Published online Jul 7, 2008. doi: 10.3748/wjg.14.3997
Revised: March 25, 2008
Accepted: April 1, 2008
Published online: July 7, 2008
AIM: To investigate dynamical and image pathological characteristics of the liver on magnetic resonance (MR) diffusion-weighted imaging (DWI) in the rabbit VX-2 tumor model.
METHODS: Forty New Zealand rabbits were included in the study and VX-2 tumor piece was implanted intrahepatically. Fifteen animals received two intrahepatic implantations while 25 had one intrahepatical implantation. DWI, T1- and T2-weighted of magnetic resonance imaging (MRI) were carried out on the 7th and the 14th d after implantation and DWI was conducted, respectively on the 21th d. Ten VX-2 tumor samples were studied pathologically.
RESULTS: The rate of lump detected by DWI, T1WI and T2WI was 78.7%, 10.7% and 53.5% (χ2 = 32.61, P < 0.001) on the 7th d after implantation and 95.8%, 54.3% and 82.9% (χ2 = 21.50, P < 0.001) on the 14th d. The signal of most VX-2 tumors on DWI was uniform and it was equal on the map of apparent diffusion coefficient (ADC). The signal of VX tumors did not decrease on the 7th d after implantation, most of them slowly growing during the week following implantation without significant cell dying within the tumor. VX-2 tumors grew increasingly within 14 d after implantation but the signal of most VX-2 tumors on DWI or on the map of ADC was uniform or uneven and ADC of VX tumors decreased obscurely or slightly because tumor necrosis was still not obvious. On the 21th d after implantation, the signal of most VX-2 tumors on DWI or on the map of ADC was uneven because tumor necrosis was evident and ADC of VX-2 tumor necrotic areas decreased. The areas of viable cells in VX-2 tumors manifested a high signal on DWI and a low signal on the map of ADC. The areas of dead cells or necrosis in VX-2 tumors manifested low signals on DWI and low, equal or high signals on the map of ADC but they manifested high signals on DWI and on the map of ADC at the same time when the areas of necrotic tumor became liquefied or cystic. The border of tumors on DWI appeared gradually distinct and internal signals of tumor became progressively uneven.
CONCLUSION: The manifestations of viable, necrotic and liquefied or cystic areas in VX-2 tumors on DWI are typical and DWI is of significant and potential values in clinical application in both the early detection and diagnosis of liver tumors.
- Citation: Yuan YH, Xiao EH, Liu JB, He Z, Jin K, Ma C, Xiang J, Xiao JH, Chen WJ. Characteristics of liver on magnetic resonance diffusion-weighted imaging: Dynamic and image pathological investigation in rabbit liver VX-2 tumor model. World J Gastroenterol 2008; 14(25): 3997-4004
- URL: https://www.wjgnet.com/1007-9327/full/v14/i25/3997.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.3997
Magnetic resonance (MR) diffusion-weighted imaging (DWI) is a new functional imaging technology developed in recent years[1–5], which is able to reflect non-woundingly water molecule diffusion in vivo. It is generally accepted that DWI is valuable in the qualitative and quantitative diagnosis of cerebral ischemia during the hyper-inchoate period[6–9]. In the past, DWI has not been extensively used in the diagnosis and evaluation of progression and prognosis of hepatic tumors, mainly because of its poor image quality. However, with the recent development of MR software and scanning technology, especially for echo planar imaging (EPI) series, the DWI poor imaging quality and slow scanning speed have been overcome[10–14]. This has led to the employment of DWI on the monitoring of focal and diffuse hepatic lesions, such as cysts, hemangiomas, hepatocellular carcinomas, metastases and liver cirrhosis. As reported by Ichikawa et al[1516], Yamashita et al[17], Taouli et al[18], and Sun et al[19], the apparent diffusion coefficient (ADC) of such lesions grew gradually on DWI, the ADC of cysts being the biggest, followed by benign tumors and malignant tumors. Colagrande et al[20] demonstrated that the coagulation necrosis manifested a low signal when compared to the normal parenchyma. Kamel et al[21] confirmed in 8 patients with hepatocellular carcinoma that the apparent diffusion coefficient (ADC) becomes higher with the extent of tumor necrosis and that the signals of 6 tumors were higher than normal parenchyma on DWI. The signals of liquor puris in abscesses were moderate or high on DWI but they were lower than the signals of cysts and cystic metastatic tumors because of their high consistency, and the signals of abscess walls were equal to those of normal parenchyma around[22–26].
From what has been discussed above, DWI, especially ADC, has potential values in reflecting characteristics of hepatic pathological changes and differentiating benign tumors from malignant tumors[27–31]. However, to date, no dynamic and image pathological investigation on the characteristics of hepatocellular carcinomas on DWI has been reported. Rabbit liver VX-2 tumor is the most valuable animal model of hepatocellular carcinoma in imaging investigation. The purpose of our experiment was to investigate the dynamic characteristics and the pathological mechanisms underlying the signals on DWI in rabbit VX-2 tumor models after implantation and to evaluate the superiority of DWI in detecting, diagnosing and differentiating tumors.
