Published online Apr 28, 2008. doi: 10.3748/wjg.14.2566
Revised: March 4, 2008
Published online: April 28, 2008
AIM: To study the hepatoprotective capacity of Sapindus mukorossi (S. mukorossi) and Rheum emodi (R. emodi) extracts in CCl4 treated male rats.
METHODS: The dried powder of S. mukorossi and R. emodi was extracted successively with petroleum ether, benzene, chloroform, and ethanol and concentrated in vacuum. Primary rat hepatocyte monolayer cultures were used for in vitro studies. In vivo, the hepatoprotective capacity of the extract of the fruit pericarp of S. mukorossi and the rhizomes of R. emodi was analyzed in liver injured CCl4-treated male rats.
RESULTS: In vitro: primary hepatocytes monolayer cultures were treated with CCl4 and extracts of S. mukorossi & R. emodi. A protective activity could be demonstrated in the CCl4 damaged primary monolayer culture. In vivo: extracts of the fruit pericarp of S. mukorossi (2.5 mg/mL) and rhizomes of R. emodi (3.0 mg/mL) were found to have protective properties in rats with CCl4 induced liver damage as judged from serum marker enzyme activities.
CONCLUSION: The extracts of S. mukorossi and R. emodi do have a protective capacity both in vitro on primary hepatocytes cultures and in in vivo in a rat model of CCl4 mediated liver injury.
-
Citation: Ibrahim M, Khaja MN, Aara A, Khan AA, Habeeb MA, Devi YP, Narasu ML, Habibullah CM. Hepatoprotective activity of
Sapindus mukorossi andRheum emodi extracts:In vitro andin vivo studies. World J Gastroenterol 2008; 14(16): 2566-2571 - URL: https://www.wjgnet.com/1007-9327/full/v14/i16/2566.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.2566
The liver is an organ of paramount importance. Due to its unique and considerable regenerative capacity, even a moderate cell injury is not reflected by measurable change in its metabolic functions. However, some of its functions are so sensitive that abnormalities start appearing depending upon the nature and the degree of initial damage.
The etiology of the liver disorders depends on various factors as nutritional, biochemical, bacteriological, viral, or environmental aberration. The liver plays a significant role not only in the metabolism and disposition of the chemicals to which it is exposed directly or indirectly, but also in the metabolism of fats, carbohydrates, proteins, and immunomodulation.
The impairment of the liver function is generally caused by xenobiotics, excessive exposure to various pharmacological and chemical agents, and protozoal or viral infections. Depending upon the severity of cellular injury, acute hepatitis can lead to chronic hepatitis, which is finally terminated to cirrhosis or malignant lesions in untreated cases. In case of deranged liver functions, the chemical composition of liver or its subcellular organelles are possibly altered. A slight alteration in hepatic structure and function may result in portal hypertension, ascities, jaundice, and increased bleeding, and causes multiple metabolic changes affecting other organs as well. Medical survey indicates exceptionally high occurrence of hepatic diseases and has become one of the most serious problem in the area of public health. Acute viral hepatitis is a diffuse inflammatory lesion of the liver usually accompanied by clinical and biochemical abnormalities and often caused by the well-characterized hepatitis virus. Acute hepatitis closely resembles viral hepatitis clinically, biochemically, and histologically. It can also be induced by chemicals or drugs.
Alcoholic liver disease (ALD) is one of the most serious consequences of chronic alcohol abuse. Liver cirrhosis, the culmination of the illness, is one of the leading causes of death in western countries[12].
According to Wang[3] chronic and excessive ethanol consumption is associated with cellular proliferation, fibrosis, cirrhosis, and cancer of the liver. An important characteristic of alcohol-induced liver injury is an impaired vitamin A nutritional status. Studies in human Hep G2 cells have shown that ethanol is cytotoxic to Hep G2 cells, which are transduced to express P-450 2E1 (CYP 2E1) and this toxicity is apoptotic in nature[4] predominantly in the liver. The main pathways for hepatic oxidation of ethanol to acetaldehyde involve alcohol dehydrogenase[5] and are associated with the reduction of NAD+ to NADH[6].
