Published online Jun 7, 2008. doi: 10.3748/wjg.14.3410
Revised: May 6, 2008
Accepted: May 13, 2008
Published online: June 7, 2008
AIM: To evaluate the effects of honey on bacterial translocation and intestinal villus histopathology in experimental obstructive jaundice.
METHODS: Thirty Wistar-Albino rats were randomly divided into three groups each including 10 animals: group I, sham-operated; group II, ligation and section of the common bile duct (BDL); group III, bile duct ligation followed by oral supplementation of honey (BDL + honey) 10 g/kg per day. Liver, blood, spleen, mesenteric lymph nodes, and ileal samples were taken for microbiological, light and transmission electrone microscopic examination.
RESULTS: Although the number of villi per centimeter and the height of the mucosa were higher in sham group, there was no statistically significant difference between sham and BDL + honey groups (P > 0.05). On the other hand, there was a statistically significant difference between BDL group and other groups (P < 0.05). The electron microscopic changes were also different between these groups. Sham and honey groups had similar incidence of bacterial translocation (P > 0.05). BDL group had significantly higher rates of bacterial translocation as compared with sham and honey groups. Bacterial translocation was predominantly detected in mesenteric lymph nodes.
CONCLUSION: Supplementation of honey in presence of obstructive jaundice ameliorates bacterial translocation and improves ileal morphology.
- Citation: Gencay C, Kilicoglu SS, Kismet K, Kilicoglu B, Erel S, Muratoglu S, Sunay AE, Erdemli E, Akkus MA. Effect of honey on bacterial translocation and intestinal morphology in obstructive jaundice. World J Gastroenterol 2008; 14(21): 3410-3415
- URL: https://www.wjgnet.com/1007-9327/full/v14/i21/3410.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.3410
Obstructive jaundice is a common clinical entity complicated by intestinal failure and endotoxemia, leading to high postoperative morbidity and mortality rates. The gastrointestinal tract performs a variety of functions in digestion, selective absorption, and secretion. However, its barrier function, which prevents spread of intraluminal bacteria and endotoxins to the organs and tissues, plays a key role[12]. Intestinal barrier failure is associated with an increased incidence of bacteria and toxin translocation from the intestinal lumen to the systemic circulation, causing systemic infection and multiple organ failure in the critically ill or injured patient[3]. Gut barrier failure may result from one or more of the three basic pathophysiologic conditions; disruption of the normal ecologic balance of the indigenous gut microflora, impaired host immune defenses, and physical disruption of the gut mucosal barrier[4].
Bacterial translocation is the passage of bacteria or endotoxins from the gastrointestinal tract to extraintestinal sites, such as mesenteric lymph nodes, liver, spleen, and/or bloodstream. In a normal, healthy individual, gut-originated bacteremia and sepsis do not occur because the host has multiple defense mechanisms to prevent the bacteria and their products from crossing the mucosal barrier and spreading to systemic tissues. Under certain experimental and clinical circumstances, this intestinal barrier function becomes overwhelmed or impaired, resulting in bacterial translocation[4]. Current advances in the pathophysiology of intestinal failure in obstructive jaundice have showed that the breakage of gut barrier is multifactorial, involving disruption of the immunologic, biological, mechanical, and biochemical barrier[1].
Honey is a supersaturated sugar solution produced by honey bees from nectar of different plants. It has a long tradition of use for wound healing since ancient times. Honey has bactericidal, bacteriostatic, antifungal, antiviral, scolicidal, antioxidant, antitumoral, and anti-inflammatory effects[5–12].
In this study, we investigated the effects of honey on bacterial translocation and intestinal morphology in experimental obstructive jaundice.
Thirty Wistar-Albino male rats, weighing 250 ± 25 g, were housed under constant temperature (21 ± 2°C) individually in wire cages with 12 h light-dark cycle. Twelve hours before anesthesia, animals were deprived of food, but had free access to water 2 h before anesthesia. No enteral or parenteral antibiotics were administered at any time. The rats that died during the experiment were excluded from the experiment and no new rat was included. The procedures in this experimental study were performed in accordance with the National Guidelines for The Use and Care of Laboratory Animals and approved by Animal Ethics Committee of Ankara Research and Training Hospital.