Animal studies were carried out under the supervision of a veterinarian according to the Guidelines of Chinese Ministry of Health for the Use of Laboratory Animals. All animals were provided by the Laboratory Animal Center of the Second Xiangya Hospital and all protocols were approved by the Animal Use and Care Committee of the Second Xiangya Hospital.
Forty New Zealand normal white rabbits were employed (22 males and 18 females), weighing 1.7-2.5 kg and aged 5-6 mo.
The rabbit VX-2 tumor strain was provided by the Fourth Military Medical University of China.
The VX-2 tumor donors were anesthetized by injecting 3% soluble pentobarbitone into auriborder vein or abdominal cavity at a dose of 1 mL/kg. The site of implantation was disinfected by Iodine, the skin was incised to expose one cauliflower of the subcutaneous tumor which was implanted before experiment and then one cauliflower was excised from it. The tumor parenchyma was exposed and quickly put into saline solution containing 40 000 unit gentamycin per 100 mL. The necrotic tissue and blood clot were discarded and the viable tumor tissue was divided into 1-2 mm2 microblocks.
The VX-2 tumor strain was subsequently implanted into the liver parenchyma of rabbits as follows. The animals were anesthetized by injecting 3% soluble pentobarbitone and the skin of abdomen was disinfected. The liver lobe chosen for implantation was exposed through incising the skin and vagina musculi recti abdominis. The tube containing the VX-2 tumor strain was implanted into the lobe by rotating and pushing it into the liver parenchyma. Then a gelatin sponge was inserted to prevent bleeding. Afterward, the peritoneum, musculus, and skin were sutured, respectively. Implantation was performed in one lobe in 25 rabbits and in two lobes in 15 rabbits. After implantation, 200 000 unit penicillin was administered by intramuscular injection for 4 d while the animal room was kept dry and ventilated.
The animals were anesthetized with 3% soluble pentobarbitone injected into auriborder vein at different doses based on the animal status to make sure that the breathing was slow and stable. T1-weighted imaging (T1WI), T2-weighted imaging (T2WI) and DWI were performed on a 1.5-Tesla Signa Twinspeed MR scanner (General Electron Medical Systems, USA), using a small diameter cylindrical brain radiofrequency coil. DWI (axial) and MRI (T1WI and T2WI, axial) were carried out on the 7th d and the 14th d after implantation and DWI (axial) was performed also on the 21th d after implantation. The scanning parameters of DWI included spin echo echo planar imaging (SE-EPI) series, b-value 100 and 300 s/mm2, repetition time (TR) 6000 ms, echo time (TE) 45 ms, 20 cm × 15 cm field of view (FOV), 8NEX, 2 mm thick layer, 0.5 mm space, 128 ×128 matrix, etc. The scanning parameters of common MRI included fast reverse, fast spin echo (FRFSE) series, T1WI (TR 400/TE 12.3 ms), T2WI (TR3000/TE80 ms), 20 cm × 15 cm FOV, 4 NEX, 5 mm thick layer, 0 mm space, 256 × 192 (T1WI) and 320 × 256 (T2WI) matrix.
Twelve samples of VX-2 tumors were processed. Ten samples were taken on the 21th d after implantation, one on the 7th d and the other one on the 14th d. The animals were euthanized with an overdose of 3% soluble pentobarbitone into auriborder vein. The VX-2 lump surrounded by normal liver parenchyma was taken under aseptic conditions. Then the lump was cut open and sliced (Figure 1). After 24 h of fixation in formaldehyde solution, all samples were embedded in mineral wax.
The distinction of tumor detection between DWI, T1WI and T2WI on the 7th d and the 14th d after implantation was respectively assessed. The statistical significance was calculated by χ2 analysis using SPSS12.0 software.
Thirty-eight of 40 rabbits were still alive 21 d after implantation, while 2 rabbits died from overdose of anesthesia. Forty-seven VX-2 tumors were detected in 47 hepatic lobes of 35 rabbits.
On the 7th d after implantation, 37 lumps, 3 lumps and 15 lumps were respectively detected by DWI, T1- and T2-weighted imaging and the detection rates were 78.7% (37/47), 10.7% (3/28) and 53.8% (15/28), respectively. The difference of detection was significant among DWI, T1- and T2 weighted imaging, between DWI and T2 weighted imaging and between T1- and T2 weighted imaging (χ2 = 32.61, P < 0.001; χ2 = 5.22, P = 0.022; χ2 = 11.79, P < 0.001). On the 14th d after implantation, 45 lumps, 19 lumps and 29 lumps were detected by DWI, T1- and T2-weighted imaging and the detection rates were 95.87% (45/47), 54.3% (19/35) and 82.9% (29/35), respectively. The difference of detection was significant among DWI, T1- and T2-weighted imaging, between DWI and T2 weighted imaging and between T1- and T2 weighted imaging (χ2 = 21.50, P < 0.001; χ2 = 3.78, P > 0.05; χ2 = 6.63, P = 0.01). On the 14th d after implantation, all 47 tumors were detected by DWI (b = 100 or b = 300 s/mm2).