The magnitude of derangement of liver by disease or hepatotoxins is generally measured by the level of glutamate pyruvate transaminase (ALT), glutamate oxaloacetate transaminase (AST), alkaline phosphatase (ALP), bilirubin, albumin, and whole liver homogenate.
Herbal drugs are playing an important role in health care programs worldwide, and there is a resurgence of interest in herbal medicines for treatment of various ailments including hepatopathy. India, the abode of Ayurvedic system of medicine, assigns much importance to the pharmacological aspects of many plants. Hepatoprotective effect of some plants like Spirulina maxima[7], Eclipta alba[8], Boehmeria nivea[9], Cichorium intybus[10], and Picrorhiza kurroa[11] has been well established. Nearly 150 phytoconstituents from 101 plants have been claimed to possess liver protecting activity[12]. At the same time, surprisingly, we do not have satisfactory plant drugs/formulations to treat severe liver diseases. Most of the studies on hepatoprotective plants are carried out using chemical induced liver damage in rodents as models. A few excellent reviews have appeared on this subject in the recent past[13].
This study is based on the natural products responsible for repairing and healing of adversely affected liver cells. In the present study, we selected two plants namely S. mukorossi and R. emodi and investigated the hepatoprotective effect of these plant extracts against CCl4 induced hepatocyte damage in vitro and liver injury in vivo.
S. mukorossi Gaerten (Sapindaceae), commonly known as Ritha or Aritha is found throughout India. The major constituents of its fruit are saponins (10%-11.5%), sugars (10%) and mucilage[14]. The fruit of the plant is reported to have expectorant, emetic, alexipharmic, and abortificiant effects. It is also used in excessive salivation, epilepsy and chlorosis[1516]. Saponins from this plant are known to be spermicidal in vitro[17]. This spermicidal property has been used in contraceptive cream[18]. The alcoholic extract (Sapindus trifoliatus Linn) is reported to possess anti-implantation activity.
R. emodi (Polygonaceae) commonly known as Indian or Himalayan Rhubarb is found in India. The major constituents of rhubarb rhizomes are anthraquinones. Rhubarb is used as a laxative, diuretic to treat kidney stones, gout, and liver diseases characterized by jaundice. Externally, it is used to heal skin sores and scabs. Paradoxically, although larger doses are used as laxative, small doses are used to treat dysenteric diarrhea[19]. Chinese use rhubarb as an ulcer remedy and consider it a bitter, cold, dry herb used to “clear heat” from the liver, stomach and blood, to expel helminthes and to treat cancer, fever, upper intestinal bleeding (ulcers), and headache[2021]. It is also used to treat toothache[22]. In Europe, rhubarb is a component of spring tonics or blood cleansing cures, including Swedish bitter[23]. Turkish or medicinal rhubarb is also one of the four major ingredients in the herbal cancer remedy.
We isolated the extracts from both plants, and a study was designed using the products of S. mukorossi and R. emodi to assess the hepatoprotective effect of these plant extracts against CCl4 induced hepatocyte damage in vitro and liver injury in vivo.
Authentic samples of S. mukorossi and R. emodi were obtained from authorized supplier M/s Munnalal Dawasas and Co. Hyderabad, Andhra Pradesh, India. The plants were previously identified and authenticated by experts in the Post Graduate and Research Department of Botany, Anwar-ul-loom College Hyderabad, Andhra Pradesh, India.
Male Wister rats weighing 175-200 g were obtained from the animal house of Deccan College of Medical Sciences, Hyderabad and housed in polycarbonate cages. The rats had free access to standard pellet chow and water ad libitum throughout the experiment with the exception of some experiments (see below) in which the animals were deprived of food, but not water, for 18-24 h before the experiments were performed. After procurement, all the animals were divided into different groups and were left for one week for acclimatization to experimentation room and were maintained on standard conditions (23°C, 60%-70% relative humidity and 12 h photo period). There were six animals in each group for observational screening and acute toxicity studies. All experimental protocols described below were approved by the ethical board.
For phytochemical analysis, approximately 100 g of fruit pericarp of S. mukorossi and rhizomes of R. emodi was collected and materials were chopped, air dried at 35-40°C and pulverized in electric grinder. The powder obtained was successively extracted with the following chemicals, petroleum ether (60-80)°C, benzene, chloroform, and ethanol, respectively.