Rats were randomly divided into three groups each including 10 animals: group I, sham-operated; group II, ligation and section of the common bile duct (BDL); group III, BDL followed by oral supplementation of honey 10 g/kg per day (Balparmak LTD, Istanbul, Turkey), once a day, with nasogastric tube (7 Gauge feeding tube) that was inserted daily and taken off after honey supplementation. Animals were sacrificed by high-dose diethyl ether inhalation on postoperative day 7. Liver, blood, spleen, mesenteric lymph nodes, and ileal samples were taken for microbiological, light and TEM (transmission electron microscopic) examination.
There isn’t a standard dose for honey in experimental studies. The dose used in previous studies ranges between 0.078 g/kg to 5 g honey/rat per day[12–15]. We gave 10 g/kg per day to each rat.
Animals were anesthetized by intramuscular injection of 30 mg/kg ketamine hydrochloride (Ketalar®; Parke-Davis, Istanbul, Turkey) and 5 mg/kg xylasine (Rompun®, Bayer, Istanbul, Turkey). Midline laparotomy was performed under sterile conditions. In the sham-operated group (group I) the common bile duct (CBD) was freed from the surrounding soft tissue and was manipulated without ligation and transection. In group II and III, CBDs of the rats were identified, double ligated with 5-0 silk, and sectioned between the ligatures. The same surgeon performed all procedures. The abdominal incisions were closed in two layers with continuous 3-0 silk sutures. Animals were allowed to feed after the operation.
The mesenteric lymph nodes (MNLs), spleen and liver were chopped with sterile instruments under aseptic conditions. Then the tissue samples were weighed and placed in tubes containing 1.5 mL broth (thioglycolate, Oxoid, UK) and homogenized. After that 0.01 mL tissue samples were inoculated on blood agar (Oxoid, UK) and Levine Eosine Methylene Blue (EMB) agar (Oxoid, UK). Plates were incubated at 37°C for examination of bacterial growth. The growth of bacteria in quantitative culture was observed at 24 h and 48 h.
Blood samples taken from inferior vena cava of rats were inoculated on the medium of aerobic and anaerobic blood culture. The aerobic and anaerobic blood cultures were observed by incubation in BACTEC 9240 blood culture system (Becton Dickinson, USA) at 37°C for seven days. Samples taken from the blood culture bottle giving positive alarm were subcultured by inoculating on blood agar and EMB agar. The subcultures were inoculated at 37°C under aerobic and anaerobic condition and examined at 24 h and 48 h.
Total bilirubin, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) levels were measured as parameters indicative of hepatic function by an autoanalyser (Olympus AU640, Japan).
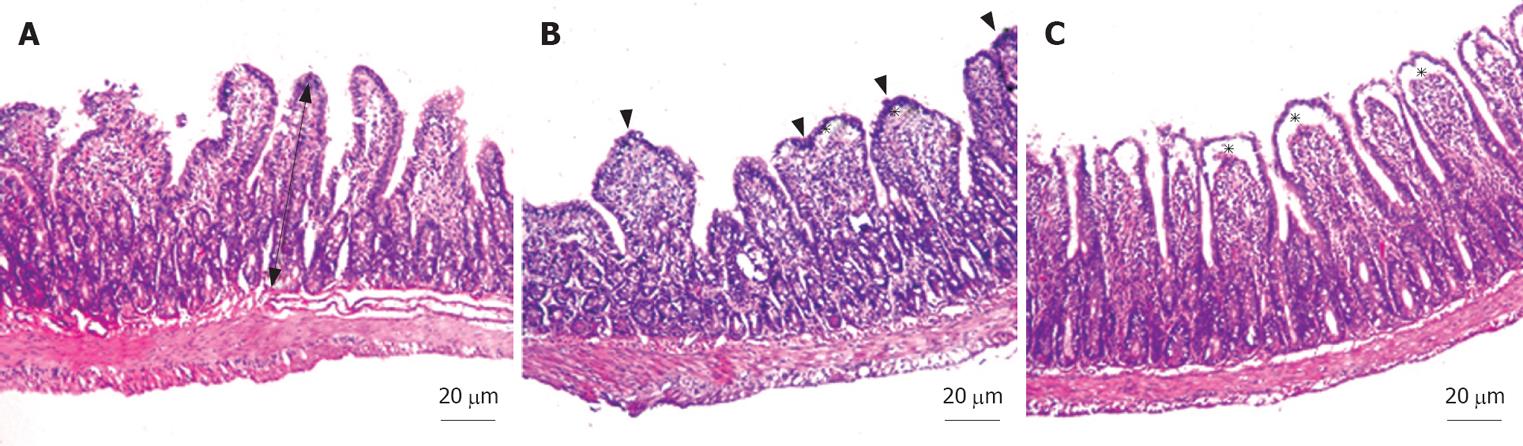
For light microscope analyses, tissue samples from the terminal ileum were obtained from all animals. In order to avoid mucosal suffering, the intestinal lumen was carefully cannulated and gently washed with normal saline solution before the sampling. The ileal samples were fixed in 10% neutral buffered formalin, embedded in paraffin, sectioned at 5 &mgr;m by Leica RM 2125 RT, and stained with hematoxylin and eosin (HE) for routine light microscopic examination. Histopathological examinations were performed by a pathologist who was blinded to the study design and photographs were taken with Nikon Eclipse E 600. The number of villi per centimeter (V/cm) and the total mucosal thickness were assessed in all groups. The mucosal thickness was measured in a minimum of 20 well-preserved villi in each randomly selected sample from each tissue block.