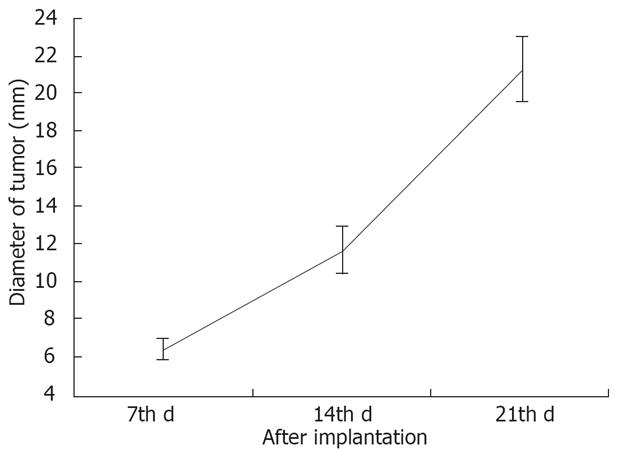
The average diameter of VX-2 tumors detected by DWI, T1- and T2 weighted imaging was 6.49 (3.00-12.90) mm, 11.78 (5.00-25.10) mm and 21.44 (7.50-36.70) mm respectively on the 7th, the 14th d and the 21th d after implantation (Figure 2).
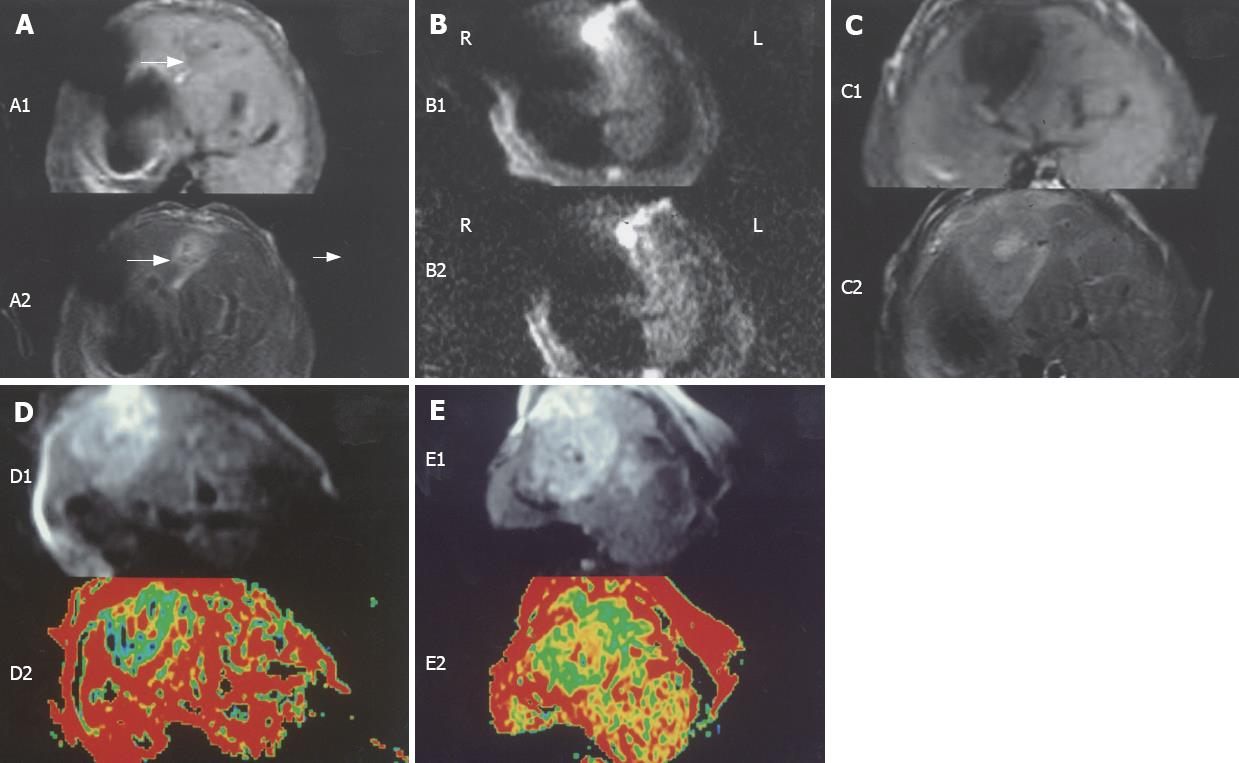
The signals of most VX-2 tumors were low and the borders were unclear on T1-weighted imaging (5/15), whereas the signals were high and the borders were clear on T2-weighted imaging (14/15) seven days after implantation (Figure 3). The signals of VX-2 tumors were high with unclear borders on T1-weighted imaging (18/19), while they were high with clear borders on T2-weighted imaging (14/29) two weeks following implantation (Figure 3). DWI appearance of hepatic VX-2 tumors is summarized in Tables 1 and 2.