The extracts were then powdered by using rotary evaporator under reduced pressure. Fruit pericarp of S. mukorossi yielded 38 g, 28 g, 34 g, and 35 g and rhizomes of R. emodi yielded 19 g, 17 g, 21 g, and 22 g powdered extracts with petroleum ether, benzene, chloroform, and ethanol, respectively.
The extracts were obtained by percolation using 70% of ethanol as solvent at room temperature; according to process A of Farmacopeia dos Eastados Unidos do Brasil (1959) (AOAC 1990). The extracts were evaporated at 40°C under vacuum and the residue was freeze-dried. The dry extracts of the fruit pericarp of S. mukorossi and rhizomes of R. emodi were tested for the presence of saponins and anthraquinones.
Each extract of the fruit pericarp of S. mukorossi (SM) and rhizomes of R. emodi (RE) were column chromatographed over Silica gel (200 mesh), eluting with CHCl3-MeOH (70:30, 60:40, 50:50, 25:75) and compound fractions of (250 mL each) were collected and monitored by TLC. These column chromatographed compound fractions were further filtered to yield saponins from S. mukorossi and anthraquinones from R. emodi, which were separated by paper chromatography and preparative TLC to yield saponins [(SM-A (petroleum ether), SM-B (benzene), SM-C (chloroform) & SM-D (ethanol)], and anthraquinones [(RE-A (petroleum ether), RE-B (benzene), RE-C (chloroform) & RE-D (ethanol)], respectively. All the filtrates obtained were dried by evaporation (Rotometer, 40°C), the dried extracts were individually dissolved in 10 mL ethanol (95%) and then subjected to complete drying process and weighed according to the AOAC (1990) method[20].
It is emphasized that hepatotoxins that cause acute hepatitis should have close resemblance with the viral hepatitis, clinically, biochemically, and histologically. Certain drugs are also responsible for chronic hepatic disease such as chronic hepatitis, fatty liver, cirrhosis, and several vascular lesions of the liver. In many instances drug induced hepatitis is indistinguishable from viral hepatitis. Chemically induced hepatic injury for experimental studies should be severe enough to cause cell death or to modify hepatic functions. The mechanism of acute hepatic injury depends upon the chemical compound and the species of animals used. We have studied hepatoprotective activity against carbon tetrachloride (CCl4) induced hepatotoxicity.
CCl4 is one of the most powerful hepatotoxin in terms of severity of injury. It causes toxic necrosis leading to biochemical changes having clinical features similar to those of acute viral hepatitis[2425]. Liver injury was produced by administration of CCl4 mixed with liquid paraffin. Animals were given single doses of CCl4 100 &mgr;L/kg p.o. per day through out the experimental setup. Control animals received an equal volume of liquid paraffin.
In vitro: Fasting Wistar male adult rats weighing 280-300 g were used. Liver cells were isolated by using a modified procedure of Kiso et al[27]. The animal was cleaned thoroughly using rectified alcohol and anaesthetized with ether. Dissection of the animal was carried out under aseptic conditions using sterilized instruments. A midline incision was made on the abdomen of the anaesthetized animal. The portal vein was canulated with needle no 25 connected to an infusion set. The needle was tied in place and the inferior vena cava was cut below the renal vein. Perfusion of the liver was started immediately with Ca2+-Mg2+ free Hanks buffer salt solution (pH 7.4 at 37°C) which was prepared according to the procedure of Ohno (1965). When the liver was thoroughly perfused (i.e. has turned white), the flow of HBSS was stopped and the needle was removed. The liver was transferred to a sterile Petri dish containing Ca2+-Mg2+ free HBSS and minced into small pieces, which were transferred to a conical flask containing 10 mL of 0.075% collagenase in HBSS. This was placed on a magnetic stirrer at 37°C for 10 min. The cell suspension thus obtained was centrifuged at 50 g for 10 min. The supernatant was aspirated and the cell suspended in the Ca2+-Mg2+ free HBSS. The cells were washed twice and counted in the presence of trypan blue dye. Viability of the cells in each of the experiment performed was found to be 90%. The isolated hepatocytes were cultured in Eagles MEM, supplemented with 10% inactivated serum at density of 0.5 × 109 cells/L in sterile disposable culture bottles and incubated in a humified incubator at 37°C under 5% CO2. The viability of hepatocytes was studied after 6, 12, and 24 hrs. The hepatocytes which settled down were observed for their growth. Cytotoxicity of S. mukorossi and R. emodi extracts was tested in primary hepatocytes monolayer cultures. Neither of the extracts caused significant enzymes release or were cytotoxic (Table 1).
