Published online Jun 14, 2008. doi: 10.3748/wjg.14.3518
Revised: May 13, 2008
Accepted: May 20, 2008
Published online: June 14, 2008
AIM: To investigate the proportion of patients with moderate-severe erosive esophagitis (EE) who will have Barrett’s esophagus (BE) after healing of inflammation.
METHODS: Patients with EE of Los Angeles (LA) class B, C and D who underwent follow-up endoscopy documenting complete mucosal healing.
RESULTS: A total of 86/169 patients were suspected of having BE (38 before healing and 48 after healing of EE) and, 46/86 eventually had the histological confirmation. At index esophago-gastro-duodenoscopy (EGD), BE was suspected in 38/169 (22%), and ultimately, histologically confirmed in 20 of these. In 11 patients where biopsies were performed in the presence of inflammation, BE was detected in 2 and missed in 5 (including 2 dysplasias). In 131/169 patients (77.5%), BE was not suspected at index EGD. After healing of EE though, 48 patients had suspicion of BE who underwent biopsies, and in 26 of these histology was positive for BE. The length of inflammation had a linear correlation with the length of BE (P = 0.01). Out of multiple variables to predict BE, only the suspicion at index endoscopy was statistically significant (P = 0.01).
CONCLUSION: BE was seen in 46/169 (27%) patients with EE of LA class B, C and D. The length of EE can predict the length of underlying BE segment. Even when suspected, BE and associated dysplasia can be missed in the presence of inflammation; therefore, repeat evaluation should be considered after complete healing of esophagitis.
- Citation: Gilani N, Gerkin RD, Ramirez FC, Hakim S, Randolph AC. Prevalence of Barrett’s esophagus in patients with moderate to severe erosive esophagitis. World J Gastroenterol 2008; 14(22): 3518-3522
- URL: https://www.wjgnet.com/1007-9327/full/v14/i22/3518.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.3518
Gastroesophageal reflux disease (GERD) affects 10-20 percent population in Western nations and may be complicated by erosive esophagitis (EE), Barrett’s esophagus (BE), and esophageal adenocarcinoma among others[1]. In a large population-based cohort study, the estimated incidence of esophagitis was 2.4 per 1000 patient-years[2] and the importance of this complication rests in its potential for masking underlying BE, a condition clearly associated with adenocarcinoma[3]. BE appears to have a higher prevalence in middle-aged to elderly men[4–6]. In one study involving a population of veteran patients, the prevalence of BE was 13% in the setting of typical gastro-esophageal reflux symptoms[7]. The current opinion, once EE is found on endoscopy, is to provide effective anti-secretory therapy to eliminate the confounding factor (both visual and histological) of esophagitis, in order to correctly diagnose BE and any associated dysplasia. However, this practice is not universally accepted by the gastroenterology physicians due to limited data on the prevalence of BE in patients with EE[8]. We aimed to investigate the prevalence of BE in a group of patients with moderate-severe EE, after achieving complete healing on follow-up endoscopy.
Records of all esophago-gastro-duodenoscopies (EGDs) performed at our institution between January 1998 and June 2006 were retrospectively reviewed from our endoscopy database. Our standardized, electronic report protocol contains all the essential elements included in the analysis (length of EE, Los Angeles classification of EE, length of suspected BE segment, size of hiatus hernia, etc). Other required information was obtained from patient’s electronic medical records. All EGDs were either performed independently by a board certified, faculty gastroenterologist or by an in-training fellow under direct supervision of a faculty member. Photo documentation was obtained in all cases. Our department mandates completion of all elements of endoscopy reporting and adherence to the standardized biopsy protocol. In addition, patients with EE of class C and D are routinely (irrespective of the suspicion of BE) followed endoscopically, while class B patients undergo a follow-up EGD only if a normal Z-line cannot be clearly identified. For follow-up purposes, patients are routinely given written instructions at the conclusion of the initial visit in addition to a mailed letter, and a reminder phone call by the department clerk the day before their follow-up appointment. The patient population using our department’s services comprises of 80% Caucasians, 15 % Hispanics and 5% African-Americans.