| Tumors detected | Border | Signal | Signal of center | ADC map | ||||||
| Clear | Unclear | Even | Uneven | High | Equal | Low | Equal | Low | ||
| A | 37 | 5 | 32 | 30 | 7 | 1 | 32 | 4 | 29 | 8 |
| B | 45 | 28 | 17 | 25 | 20 | 2 | 32 | 11 | 20 | 25 |
| C | 47 | 45 | 2 | 9 | 38 | 5 | 14 | 28 | 4 | 43 |
| Tumors detected | Border | Signal | Signal of center | ADC map | ||||||
| Clear | Unclear | Even | Uneven | High | Equal | Low | Equal | Low | ||
| A | 37 | 5 | 32 | 29 | 8 | 0 | 33 | 4 | 33 | 4 |
| B | 45 | 30 | 15 | 25 | 20 | 2 | 32 | 11 | 19 | 26 |
| C | 47 | 46 | 1 | 9 | 38 | 3 | 16 | 28 | 3 | 44 |
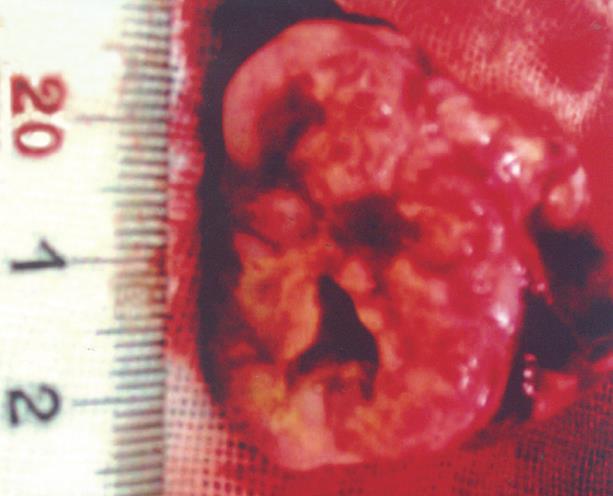
On the 7th and 14th d after implantation, intrahepatic VX-2 tumors appeared pale without identifiable margin or envelope. No lumps were detected in the hepatic peritumoral parenchyma, whereas residuum of gelation sponge in or around VX-2 tumors was detected. On the 21th d after implantation, many encroachments and metastatic tumors were found in dissection, including abdominal wall encroachment in 15 cases, abdominal dropsy in 16, mesenterium encroachment in 15, lung metastases in 19, pleural effusion in 12 and diaphragm encroachment in 2. The extrahepatic surface was uneven and VX-2 tumors were pale, with a clear distinction between tumoral tissues and normal surrounding parenchyma. The tumors were hard and there was no clear amiculas. Cavitates of unequal size were found in the lumps of some cases because of kermesinus liquid running off after the tumors were cut open and there was some residuum of gelation sponge in or around the lumps of some tumors (Figure 4).
Macroscopically, the surface of the peritumoral hepatic parenchyma was brown-grey; 11 small lumps, observed on the liver surface, proved to be metastatic by pathology. The texture of normal hepatic parenchyma was soft after being cut open. Microscopically (× 100), the peritumoral architecture and cell morphology were normal and necrotic zones were not observed. However, there were some different thickening blood vessels and inflammatory cells as well as areas of edema or ballooning degeneration. At higher magnification (× 400), the size of cell was equal on the whole and there was abundant endochylema in cells. Abnormal caryocinesia was not observed in cell nucleuses.
The outer layer areas and the periphery areas in VX-2 tumors appeared grey, fish and hard without distinct borders and amiculas between the area of normal hepatic parenchyma and the area of VX-2 tumor outer layer (Figure 4). The distribution of necrotic areas differed between the cancer center and its periphery, being prevalent in the tumor core and significantly less at its periphery or its outer layer. Tumor necrotic tissue looked like tofukasu and tumor viable tissue looked like fish (Figure 4).