For TEM (transmission electron microscopic) analyses, samples were fixed with phosphate buffered (pH 7.3) 2.5% glutaraldehyde and 2% PFA mixture solution for 2 h at room temperature. They were washed with phosphate buffered saline solution (PBS, pH 7.3) and were fixed with 1% osmium tetraoxide for 2 h as secondary fixation. After washing, they were embedded in Araldite 6005 and were cut with Leica EM FCS (Wien, Austria) ultramicrotome. One &mgr;m semi-thin sections were stained with Toluidin blue-Azur II to select the region of interest for the following procedures. Sixty to 70 nm thin sections were stained with uranil acetate and lead citrate. They were examined and photographed using a LEO 906 E TEM (80 Kv, Oberkochen, Germany). The pathologist was blinded about the groups.
Differences between the numbers of positive cultures of the groups were evaluated by chi-square test. Scores of total mucosal thickness and number of villi per centimeter were presented as mean ± SD and compared by One-Way ANOVA or Kruskal-Wallis variance analysis. If the P values of the variance analyses were statistically significant, differences between groups were analyzed with the Mann-Whitney U test. Statistical analyses were performed with the Statistical Package for the Social Sciences (SPSS), version 13.0 for Windows (SPSS Inc., Chicago, USA). P < 0.05 was considered to be statistically significant.
All rats were sacrificed on postoperative day 7. Two rats from group II (BDL group) and one from group III (BDL + honey group), totally 3 rats, died during the early postoperative period probably due to anesthesia. The liver function tests and bilirubin levels were normal in sham group and high in BDL and BDL + honey groups.
In all specimens of the sham group, the histological features showed regular appearance of ileal tissue. When we evaluated the specimens systematically, including assessment of villous architecture, surface and crypt epithelia, lamina propria constituents and submucosal structures, no alteration was found in sham group (Figure 1A). The specimens of the BDL group presented villous blunting associated with reduced mucosal thickness. We identified subepithelial edema mostly located at the tip of the villi, but also extended throughout the villus, with epithelial layer moderately lifted from the lamina propria. We observed that the crypts were generally preserved. The number of villi per centimeter (V/cm) (villus density) was decreased in BDL group (Figure 1B). In group III, the subepithelial edema still existed, but villous blunting was not evident. Farther, the crypts generally appeared to be preserved (Figure 1C). Although the number of villi per centimeter and the height of the mucosa were higher in sham group, there was no statistically significant difference between sham and BDL + honey groups (P > 0.05). On the other hand, there was a statistically significant difference between BDL group and other groups (P < 0.05). Mean number of villi per centimeter and mean mucosal height of the groups are given in Table 1.