LA classification of EE defines class A as one or more mucosal breaks confined to the mucosal folds, each no longer than 5 mm; class B, at least one mucosal break > 5 mm, confined to the mucosal folds, not contiguous between the tops of 2 folds; class C, at least one mucosal break contiguous between the tops of 2 or more mucosal folds but not circumferential (≤ 75% of the luminal circumference) and class D, one or more circumferential mucosal breaks (comprising > 75% of esophageal luminal circumference). Inclusion criteria: all patients with EE of LA classes B, C, or D, who after receiving intensive anti-secretory therapy using double dose proton pump inhibitors (institutional formulary restricted to the use of omeprazole, lansoprazole and rabeprazole) had achieved complete mucosal healing, documented on at least one follow-up EGD. Exclusion criteria: patients with EE of LA class A, those without EGD follow up, those who failed to achieve complete mucosal healing after PPI therapy, those with known BE, and those with an EGD performed at our institution prior to the index examination. Demographic data including age, race, sex, and body mass index were noted. At index EGD length and LA class of EE, presence/absence of hiatus hernia and its estimated size were recorded. If biopsy specimens were obtained at index procedure due to suspected BE, the results of these were noted and compared with results of biopsies taken at follow-up endoscopy. Finally, if BE was endoscopically suspected, its length, and results of four quadrant biopsies taken at 2 cm intervals (a uniform practice in our department), when applicable, were documented. For non-circumferential BE (tongue like salmon colored projections above the esophago-gastric junction), the mucosal sampling was obtained in a similar fashion from the involved area. BE was suspected endoscopically by the presence of salmon-colored columnar-appearing mucosa in the tubular esophagus, and confirmed by the presence of specialized intestinal metaplasia containing goblet cells, on histology. BE was labeled as short segment (SSBE) or long segment (LSBE) based on the length of the columnar appearing mucosal segment of < 3 cm, or ≥ 3 cm, respectively. The presence of any grade of dysplasia on histology was recorded. This study was approved by our institutional review board (IRB).
Continuous variables were reported as mean ± standard error of the mean (SEM). Categorical data were listed as percentages (%). For continuous outcomes, analysis of variance (ANOVA) was used to determine differences between groups. Logistic regression was performed to identify variables predictive of BE. These variables included age, length and severity of EE at index endoscopy, size of hiatus hernia, body mass index, and suspicion of BE at index endoscopy. Also, linear regression was used to determine predictors of the length of BE found after healing. A two-tailed P < 0.05 was considered statistically significant.
A total of 546 patients had the endoscopic diagnosis of EE at our institution between January, 1998 and June, 2006. Of these, 377 were excluded from the study (EE class A, n = 168; prior EGD or history of BE, n = 91; EE class B with visibly normal Z-line, n = 68; failure to follow up, n = 18; incomplete healing, n = 18; insufficient documentation, n = 14). After excluding above, 169 patients met the inclusion criteria of LA class B (36/169, 21.3%), C (83/169, 49.1%), or D (50/169, 29.6%) EE, and subsequently demonstrated complete healing on at least one follow-up EGD (mean duration: 12 wk; range: 2-52 wk; only 2 patients underwent FU EGD at 52 wk). The indications for the index EGD varied as listed in Table 1. The demographic information of the study population is listed in Table 2. The characteristics of the excluded patients were similar to the patients included in the study. Patients initially diagnosed with EE were mostly (98%) Caucasian men, and those diagnosed with BE were all men. The average age, BMI, and size of hiatus hernia were similar between BE and non-BE groups (Table 2). A hiatus hernia was more prevalent in patients diagnosed with BE than non-BE patients (90.2% vs 72.6%, respectively; P < 0.001). The mean length of EE segment in the study population was 4.44 ± 0.35 cm; the mean length of EE in class B was 2.94 ± 0.56 cm; class C, 3.83 ± 0.38 cm and class D, 6.30 ± 0.77 cm. Interestingly, the mean length of EE segment increased as the severity of LA class increased (D > B/C; P = 0.002).
