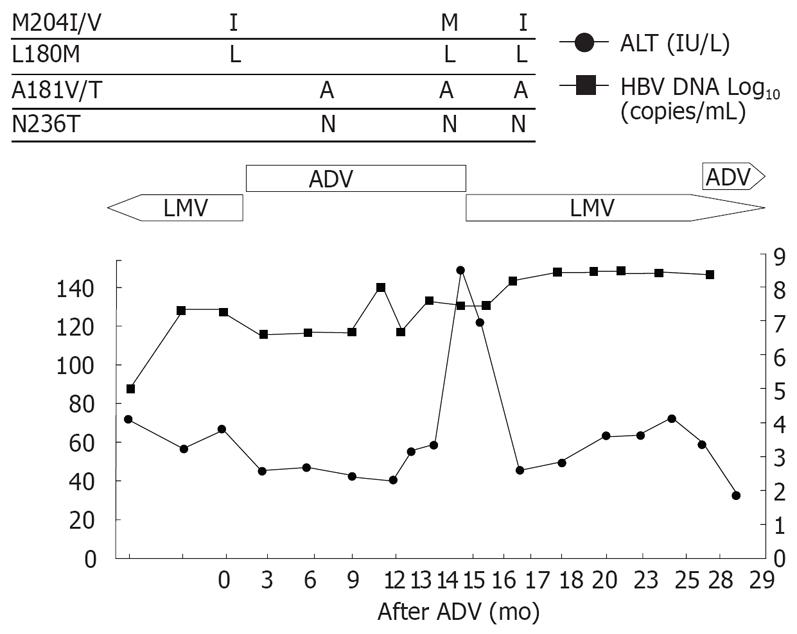
Patient 4 (Figure 4), a 50-year-old man with cirrhosis, received lamivudine for 14 mo. He had viral breakthrough with mild elevation of ALT, therefore lamivudine was changed to adefovir. However, he had no viral response though he showed a normal ALT level during the adefovir treatment. Adefovir was switched to lamivudine due to the high viral load. The ALT level was stable despite no decrease in HBV-DNA. After 2 mo of lamivudine retreatment, HBV-DNA rebound with ALT flare developed. HBV mutation rtM204I was reappeared at this time. Adefovir was added to the ongoing lamivudine treatment.
Figure 4 Viral responses and clinical courses in 50-year-old man with compensated cirrhosis (patient 4).
The underline represents sequential evolution of lamivudine- or adeforvir- resistant genotypic mutations in HBV polymerase gene. LMV: Lamivudine; ADV: Adefovir; ALT: Alanine aminotransferase.
All the patients were positive for HBeAg and had no seroconversion of HBeAg during the sequential antiviral treatment except for patient 3. Patient 3 had HBeAg loss after the lamivudine/adefovir combination therapy. No death occurred, however, two of them showed hepatic decompensation following the reemergence of YMDD mutations and viral breakthrough.
DISCUSSION
These cases showed that reintroduction of lamivudine could induce rapid reemergence of lamivudine-resistant mutations in chronic hepatitis B patients with prior lamivudine resistance. Lamivudine is well tolerated and significantly reduces HBV-DNA level[10]. However, lamivudine resistance associated with mutations in the polymerase gene, particularly in rtM204I/V known as YMDD mutant, occurs at a rate of 14%-30% annually[34]. Development of lamivudine resistance is associated with high baseline HBV-DNA level, duration of lamivudine treatment, precore variant, insufficient serum HBV-DNA suppression and elevated serum ALT level during treatment[11–13]. All the cases had positive HBeAg and high viral load at the time of lamivudine resistance. The high viral replication status may predispose to develop antiviral resistant mutations and subsequent viral breakthrough.
Adefovir is an effective rescue therapy for lamivudine-resistant HBV and significantly improves biochemical, virological, and histological features of lamivudine-resistant patients[56]. Compared with lamivudine, adefovir is associated with a low rate of antiviral resistance[14]. However, adefovir resistance occurs more common in patients with preexisting lamivudine resistance[7]. Among our cases, rtN236T mutation was detected in two patients at the time of viral breakthrough after adefovir rescue therapy. The other two showed no virologic response, and rtA181V was found in one patient.
YMDD mutations, mainly rtM204I in our cases, disappeared after the adefovir treatment, suggesting that cessation of antiviral therapy leads to disappearance of drug-resistant mutations. However, it was reported that lamivudine-resistant HBV mutants reappear rapidly after reintroduction of lamivudine[15]. Once antiviral-resistant HBV mutants have been selected, the mutants are archived even if treatment is stopped. In our cases, YMDD mutation reemerged within 3 mo after reintroduction of lamivudine, suggesting that re-treatment to which the virus has been previously resistant has a limited efficacy even after wild-type YMDD has restored. It could not be excluded that the adefovir-resistant mutations may affect the rapid emergence of lamivudine-resistant mutation.
The emergence of lamivudine resistance can result in viral breakthrough, flares of hepatitis and worsening of the initial histological improvement[12]. In patients with cirrhosis, severe exacerbation of hepatitis may result in hepatic failure[16]. It was reported that patients with YMDD mutations experience a higher rate of hepatitis flares than those without YMDD mutations[17], which is possibly because the YMDD locus takes part in cellular immune response in some cases.
Serum HBV DNA level was higher than baseline in the cases after emergence of the YMDD mutant before exacerbation occurred. Two patients experienced a very rapid deterioration of hepatic function accompanying the reemerging YMDD mutants only a few months after lamivudine reintroduction. These findings clearly demonstrate that sequential monotherapy, particularly retreatment with the antiviral agent to which the HBV has been previously resistant, should be avoided.
Combination therapy with adefovir and lamivudine or other antiviral agents could be a better option to prevent the emergence of antiviral-resistant mutations in patients with lamivudine- or adefovir -resistance. Combination therapy is not likely to improve virologic response, but rather decrease the rate of viral resis-tance[1819]. Unfortunately, combination therapy could not be commonly used in Korea so far because of the high cost of antiviral agents. Other antiviral agents, such as tenofovir or pegylated interferon, could be promising agents for multi-drug resistant HBV[2021]. All the patients in this study received combination therapy with adefovir and lamivudine or entecavir as a rescue therapy for lamivudine resistance. After the combination treatment, the HBV DNA and ALT level dropped and hepatic function was restored. It was reported that entecavir has a potent antiviral efficacy against naïve HBV and lamivudine-refractory HBV[22]. Case 1 received adefovir and entecavir as the rescue therapy. These two agents have antiviral activity on wild type or YMDD mutants without cross resistance. The other three patients received adefovir and lamivudine combination treatment. Case 3 showed a rapid reduction in HBV DNA level and HBeAg seroconversion soon after the combination therapy. Long-term data are not available from cases 2 and 4. However, they showed a significant decrease in HBV DNA level and improvement in hepatic function after combination therapy.
Taken together, lamivudine retreatment may induce rapid reemergence of the YMDD mutation and significant viral rebound and subsequent hepatic flare in patients with preexposed YMDD mutants. Sequential lamivudine treatment of those with a prior history of YMDD mutation should be abandoned.