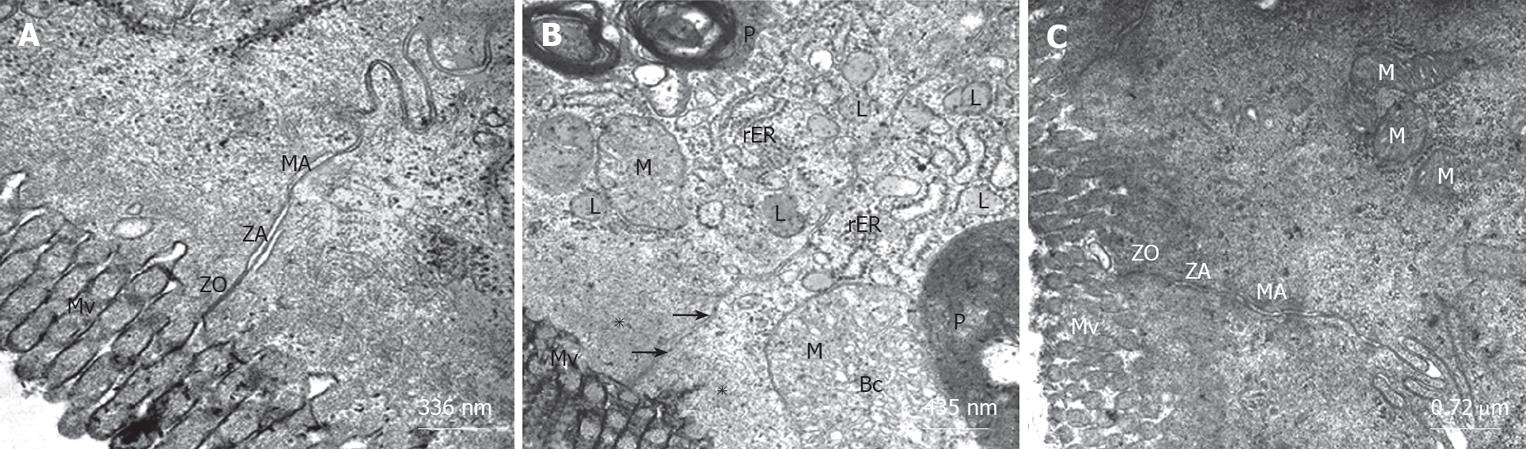
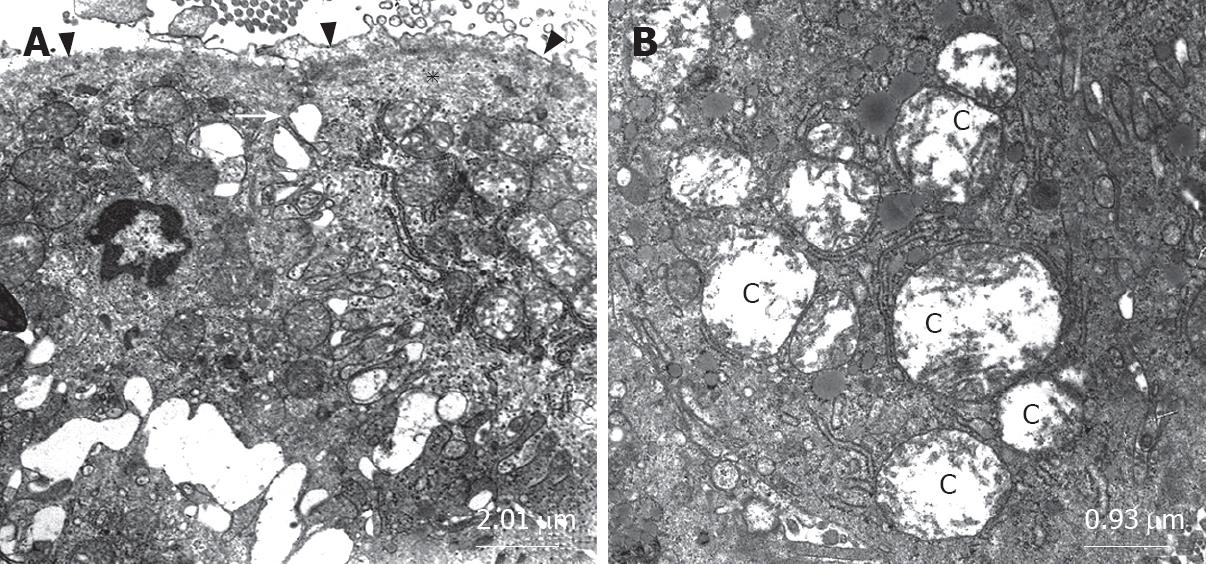
The ultrastructure of intestinal epithelial junctional complexes was observed by electron microscopy. In the sham group, enterocytes were tightly bound to the luminal surface by junctional complexes. Zonulae occludens, zonulae adherentes and maculae adherentes appeared normal in the sham group. The luminal surface was covered with microvilli (Figure 2A). When we evaluated the BDL group, we observed desquamated epithelial tissue, cytoplasmic vacuoles, phagosomes and disrupted structure of the tight junction between epithelial cells possibly due to apical surface edema. Zonulae occludens located within the plasma membranes of adjacent epithelial cells diverged (Figures 2B and 3A) and the mitochondria were swollen with electrolucent matrix and ballooned cristae. Markedly swollen mitochondria with peripherally placed, disoriented and disintegrating cristae and cavitations of the matrix were also observed in BDL group (Figures 2B and 3B). The structure of the microvilli and mitochondria were regular in the BDL + honey group. The junctional complexes had normal appearance (Figure 2C).
The rates of bacterial translocation (BT) in all groups are summarized in Table 2. Sham and BDL + honey groups had similar incidence of BT. BDL group had significantly higher rates of BT as compared with sham and BDL + honey groups. Only BT to spleen was not significantly different between the BDL and BDL + honey groups. BT was predominantly detected in MLNs.