| Indications | Number of patients |
| HB | 65 |
| HB + dysphagia | 18 |
| HB + abd. Pain | 5 |
| HB + hemetemesis | 2 |
| HB + N/V | 1 |
| HB + anemia | 1 |
| Dysphagia | 29 |
| Hemetemesis | 16 |
| N/V | 6 |
| Dyspepsia | 6 |
| Anemia | 5 |
| Melena | 4 |
| Abdominal pain | 3 |
| Odynophagia | 2 |
| Globus sensation | 1 |
| Chest pain | 1 |
| Cough/pyrosis | 1 |
| Variceal screening | 1 |
| Hemoccult positive stools | 1 |
| PEG | 1 |
| Patient demographics | BE group | Non BE group |
| (n = 46) | (n = 123) | |
| Mean age (yr) | 57.2 | 58.6 |
| Men (%) | 100 | 95.9 |
| Race | ||
| Caucasian (%) | 87 | 87 |
| Hispanic (%) | 10.9 | 10.6 |
| Black (%) | 2.2 | 2.4 |
| BMI (kg/m2 ) | 28.6 | 28.8 |
| Hiatus hernia size1 (mean, cm) | 3 | 2.5 |
| % HH (P < 0.001) | 90.2 | 72.6 |
A total of 86/169 (50.9%) patients were suspected of BE (either before or after healing of EE), and 80 of these who underwent repeat biopsies, histological confirmation was obtained in 46 (57.5%). Histological confirmation of BE in patients with suspected LSBE was 12/16 (75%) and in patients with suspected SSBE was 34/64 (53.1%).
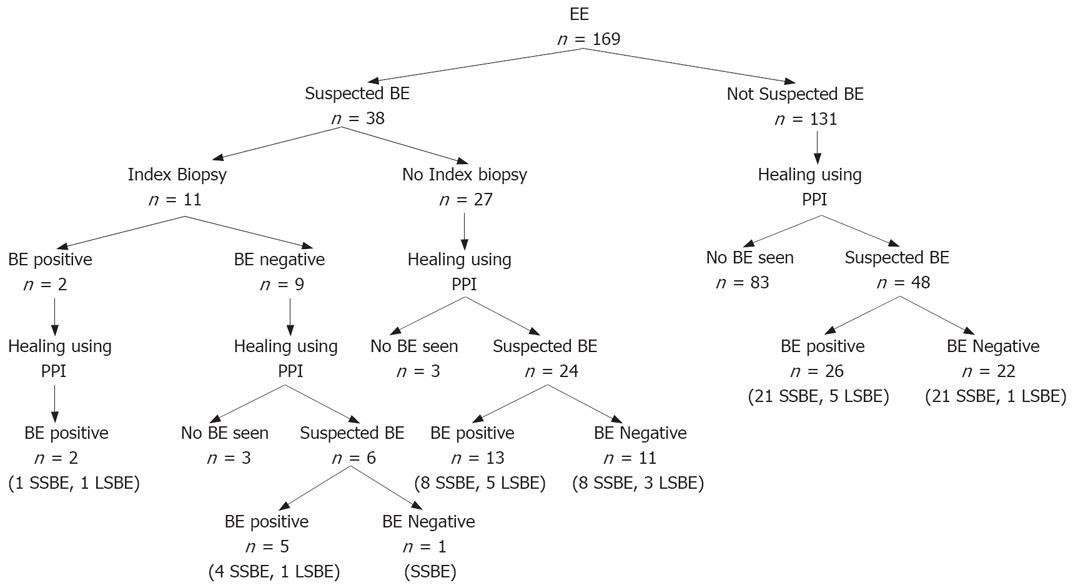
In 38 (22.5%) patients, BE was visually suspected at index EGD and in 11 of these biopsies were performed during the same procedure. Interestingly, BE could initially be confirmed only in 2 of these 11; however, after healing, 6 still had suspicion of BE and repeat biopsies confirmed specialized intestinal metaplasia in 5 of them (two patients also had a low grade and a high grade dysplasia each). In the remaining 27 with suspicion of BE at index EGD who did not undergo index biopsies, 24 were still suspected of BE after healing of EE, and in 13/24 histology was positive for BE on repeat biopsies (Figure 1).