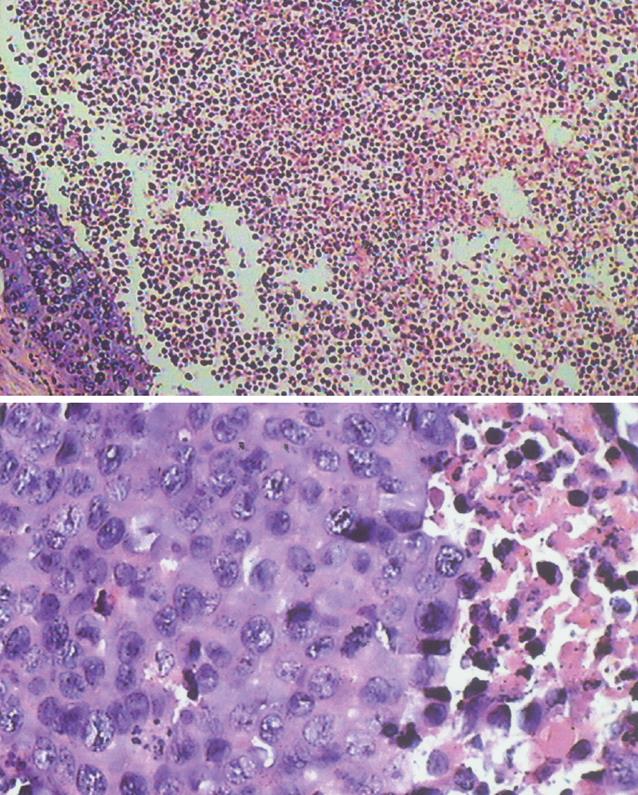
At low magnification (× 100), a plenty of tumor nests in lumps were observed and they showed inequality in size, and round or ellipse in shape. There were more blood capillaries and little connective tissue in lumps. Calcification changes and residuum of gelation sponge could be observed in some cases. There were unequal necroses in the area of most VX-2 tumor center but there was little necrosis in the area of tumor periphery (Figure 5).
At higher magnification (× 400), the size of most tumor cells was not equal and tumor nests differed in size, and round or ellipse in shape. There was little intercellular substance in tumors and some cell membranes appeared poorly defined. The size of cell nucleuses was uneven and the number of cell nucleuses was not equal so that the karyoplasmic ratio of cells was altered. Most of cell nucleuses were stained deeply and some of them had obvious caryocinesia. Some apoptotic cells were also observed.
Diffusion is caused by water molecular random motion, so-called “Brownian motion”[24–27]. By adding a powerful polar and quick switching gradient radiofrequent (RF) pulse, it is possible to amplify these phase changes in order to detect water molecule diffusion motion, known as diffusion-weighted imaging (DWI)[16182025]. By using such an approach, the signals of hepatocellular carcinoma, metastases and hemangiomas were higher than that of the surrounding hepatic parenchyma, as reported by Ichikawa et al[1516], Taouli et al[18] and Yang et al[32]. This phenomenon can be explained by differences in terms of water molecular motions, which are limited in hepatocellular carcinoma, metastases and hemangiomas when compared to normal hepatic tissues. Changes of their phase position in the magnetic field were therefore smaller and signal deamplification is reduced. Although the limitation of water molecular motion in the hemangioma is smaller than that of hepatocellular carcinoma or metastasis, the signal of the former was higher than that of the latter because there were more water molecules in the hemangioma.
As VX-2 tumor is a solid tumor and its body mainly consists of tumor cells, tumor nests and other cells, its water molecular diffusion motion is restricted. The signals of VX-2 tumor on DWI are higher than that of normal hepatic parenchyma because the signal deamplification of VX-2 tumor is small. On the 7th, the 14th d and the 21th d after implantation in our experiment, the signals of most VX-2 tumors were higher than that of normal parenchyma, and they looked like “lamp bulbs” (Figure 3). Their margins were usually distinct and their ADC values were lower than that of normal parenchyma, confirming the published data.
On the 7th d after implantation, the ability of detecting tumors by DWI was significantly higher than that of T1-weighted imaging (78.7% vs 10.7%) or T2-weighted imaging (78.7% vs 53.5%) and the signal contrast on DWI between VX-2 tumor and normal parenchyma was more evident when compared to T1-weighted or T2-weighted imaging (Figure 3A and B). DWI has important and potential clinical value in detecting tumors earlier because a powerful polar and quick switching gradient RF pulse besides MRI routine RF is added, molecular phase changes are amplified, which has not been reported in literatures before. The signals of most VX-2 tumors on DWI were high and uniform, including the central area of tumor on the 7th d after implantation; the signals on the map of ADC were equal even if most borders were unclear (Figure 3), probably because, at this time, the blood provision of VX-2 tumors is sufficient and there is no coagulative necrosis or colliquation.
On the 14th d after implantation, the signals of DWI in 31% (b-value 100 s/mm2) and 38% (b-value 300 s/mm2) of all tumor models were uneven and the borders were relatively distinct. The low signals detected in the central areas of 11 cases proved by pathology to be caused by unabsorbed gelatin sponge. At this time, no coagulative necrosis or colliquation were observed. Inflammation and cellular edema tended to decrease at d 14 after implantation in comparison with day 7.