| Groups | Liver | Spleen | MLNs | Blood |
| Sham (Group I) | 0/10 (0.0%) | 0/10 (0.0%) | 1/10 (10.0%) | 0/10 (0.0%) |
| BDL (Group II) | 6/8 (75.0%) | 4/8 (50.0%) | 7/8 (87.5%) | 4/8 (50.0%) |
| BDL + Honey (Group III) | 1/9 (11.1%) | 2/9 (22.2%) | 2/9 (22.2%) | 0/9 (0.0%) |
| P values | ||||
| I vs II | 0.002 | 0.023 | 0.002 | 0.023 |
| II vs III | 0.013 | > 0.05 | 0.012 | 0.029 |
| I vs III | > 0.05 | > 0.05 | > 0.05 | > 0.05 |
The most commonly isolated bacteria was Escherichia coli. The other isolated microorganisms were Enterococcus spp., Staphylococcus spp., Proteus spp., Staphylococcus aureus and Enterobacter cloacae.
Bacterial translocation is the migration of bacteria or bacterial products from the intestinal lumen to mesenteric lymph nodes or other extraintestinal organs and sites. In addition to nutrient absorption, the gut functions as a barrier to prevent the spread of intraluminal bacteria and endotoxin to systemic organs and tissues[41617].
Bile inhibits bacterial overgrowth, has a trophic effect on the intestinal mucosa, decreases epithelial internalization of enteric bacteria, exerts detergent actions with anti-adherence effects, and binds endotoxins. Therefore, the absence of bile in the intestine facilitates BT and enhances endotoxin-induced BT[16]. Obstructive jaundice is almost universally believed to promote bacterial translocation. Absence of bile from the lumen of gut is also associated with a quantitative increase in small intestinal microflora[18].