A total of 131 EE patients were not suspected of having BE at index EGD. After healing of EE though, BE was suspected in 48/131 (who underwent biopsies). Of these, 26 were histologically confirmed as having BE (Figure 1).
Overall, 46/169 (27%) from the study population had BE confirmed by histology. The breakdown by LA class included 8 (17.4%) from B (6 SSBE, 2 LSBE), 25 (54.3%) from C (20 SSBE, 5 LSBE) and 13 (28.3%) from class D (8 SSBE, 5 LSBE) EE. This represented 22.2% of all patients from class B, 30.1% from C and 26% from class D esophagitis.
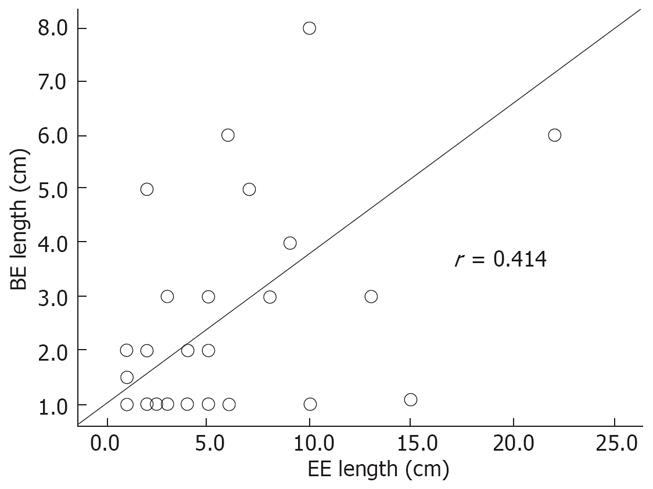
Logistic regression performed to identify variables that could predict the presence of BE (including age, BMI, length and severity of EE, size of hiatus hernia, and suspicion of BE at index) found only “suspicion of BE at index” to be significant. Patients in whom BE was suspected at index EGD were 4.5 times more likely to have histological confirmation than those in whom it was not suspected at index EGD (P = 0.01). Linear regression was also performed and it was found that the length of EE correlated with the length of BE (found after healing of esophagitis); there appeared to be a linear relationship between these two variables (P = 0.009) as shown in Figure 2.
BE is an important, potentially pre-malignant complication of gastro-esophageal reflux disease. The major reason to evaluate patients with longstanding GERD is to recognize BE[9]. The possible role of GERD induced EE leading to BE has not been clearly established, but possible cellular injury and subsequent healing with columnar epithelium has been hypothesized[10]. On the other hand, this is also possible that the pathogenic mechanisms for the two entities (i.e., EE and BE), are different, but are often seen together due to shared patient characteristics and risk factors. Interestingly though, BE has also been identified in up to 25% of asymptomatic or minimally symptomatic individuals[11].