| Sample identity | Test concen (&mgr;g/mL) | Cytotoxicity (% release of enzyme in the medium) | |||
| LDH | GPT | ||||
| Sp. activity | %leakage | Sp. activity | %leakage | ||
| Control | - | 5.05 ± 0.22 | - | 103 ± 2.5 | - |
| S. mukorossi | 10 | 5.00 ± 2.21 | NS | 111 ± 2.4 | NS |
| 50 | 4.97 ± 0.23 | NS | 108 ± 3.1 | NS | |
| 100 | 5.18 ± 0.31 | NS | 100 ± 1.9 | NS | |
| R. emodi | 10 | 5.03 ± 0.11 | NS | 119 ± 2.0 | NS |
| 50 | 5.12 ± 0.17 | NS | 107 ± 2.5 | NS | |
| 100 | 5.08 ± 0.14 | NS | 114 ± 2.3 | NS | |
Hepatocytes were prepared from male Wister rats by the collagenase perfusion technique[26]. Cells were purified by several centrifugations and inoculated at density of 0.5 × 108 cells/L on collagen coated plates. One day after the isolated rat hepatocytes were plated, cells were exposed to medium containing 7 mmol/L CCl4 with or without the sample to be tested for the hepatoprotective activity[27]. After the exposure to CCl4 for 1 h the culture medium was collected and used for the determination of different parameters.
Lipid peroxidation was assessed as TBA-reactive substances using malondialdehyde (MDA) as reference. Rat hepatocytes 1 × 109 cells/L were incubated in a final volume of 1.0 mL HBSS buffer containing test materials in presence of 200 mol/L FeSO + 100 &mgr;mol/L H2O2.
The biochemical estimations were carried out by using the usual techniques (Tables 1 and 2).
| Sample identity | Test concen (&mgr;g/mL) | Hepatoprotective effect | |||||
| GSH levels (cells) | [% restoration of enzyme leakage against toxin challenge (CCl4) of enzyme leakage] | ||||||
| &mgr;g/3 x 106 cells | % recovery | LDH | GPT | ||||
| Sp. activity | % restoration | Sp. activity | % restoration | ||||
| Control | - | 5.61 ± 0.13 | - | 3.22 ± 0.25 | 100 | 94 ± 3.0 | 0 |
| CCl4 | 2.5 mmol/L | 2.52 ± 0.14 | - | 16.0 ± 0.22 | 0 | 198 ± 2.5 | 0 |
| CCl4 + | 10 &mgr;g | 2.95 ± 0.22 | 16 | 10.2 ± 2.21a | 46 | 135 ± 2.4a | 60 |
| S. mukorossi | 50 &mgr;g | 3.05 ± 0.17 | 20a | 8.10 ± 0.23b | 61 | 123 ± 3.1a | 72 |
| 100 &mgr;g | 3.31 ± 0.19 | 34a | 6.80 ± 0.31b | 71 | 105 ± 1.9b | 89 | |
| CCl4 + | 10 &mgr;g | 3.12 ± 0.13 | 24a | 10.51 ± 0.11a | 43 | 143 ± 2.0a | 52 |
| R. emodi | 50 &mgr;g | 3.27 ± 0.21 | 32a | 7.34 ± 0.17b | 67 | 129 ± 2.5a | 66 |
| 100 &mgr;g | 3.30 ± 0.13 | 33a | 6.08 ± 0.14b | 77 | 108 ± 2.3a | 86 | |
| CCl4 + Silymarin | 30 &mgr;g | 4.61 ± 0.22 | 69a | 6.00 ± 0.50b | 78 | 107 ± 2.3a | 87 |
In vivo: Detailed evaluation of extracts of S. mukorossi and R. emodi for hepatoprotective activity was carried out against CCl4. The animals were divided into five groups of six animals each. Group 1 served as vehicle control and was administered with normal saline. Group 2 rats were given CCl4 1.0 mL/kg, p.o checking the biochemical parameters periodically for hepatotoxicity. Group 3 rats were given CCl4 + extracts of S. mukorossi 2.5 g/kg, p.o. Group 4 rats were given CCl4 + extracts of R. emodi 3.0 g/kg, p.o. Group 5 rats were given CCl4 + Silymarin 50 g/kg, p.o. Blood was collected from the orbital sinus in all animals 2 h after last treatment and serum separated for different estimations (Table 3). The rats were anesthetized and sacrificed after the experimental period by cervical decapitation. The liver tissue was examined histopathologically.