Translocation from the intestine is most commonly detected by measuring the presence of viable bacteria in the tissues. This can reflect not only the integrity of the intrinsic barrier function of the mucosa but also the numbers and types of microbes in the lumen and the ability of the host to kill the bacteria that translocate[19].
Honey is a supersaturated sugar solution produced by honey bees from the nectar of plants. Some of the components of the honey are added by the bees during the maturation process or are derived from the plants[20]. The antimicrobial properties of honey are well documented[5–9]. The antibacterial activity of honey lies partially in its high osmolarity due to its high sugar content, and in its acidity due mostly to the presence of gluconic acid. Although hydrogen peroxide is thought to be the main antibacterial factor in honey, the presence of non-peroxide activity was also notable. This activity is usually attributed to the presence of organic components such as syringic acid, methyl syringate, pinocembrin, pinobanksin, caffeic acid, ferulic acid, vanillic acid, cinnamic acid, and benzoic acid[21].
The physicochemical properties of honey not only contribute to its antibacterial properties but also to its wound healing capabilities. The anti-inflammatory action of honey has been investigated, but no definite mechanism has been identified. Honey provides glucose supply for leucocytes. It also provides substrate for glycolysis, which is the major mechanism for energy production in the macrophages. Honey may modulate the activation state of immunocompetent cells (e.g. monocytes) within the wound. These data suggest that honey may have a number of effects on the molecular mechanisms of wound healing[20].
As we mentioned before, the physical barrier function of the mucosa appears to have primary importance for preventing or limiting bacterial translocation, especially in a host with a normal gut flora, whereas the immune system appears to serve a secondary or supportive role to the intestinal mucosal barrier[4]. In our study, mean number of villi per centimeter, mean mucosal height, and electron microscopic changes of the honey group were significantly better than the data observed in the BDL group. In the sham and BDL + honey groups, enterocytes were tightly bound to the luminal surface by junctional complexes. Zonulae occludens, zonulae adherentes and maculae adherentes appeared normal in these groups. The luminal surface was covered with microvilli. When we evaluated the BDL group, we observed desquamated epithelial tissue, cytoplasmic vacuoles, phagosomes and disrupted structure of the tight junction between epithelial cells possibly due to apical surface edema. Zonulae occludens located within the plasma membranes of adjacent epithelial cells were diverging and the mitochondria were swollen with electrolucent matrix and ballooned cristae. Reduced bacterial translocation rates in honey group could be explained by decreased atrophy of intestinal mucosal villi and somewhat regular structure of enterocytes and microvilli. We concluded that wound healing properties and cytoprotective effects of honey might be the reason of the decreased atrophy of intestinal mucosal villi. On the other hand, antimicrobial effects of honey on enteric bacteria could also decrease the overgrowth of these bacteria and reduce bacterial translocation.
Assimakopoulos et al[22] investigated the oxidative alterations in the intestinal mucosa of patients with obstructive jaundice and found that obstructive jaundice in humans induced intestinal oxidative stress, which might be a key factor contributing to intestinal barrier failure and the development of septic complications in this patient population. In another study, these authors showed that intestinal mucosal atrophy in obstructive jaundice was based on inhibition of proliferation and promotion of apoptotic death of enterocytes, and reactive oxygen species might be responsible for this effect[23].
The antioxidant properties of honey have been well-documented in recent studies[24–27]. Schramm et al[25] found that phenolic antioxidants from processed honey were bioavailable, and these antioxidants increased antioxidant activity of plasma. Gheldof et al[11] also showed that the in vivo serum antioxidant capacity increased significantly following consumption of buckwheat honey in human. These studies showed that the antioxidant effect of honey was not only local, but also a systemic effect. According to the results of studies about antioxidative effects of honey and intestinal oxidative stress in obstructive jaundice, we concluded that the protective effect of honey on intestinal villi and mucosal structure might be attributable to antioxidative effects of honey in our study. Since we investigated only the effects of honey on bacterial translocation and intestinal villus atrophy, not the mechanism of this effect, we did not evaluate oxidative stress parameters. These parameters should be analyzed in further studies that investigate the mechanism of this effect of honey.