In a multi-center study of non-veteran patients (60% men, 78% Caucasians) presenting for colonoscopy, the prevalence of BE was 6.8% in those with or without the symptoms of heartburn, and rose up to 15% if they had EE on the endoscopy[12]. In this study, patients with minor grades of esophagitis were not re-evaluated after medical therapy. In a study by Hanna et al[13], 176 patients with EE but without apparent BE were followed for a mean of 11 wk while on a standard dose of PPI. At follow-up 116 (67%) showed complete healing of EE. Of these, 32 (27.6%) were suspected of having BE, but histology was confirmatory only in 16 (50%). Overall, BE was seen in 21 cases (12%), most of which had short-segment BE. In contrast, our study shows 27% prevalence of BE in association with higher grades of EE (some requiring more than one follow-up endoscopy to document complete healing). Again, in the study by Hanna et al, patients with visually suspected BE were excluded, and furthermore, 33% of their patients did not achieve complete healing at follow up EGD and biopsies. These two factors could have accounted for the relatively low proportion of BE patients in their study. Interestingly, in our study, if we exclude patients with suspected BE (left arm of Figure 1) at index EGD, the prevalence of BE in the remaining patients (right arm of Figure 1) drops to 19.84% with most of these having SSBE (80.76%). In our study, the histological confirmation rate for BE was 18.18% (2/11) when biopsies were performed in the presence of inflammation and 56.4% (44/78) when performed after complete healing of EE. In two patients, BE with dysplasia was missed when biopsies were taken in the presence of inflammation. This does raise additional concerns that sampling for BE can be more difficult in those with EE and the potential for missing dysplastic areas could be higher. In our study, a significant number of patients with class B esophagitis did not undergo a follow-up EGD, as endoscopists felt confident that BE was not present.
In our analysis, as the severity of EE increased, so did the average length of erosions. Our data also suggest that the length of BE (if found at follow-up) can be predicted by the length of inflammation seen at the index examination. The strongest predictor for the presence of BE was the suspicion of it at index endoscopy. When visual diagnosis of BE was made, the final diagnosis of BE could be established only in 53% (46/86); although a sampling error cannot be ruled out, similar findings have also been reported by other investigators[1415]. It will be interesting to see if newly available mucosal enhancing techniques (chromoendoscopy, high definition/magnification, narrow band imaging or confocal endomicroscopy) with the ability of targeted biopsies could increase the yield of diagnosing BE with or without the presence of EE.
The limitations of this study include the retrospective nature of the analysis and the fact that the patient population comprises mostly of older men who are at increased risk of EE[16] and BE[17]. Therefore, these data might not be applicable to populations who have higher preponderance of women and relatively younger patients. Furthermore, in the study, the possibility of higher inter-observer variability of EE grading cannot be ruled out.
In conclusion, BE was seen in 27% of patients with moderate-severe grades of EE. Esophageal inflammation can mask underlying BE or dysplasia, and make biopsies less accurate. The length of EE at index endoscopy may predict the length of BE at follow-up endoscopy. Therefore, it is suggested that follow-up endoscopy be performed, and evaluation for potential BE made once complete endoscopic healing of moderate-severe EE is achieved.
Gastro-esophageal reflux disease may be complicated by erosive esophagitis (EE) and Barrett’s esophagus (BE). BE is clearly associated with esophageal adenocarcinoma.
To investigate whether patients with moderate-severe EE are at increased risk of BE and whether the presence of inflammation affects detection of underlying BE.
Present study shows higher prevalence of BE with advanced grades of EE, once complete healing of inflammation is achieved. There is potential for missing Barrett’s and even dysplasia if biopsies are obtained in the presence of active inflammation.
BE appeared to be more prevalent in patients with moderate-severe EE. Endoscopic biopsies (if needed) at a follow-up examination should only be performed once complete mucosal healing is documented.