On the 21th d after implantation, the signals of VX-2 tumors were high and 95% of margins were distinct on DWI while the signals on the map of ADC were low, with equally distinct borders. However, the histopathological examination did not show amiculas or distinct borders between VX-2 tumors and normal parenchyma (× 100 and × 400). A possible explanation of the high signals and distinct borders of tumors on DWI is related to the obvious limitations of water molecular motion in VX-2 tumors. The VX-2 tumor periphery area manifested mostly uniform, producing a high signal on DWI and low signal on the map of ADC. Samples in the area of VX-2 tumor periphery looked like grey fish macroscopically (Figure 4), containing viable tumor cells which showed inequality in size, and round or ellipse tumor nests under microscope (Figure 5). The intra-tumor signals were uneven on DWI with low and unequal areas in 28 lumps (Figure 3), where coagulative necrosis was confirmed by pathology (Figure 5). Because VX-2 tumors grow quickly, blood provision becomes insufficient causing local or lamellar coagulation necroses in the tumor. This result confirms the published data by Colagrande et al[20], Kamel et al[21] and Geschwind et al[22]. The limitation of water molecule diffusion is inversely proportional to the extent of the tumor necrosis, because intact cell membranes can restrict the diffusion of water molecules when tumor cells are viable. The necrotic areas generated unequal signals (Figure 3D and E), being significantly smaller than the areas of viable tumor. Histologic analysis demonstrated unequal size, endochylema disproportion and pathological caryocinesis of most viable tumor cells. Areas of high signal on DWI were observed intratumorally in 5 cases (b-value 100 s/mm2) and 3 cases (b-value 300 s/mm2) (Figure 3D). Histopathological analysis showed that these areas were due to colliquation after necrosis. The signals of constitution on DWI were affected not only by diffusion of water molecules, but also by its T2-value contribution. When the T2-value of this constitution is long (value long?) and the b-value is small in diffusion-weighted imaging scanning, the signal of constitution will be affected significantly by the long T2-value contribution, the so called “shine-through”. As reported by Yamashita et al[17], Taouli et al[18] and Yang et al[32], the limitation of water molecular motion in hepatic cysts was lower than that of the normal parenchyma; however, the signals of the cysts were higher on DWI because the number of water molecules in hepatic cysts is higher than the hepatic parenchyma. The great amount of extracellular water molecules within the necrotic region caused by cell lysis allows free diffusion to take place, justifying the resultant high signals on DWI.
As shown in Figure 2, the growth velocity of VX-2 tumor from day 7 to 14 after implantation was lower than that from d 14 to 21. At the same time, several infiltrating and metastatic tumors were found by dissection on the 21th d after implantation. According to our experience, it is a period of quick growing for VX-2 tumor from the 14th d to the 21th d after implantation and it is suitable for rabbit VX-2 tumor models to be treated interventionally or by other therapies.
Overall, the appearance of viable, necrotic and liquefied or cystic areas of VX-2 tumor on DWI is typical. Therefore, DWI could be applied in clinics for the early detection and diagnosis of liver tumors. The areas of viable cells in VX-2 tumors are associated with high signals, distinct borders, so called “lamp bulb” on DWI and low signals on the map of ADC. On the contrary, the necrotic areas in VX-2 tumors show low signals on DWI and equal or low signals on the map of ADC. Finally, high signals on DWI and on the map of ADC are produced when the areas of necrotic tumor are liquefied or have become cystic.
Magnetic resonance (MR) diffusion-weighted imaging (DWI) is a new functional imaging technology developed in recent years, which is able to reflect non-woundingly water molecule diffusion in vivo and is valuable in the qualitative and quantitative diagnosis of cerebral ischemia during the hyper-inchoate period. Ichikawa et al, Yamashita et al, Taouli et al, Sun et al and Yang et al have reported the characteristics of hepatic lesions on DWI, such as cysts, hemangiomas, hepatocellular carcinomas, metastases and liver cirrhosis. However, because of its poor image quality, DWI has not been extensively used in the diagnosis and evaluation of progression and prognosis of hepatic tumors in the past. Moreover, to date, a dynamic and image pathological investigation in the characteristics of hepatocellular carcinomas on DWI has never been reported. We believe that DWI has potential values in reflecting characteristics of hepatic pathological changes and differentiating benign tumors from malignant tumors with the recent development of MR software and scanning technology, especially for echo planar imaging (EPI) series.
Many studies of hepatic pathological changes on DWI have been reported. ADC values of benign lesions, such as hepatic cysts and hemangiomas, were higher than those of malignant lesions, such as hepatocellular carcinomas and metastases on DWI and many studies also indicated that the signals of tumor coagulative necrotic areas were lower than that of tumor viable areas. Moreover, Colagrande et al demonstrated that the coagulation necrosis manifested a low signal when compared to the normal parenchyma. There has been no dynamical and image pathological investigation on the characteristics of the liver on DWI in the rabbit VX-2 tumor model.
This study clearly demonstrates that the areas of viable cells in VX-2 tumors manifested a high signal on DWI and a low signal on the map of apparent diffusion coefficient (ADC), the areas of dead cells or necrosis in VX-2 tumors manifested a low signal on DWI and a low, equal or high signal on the map of ADC, but they manifested high signals on DWI and on the map of ADC at the same time when the areas of necrotic tumors had become liquefied or cystic. The manifestations of viable, necrotic and liquefied or cystic areas in VX-2 tumors on DWI were typical. The rate of lump detected by DWI was much higher than that by T1WI or T2WI after implantation. DWI has significant and potential clinical application values in detecting and differentiating viable tumors from necrotic tumors and in the early detection and diagnosis of liver tumors.