Since a normal functioning immune system is another important factor for adequate gut barrier function[4], honey may also reduce bacterial translocation by its modulatory effects on immunocompetent cells[2829].
In this study, we demonstrated that honey reduced bacterial translocation rates and protected intestinal villus structure in experimental obstructive jaundice model. These effects of honey might be attributable to its antibacterial, antioxidant, anti-inflammatory, and immunomodulatory activities. Further studies are needed for evaluation of the exact mechanism of this effect. After the results of these studies, honey might be used for preventing harmful effects of obstructive jaundice in clinical settings.
Spontaneous bacterial infection and septicemia due to increased bacterial translocation in patients with obstrictive jaundice result in significant morbidity and mortality.
The present study investigated the effects of honey on bacterial translocation and intestinal morphology in experimental obstructive jaundice.
Obstructive jaundice is a common clinical entity complicated by intestinal failure and endotoxemia, leading to high postoperative morbidity and mortality. Our experience from the present study shows that honey can be used safely in this situation.
This study demonstrated that honey reduced bacterial translocation rates and protected intestinal villus structure in experimental obstructive jaundice model. Honey might be used for preventing harmful effects of obstructive jaundice in clinical settings.
The rationale behind this study is that the authors have previously shown that honey has reduced bacterial translocation rates and protected intestinal villus structure in experimental obstructive jaundice.
| 1. | Assimakopoulos SF, Vagianos CE, Charonis A, Nikolopoulou VN, Scopa CD. Intestinal failure in obstructive jaundice. World J Gastroenterol. 2005;11:3806-3807. |
| 2. | Kayama S, Mitsuyama M, Sato N, Hatakeyama K. Overgrowth and translocation of Escherichia coli from intestine during prolonged enteral feeding in rats. J Gastroenterol. 2000;35:15-19. |
| 3. | De-Souza DA, Greene LJ. Intestinal permeability and systemic infections in critically ill patients: effect of glutamine. Crit Care Med. 2005;33:1125-1135. |
| 4. | Magnotti LJ, Deitch EA. Burns, bacterial translocation, gut barrier function, and failure. J Burn Care Rehabil. 2005;26:383-391. |
| 5. | Molan PC. Honey as an antimicrobial agent. Bee products: Properties, Application and Apitherapy. New York: Plenum Press 1996; 27-37. |
| 6. | Jeddar A, Kharsany A, Ramsaroop UG, Bhamjee A, Haffejee IE, Moosa A. The antibacterial action of honey. An in vitro study. S Afr Med J. 1985;67:257-258. |
| 7. | Efem SE, Udoh KT, Iwara CI. The antimicrobial spectrum of honey and its clinical significance. Infection. 1992;20:227-229. |
| 8. | Irish J, Carter DA, Shokohi T, Blair SE. Honey has an antifungal effect against Candida species. Med Mycol. 2006;44:289-291. |
| 9. | Al-Waili NS. Topical honey application vs. acyclovir for the treatment of recurrent herpes simplex lesions. Med Sci Monit. 2004;10:MT94-MT98. |
| 10. | Kilicoglu B, Kismet K, Koru O, Tanyuksel M, Oruc MT, Sorkun K, Akkus MA. The scolicidal effects of honey. Adv Ther. 2006;23:1077-1083. |
| 11. | Gheldof N, Wang XH, Engeseth NJ. Buckwheat honey increases serum antioxidant capacity in humans. J Agric Food Chem. 2003;51:1500-1505. |