| Treatment | Dose (mg/kg, p.o.) | Serum parameters | |||
| ALT (IU/L) | AST (IU/L) | ALP (IU/L) | Bilirubin (mg) | ||
| Vehicle control | - | 127.73 ± 10.65 | 100.26 ± 11.50 | 40.11 ± 2.20 | 0.11 ± 0.02 |
| Vehicle + CCl4 | - | 1142.36 ± 80.97 | 833.59 ± 34.85 | 64.06 ± 3.00 | 0.82 ± 0.08 |
| S. mukorossi CCl4 + | 2.5 | 524.18 ± 86.30b | 408.72 ± 40.07a | 47.38 ± 1.02b | 0.40 ± 0.02b |
| R. emodi CCl4 + | 3 | 384.69 ± 44.39b | 314.90 ± 44.41b | 49.00 ± 2.19 | 0.32 ± 0.02b |
| Silymarin CCl4 + | 50 | 563.47 ± 49.22b | 421.54 ± 30.82b | 47.96 ± 2.49b | 0.42 ± 0.02b |
The data obtained was subjected to statistical analysis using ANOVA for comparing different groups (Armitage, 1987) and Dunnett’s t test for control and test groups (Dunnett, 1964). The two tailed unpaired student t test for comparing means before and after treatment and one tailed unpaired student t test for comparing control and drug treated group, ED50 value with 95% confidence limits (CL) by regression analysis using log dose response (Swinscow, 1980 & Ghosh, 1984) were used. P < 0.05 or less was taken as the criterion of significance.
S. mukorossi and R. emodi extracts showed significant hepatoprotective activity against CCl4 induced liver injury in primary hepatocytes cultures (Table 2). The hepatotoxic effects of CCl4 are attributed to its metabolism by P450 to yield toxic trichloromethyl radicals that can act as free radical initiators[28]. These radicals are believed to induce injury either by interacting with the unsaturated fatty acids of cell membranes, thereby causing lipid peroxidation, or by binding covalently to important macromolecules such as proteins, lipids, or DNA[2930]. The extracts of S. mukorossi and R. emodi reduced the levels of LDH and GPT released from CCl4 injured rat hepatocytes into the medium in a concentration dependent manner, thus signifying their hepatoprotective activity.
In CCl4 intoxicated rats, serum activities of AST, ALT, ALP, and bilirubin were increased significantly when compared to the control (Table 3). The CCl4 treated group showed a marked increase in serum bilirubin (mg %) (0.82 ± 0.08), ALT (IU/L) (1142.36 ± 80.97), AST (IU/L) (833.59 ± 34.85), and ALP (IU/L) (64.06 ± 3.00) activity indicating the injury caused by CCl4. Treatment with the extracts of S. mukorossi and R. emodi significantly decreased the above elevated parameters and the normal architectural liver pattern was restored as given below.