This is an interesting study, where the length of EE at index endoscopy may predict the length of BE at follow-up endoscopy. It will be some value for clinical practice.
| 1. | Dent J, El-Serag HB, Wallander MA, Johansson S. Epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2005;54:710-717. |
| 2. | Lassen A, Hallas J, de Muckadell OB. Esophagitis: incidence and risk of esophageal adenocarcinoma--a population-based cohort study. Am J Gastroenterol. 2006;101:1193-1199. |
| 3. | Anderson LA, Watson RG, Murphy SJ, Johnston BT, Comber H, Mc Guigan J, Reynolds JV, Murray LJ. Risk factors for Barrett's oesophagus and oesophageal adenocarcinoma: results from the FINBAR study. World J Gastroenterol. 2007;13:1585-1594. |
| 4. | Kubo A, Corley DA. Marked multi-ethnic variation of esophageal and gastric cardia carcinomas within the United States. Am J Gastroenterol. 2004;99:582-588. |
| 5. | Spechler SJ. Barrett's esophagus. Semin Gastrointest Dis. 1996;7:51-60. |
| 6. | Musana AK, Resnick JM, Torbey CF, Mukesh BN, Greenlee RT. Barrett's esophagus: incidence and prevalence estimates in a rural Mid-Western population. Am J Gastroenterol. 2008;103:516-524. |
| 7. | Westhoff B, Brotze S, Weston A, McElhinney C, Cherian R, Mayo MS, Smith HJ, Sharma P. The frequency of Barrett's esophagus in high-risk patients with chronic GERD. Gastrointest Endosc. 2005;61:226-231. |
| 8. | Veldhuyzen van Zanten SJ, Thomson AB, Barkun AN, Armstrong D, Chiba N, White RJ, Escobedo S, Sinclair P. The prevalence of Barrett's oesophagus in a cohort of 1040 Canadian primary care patients with uninvestigated dyspepsia undergoing prompt endoscopy. Aliment Pharmacol Ther. 2006;23:595-599. |
| 9. | DeVault KR, Castell DO. Updated guidelines for the diagnosis and treatment of gastroesophageal reflux disease. The Practice Parameters Committee of the American College of Gastroenterology. Am J Gastroenterol. 1999;94:1434-1442. |
| 10. | Hamilton SR, Yardley JH. Regnerative of cardiac type mucosa and acquisition of Barrett mucosa after esophagogastrostomy. Gastroenterology. 1977;72:669-675. |
| 11. | Gerson LB, Shetler K, Triadafilopoulos G. Prevalence of Barrett's esophagus in asymptomatic individuals. Gastroenterology. 2002;123:461-467. |
| 12. | Rex DK, Cummings OW, Shaw M, Cumings MD, Wong RK, Vasudeva RS, Dunne D, Rahmani EY, Helper DJ. Screening for Barrett's esophagus in colonoscopy patients with and without heartburn. Gastroenterology. 2003;125:1670-1677. |
| 13. | Hanna S, Rastogi A, Weston AP, Totta F, Schmitz R, Mathur S, McGregor D, Cherian R, Sharma P. Detection of Barrett's esophagus after endoscopic healing of erosive esophagitis. Am J Gastroenterol. 2006;101:1416-1420. |
| 14. | Eloubeidi MA, Provenzale D. Does this patient have Barrett's esophagus? The utility of predicting Barrett's esophagus at the index endoscopy. Am J Gastroenterol. 1999;94:937-943. |
| 15. | Jego M, Volant A, Faycal J, Doucet L, Andlauer E, Delalande AH, Cholet F, Nousbaum JB, Gouerou H, Robaszkiewicz M. Prevalence and topography of intestinal metaplasia in columnar lined esophagus. Gastroenterol Clin Biol. 2007;31:601-606. |
| 16. | Du J, Liu J, Zhang H, Yu CH, Li YM. Risk factors for gastroesophageal reflux disease, reflux esophagitis and non-erosive reflux disease among Chinese patients undergoing upper gastrointestinal endoscopic examination. World J Gastroenterol. 2007;13:6009-6015. |
| 17. | Guardino JM, Khandwala F, Lopez R, Wachsberger DM, Richter JE, Falk GW. Barrett's esophagus at a tertiary care center: association of age on incidence and prevalence of dysplasia and adenocarcinoma. Am J Gastroenterol. 2006;101:2187-2193. |