Physicians can apply this knowledge to evaluate obviously progressive hepatic tumors and differentiate accurately the areas and degrees of necrotic tumors from that of viable tumors.
This is an interesting, well designed and written study on the clinical significance of the liver on diffusion-weighted imaging and the manuscript contained important information on the manifestations of viable, necrotic and liquefied or cystic areas in tumors and clinical application values in the early detection and diagnosis of liver tumors.
| 1. | Morimoto M, Shirato K, Sugimori K, Kokawa A, Tomita N, Saito T, Imada T, Tanaka N, Nozawa A, Numata K. Contrast-enhanced harmonic gray-scale sonographic-histologic correlation of the therapeutic effects of transcatheter arterial chemoembolization in patients with hepatocellular carcinoma. AJR Am J Roentgenol. 2003;181:65-69. |
| 2. | Kubota K, Hisa N, Nishikawa T, Fujiwara Y, Murata Y, Itoh S, Yoshida D, Yoshida S. Evaluation of hepatocellular carcinoma after treatment with transcatheter arterial chemoembolization: comparison of Lipiodol-CT, power Doppler sonography, and dynamic MRI. Abdom Imaging. 2001;26:184-190. |
| 3. | Ebied OM, Federle MP, Carr BI, Pealer KM, Li W, Amesur N, Zajko A. Evaluation of responses to chemoembolization in patients with unresectable hepatocellular carcinoma. Cancer. 2003;97:1042-1050. |
| 4. | Kim HC, Kim AY, Han JK, Chung JW, Lee JY, Park JH, Choi BI. Hepatic arterial and portal venous phase helical CT in patients treated with transcatheter arterial chemoembolization for hepatocellular carcinoma: added value of unenhanced images. Radiology. 2002;225:773-780. |
| 5. | Shankar S, vanSonnenberg E, Morrison PR, Tuncali K, Silverman SG. Combined radiofrequency and alcohol injection for percutaneous hepatic tumor ablation. AJR Am J Roentgenol. 2004;183:1425-1429. |
| 6. | Minami Y, Kudo M, Kawasaki T, Kitano M, Chung H, Maekawa K, Shiozaki H. Transcatheter arterial chemoembolization of hepatocellular carcinoma: usefulness of coded phase-inversion harmonic sonography. AJR Am J Roentgenol. 2003;180:703-708. |
| 7. | Zhang Z, Wu M, Chen H, Chen D, He J. Percutaneous radiofrequency ablation combined with transcatheter arterial chemoembolization for hepatocellular carcinoma. Zhonghua Waike Zazhi. 2002;40:826-829. |
| 8. | Seki T, Tamai T, Ikeda K, Imamura M, Nishimura A, Yamashiki N, Nakagawa T, Inoue K. Rapid progression of hepatocellular carcinoma after transcatheter arterial chemoembolization and percutaneous radiofrequency ablation in the primary tumour region. Eur J Gastroenterol Hepatol. 2001;13:291-294. |
| 9. | Kubota K, Hisa N, Nishikawa T, Fujiwara Y, Murata Y, Itoh S, Yoshida D, Yoshida S. Evaluation of hepatocellular carcinoma after treatment with transcatheter arterial chemoembolization: comparison of Lipiodol-CT, power Doppler sonography, and dynamic MRI. Abdom Imaging. 2001;26:184-190. |
| 10. | Chan JH, Tsui EY, Luk SH, Yuen MK, Cheung YK, Wong KP. Detection of hepatic tumor perfusion following transcatheter arterial chemoembolization with dynamic susceptibility contrast-enhanced echoplanar imaging. Clin Imaging. 1999;23:190-194. |
| 11. | Tsui EY, Chan JH, Cheung YK, Cheung CC, Tsui WC, Szeto ML, Lau KW, Yuen MK, Luk SH. Evaluation of therapeutic effectiveness of transarterial chemoembolization for hepatocellular carcinoma: correlation of dynamic susceptibility contrast-enhanced echoplanar imaging and hepatic angiography. Clin Imaging. 2000;24:210-216. |
| 12. | Lovblad KO, Wetzel SG, Somon T, Wilhelm K, Mehdizade A, Kelekis A, El-Koussy M, El-Tatawy S, Bishof M, Schroth G. Diffusion-weighted MRI in cortical ischaemia. Neuroradiology. 2004;46:175-182. |
| 13. | Na DG, Thijs VN, Albers GW, Moseley ME, Marks MP. Diffusion-weighted MR imaging in acute ischemia: value of apparent diffusion coefficient and signal intensity thresholds in predicting tissue at risk and final infarct size. AJNR Am J Neuroradiol. 2004;25:1331-1336. |
| 14. | O'Donnell ME, Tran L, Lam TI, Liu XB, Anderson SE. Bumetanide inhibition of the blood-brain barrier Na-K-Cl cotransporter reduces edema formation in the rat middle cerebral artery occlusion model of stroke. J Cereb Blood Flow Metab. 2004;24:1046-1056. |