| 12. | Mabrouk GM, Moselhy SS, Zohny SF, Ali EM, Helal TE, Amin AA, Khalifa AA. Inhibition of methylnitrosourea (MNU) induced oxidative stress and carcinogenesis by orally administered bee honey and Nigella grains in Sprague Dawely rats. J Exp Clin Cancer Res. 2002;21:341-346. |
| 13. | Gharzouli K, Amira S, Gharzouli A, Khennouf S. Gastroprotective effects of honey and glucose-fructose-sucrose-maltose mixture against ethanol-, indomethacin-, and acidified aspirin-induced lesions in the rat. Exp Toxicol Pathol. 2002;54:217-221. |
| 14. | Ali AT, al-Swayeh OA, al-Humayyd MS, Mustafa AA, al-Rashed RS, al-Tuwaijiri AS. Natural honey prevents ischaemia-reperfusion-induced gastric mucosal lesions and increased vascular permeability in rats. Eur J Gastroenterol Hepatol. 1997;9:1101-1107. |
| 15. | Onat F, Yegen BC, Lawrence R, Oktay A, Oktay S. Site of action of grayanotoxins in mad honey in rats. J Appl Toxicol. 1991;11:199-201. |
| 16. | Wiest R, Garcia-Tsao G. Bacterial translocation (BT) in cirrhosis. Hepatology. 2005;41:422-433. |
| 17. | Balzan S, de Almeida Quadros C, de Cleva R, Zilberstein B, Cecconello I. Bacterial translocation: overview of mechanisms and clinical impact. J Gastroenterol Hepatol. 2007;22:464-471. |
| 18. | Gatt M, Reddy BS, MacFie J. Review article: bacterial translocation in the critically ill--evidence and methods of prevention. Aliment Pharmacol Ther. 2007;25:741-757. |
| 19. | Alexander JW. Bacterial translocation during enteral and parenteral nutrition. Proc Nutr Soc. 1998;57:389-393. |
| 20. | Lusby PE, Coombes A, Wilkinson JM. Honey: a potent agent for wound healing? J Wound Ostomy Continence Nurs. 2002;29:295-300. |
| 21. | Aljadi AM, Yusoff KM. Isolation and identification of phenolic acids in Malaysian honey with antibacterial properties. Turk J Med Sci. 2003;33:229-236. |
| 22. | Assimakopoulos SF, Thomopoulos KC, Patsoukis N, Georgiou CD, Scopa CD, Nikolopoulou VN, Vagianos CE. Evidence for intestinal oxidative stress in patients with obstructive jaundice. Eur J Clin Invest. 2006;36:181-187. |
| 23. | Assimakopoulos SF, Scopa CD, Zervoudakis G, Mylonas PG, Georgiou C, Nikolopoulou V, Vagianos CE. Bombesin and neurotensin reduce endotoxemia, intestinal oxidative stress, and apoptosis in experimental obstructive jaundice. Ann Surg. 2005;241:159-167. |
| 24. | Gheldof N, Wang XH, Engeseth NJ. Identification and quantification of antioxidant components of honeys from various floral sources. J Agric Food Chem. 2002;50:5870-5877. |
| 25. | Schramm DD, Karim M, Schrader HR, Holt RR, Cardetti M, Keen CL. Honey with high levels of antioxidants can provide protection to healthy human subjects. J Agric Food Chem. 2003;51:1732-1735. |
| 26. | Henriques A, Jackson S, Cooper R, Burton N. Free radical production and quenching in honeys with wound healing potential. J Antimicrob Chemother. 2006;58:773-777. |
| 27. | Gheldof N, Engeseth NJ. Antioxidant capacity of honeys from various floral sources based on the determination of oxygen radical absorbance capacity and inhibition of in vitro lipoprotein oxidation in human serum samples. J Agric Food Chem. 2002;50:3050-3055. |
| 28. | Abuharfeil N, Al-Oran R, Abo-Shehada M. The Effect of Bee Honey on the Proliferative Activity of Human B-and T-Lymphocytes and the Activity of Phagocytes. Food and Agricultural Immunology. 1999;11:169-177. |
| 29. | Watanabe K, Shinmoto H, Kobori M, Tsushida T, Shinohara K, Kanaeda J, Yonekura M. Stimulation of cell growth in the U-937 human myeloid cell line by honey royal jelly protein. Cytotechnology. 1998;26:23-27. |