| 15. | Ichikawa T, Haradome H, Hachiya J, Nitatori T, Araki T. Diffusion-weighted MR imaging with a single-shot echoplanar sequence: detection and characterization of focal hepatic lesions. AJR Am J Roentgenol. 1998;170:397-402. |
| 16. | Ichikawa T, Haradome H, Hachiya J, Nitatori T, Araki T. Diffusion-weighted MR imaging with single-shot echo-planar imaging in the upper abdomen: preliminary clinical experience in 61 patients. Abdom Imaging. 1999;24:456-461. |
| 17. | Yamashita Y, Tang Y, Takahashi M. Ultrafast MR imaging of the abdomen: echo planar imaging and diffusion-weighted imaging. J Magn Reson Imaging. 1998;8:367-374. |
| 18. | Taouli B, Martin AJ, Qayyum A, Merriman RB, Vigneron D, Yeh BM, Coakley FV. Parallel imaging and diffusion tensor imaging for diffusion-weighted MRI of the liver: preliminary experience in healthy volunteers. AJR Am J Roentgenol. 2004;183:677-680. |
| 19. | Sun XJ, Quan XY, Liang W, Wen ZB, Zeng S, Huang FH, Tang M. [Quantitative study of diffusion weighted imaging on magnetic resonance imaging in focal hepatic lesions less than 3 cm]. Zhonghua Zhongliu Zazhi. 2004;26:165-167. |
| 20. | Colagrande S, Politi LS, Messerini L, Mascalchi M, Villari N. Solitary necrotic nodule of the liver: imaging and correlation with pathologic features. Abdom Imaging. 2003;28:41-44. |
| 21. | Kamel IR, Bluemke DA, Ramsey D, Abusedera M, Torbenson M, Eng J, Szarf G, Geschwind JF. Role of diffusion-weighted imaging in estimating tumor necrosis after chemoembolization of hepatocellular carcinoma. AJR Am J Roentgenol. 2003;181:708-710. |
| 22. | Geschwind JF, Artemov D, Abraham S, Omdal D, Huncharek MS, McGee C, Arepally A, Lambert D, Venbrux AC, Lund GB. Chemoembolization of liver tumor in a rabbit model: assessment of tumor cell death with diffusion-weighted MR imaging and histologic analysis. J Vasc Interv Radiol. 2000;11:1245-1255. |
| 23. | Somford DM, Marks MP, Thijs VN, Tong DC. Association of early CT abnormalities, infarct size, and apparent diffusion coefficient reduction in acute ischemic stroke. AJNR Am J Neuroradiol. 2004;25:933-938. |
| 24. | Brugieres P, Thomas P, Maraval A, Hosseini H, Combes C, Chafiq A, Ruel L, Breil S, Peschanski M, Gaston A. Water diffusion compartmentation at high b values in ischemic human brain. AJNR Am J Neuroradiol. 2004;25:692-698. |
| 25. | Mitsias PD, Ewing JR, Lu M, Khalighi MM, Pasnoor M, Ebadian HB, Zhao Q, Santhakumar S, Jacobs MA, Papamitsakis N. Multiparametric iterative self-organizing MR imaging data analysis technique for assessment of tissue viability in acute cerebral ischemia. AJNR Am J Neuroradiol. 2004;25:1499-1508. |
| 26. | Moteki T, Horikoshi H, Oya N, Aoki J, Endo K. Evaluation of hepatic lesions and hepatic parenchyma using diffusion-weighted reordered turboFLASH magnetic resonance images. J Magn Reson Imaging. 2002;15:564-572. |
| 27. | Wang JL, Xie JX. Assessment of hemodynamics and pathophysiology of acute cerebral ischemia with MR perfusion-weighted imaging and dynamic diffusion-weighted imaging. Zhonghua Fangshexue Zazhi. 1998;32:370-374. |
| 28. | Xiao XH, Kong XQ, Jiang Li, Jiang L, Wang YM, Xu HB, Li LY, Tang YF. An experimental study on acute cerebral ischemia and reperfusion with magnetic resonance diffusion weighted imaging. Zhonghua Fangshexue Zazhi. 1999;33:662-666. |
| 29. | Xie JX, Fu Y, Zhang Y. The change of water cellular diffusion motion and its clinical application. Beijing Daxue Xuebao (Yixueban). 2001;33:109-112. |
| 30. | Han HB, Xie JX. Application of EPI diffusion-weighted and Gd DTPA2 perfusion imaging in the diagnosis of brain ischemia. Zhonghua Fangshexue Zazhi. 1998;32:364-369. |
| 31. | Marks MP, de Crespigny A, Lentz D, Enzmann DR, Albers GW, Moseley ME. Acute and chronic stroke: navigated spin-echo diffusion-weighted MR imaging. Radiology. 1996;199:403-408. |
| 32. | Yang ZH, Xie JX, Zhang YW, HU BF. Study on diffusion-weighted imaging in cirrhotic liver. Zhongguo Yixue Yingxiang Jishu. 2002;9:907-909. |