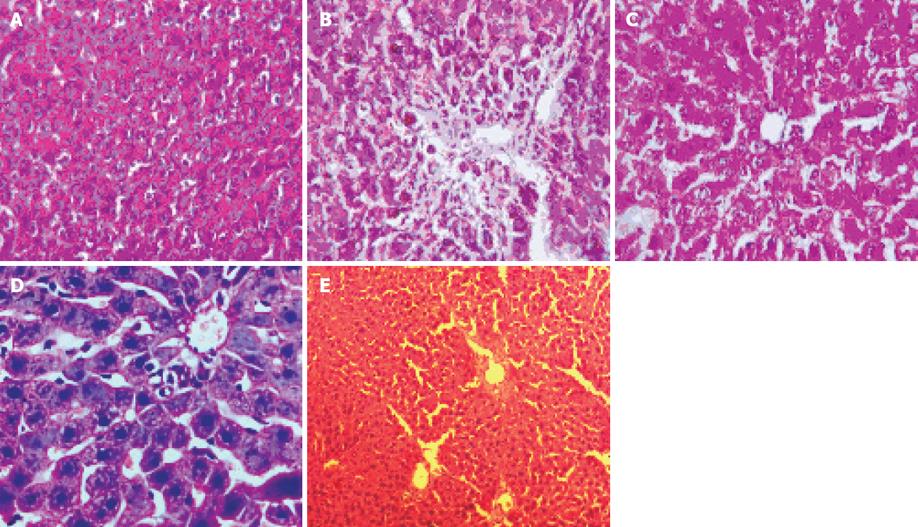
Liver section of control rat showed normal hepatocytes and normal architecture (Figure 1A). Liver sections from CCl4 treated rats demonstrated the destruction of architectural pattern, nodule formation in the lobular zone, inflamed periportal zone, and moderate inflammation of portal area (Figure 1B). Liver sections from Silymarin treated rats showed regeneration of normal hepatocytes (Figure 1C). Liver sections from R. emodi treated rats showed normal lobular architecture (Figure 1D). Liver sections from S. mukorossi treated rat showed normal lobular architecture, and no necrosis or fatty changes or inflammatory reaction were seen (Figure 1E). These histopathological findings demonstrate a hepatoprotective effect of the extracts against CCl4-mediated liver damage.
The purpose of this study was to explore the hepato-protective effect of S. mukorossi and R. emodi extracts in the hepatic damage caused by CCl4. Administration of CCl4 to normal rats increased serum levels of AST, ALT, ALP, and bilirubin. The enzymes leaking out from damaged liver cells into circulating blood represent the damage to hepatic cells.
It is well established that the toxic metabolite of CCl4, a free radical CCl3 is responsible for damage to liver cells. S. mukorossi and R. emodi extracts caused statistically significant decrease in all the above parameters at the dose of 2.5 mg/kg and 3.0 mg/kg given orally to CCl4 treated rats. Histopathological examination of the liver sections of rats treated with CCl4 showed destruction of architectural pattern, nodule formation in the lobular zone, inflamed periportal zone, moderate inflammation of portal area (Figure 1B). The group of rats treated with an extract of R. emodi showed normal lobular architecture (Figure 1D).
The group of rats treated with S. mukorossi showed normal lobular architecture and no necrosis or fatty changes or inflammatory reaction (Figure 1E). This suggests the reparative quality and maintenance of structural integrity of hepatocytic cell membrane of damaged liver cells by the extracts. The group of rats treated with Silymarin showing regeneration of normal hepatocytes was taken as standard (Figure 1C).
The ability of S. mukorossi and R. emodi to reduce the injurious effect or to preserve normal hepatic function disturbed by the hepatotoxin CCl4 is the index of its hepatoprotective effect.
These findings show the prophylactic and curative efficacy of S. mukorossi and R. emodi in maintaining the integrity and functional status of hepatocytes.
In conclusion, the data presented here indicate that the extracts of S. mukorossi and R. emodi are hepatoprotective both in CCl4 treated male rats and in CCl4 treated cultured primary rat hepatocytes. In addition, the in vivo studies carried out using the extracts also proved to be highly efficient in terms of dosage, tolerability, and restoring the liver. We now intend to look at the mechanism by which these extracts maintain the integrity of the liver.
This article is based on the natural products responsible for repairing and healing of adversely affected liver cells. Our study suggests that the extracts of S. mukorossi and R. emodi are hepatoprotective both in vitro in CCl4 damaged primary hepatocytes monolayer cultures and in vivo with doses of S. mukorossi (2.5 g/L) and R. emodi (3.0 g/L) in a CCl4 induced liver injury animal model.
Great effort has been and is still being done to minimize costs and side effects of synthetic drugs which are being used in the treatment of liver diseases.
The products are effective both in vitro and in vivo studies with minimal side effects and are cost effective. The herbal products were observed to have an excellent reparative effect on the CCl4 damaged hepatocytes.
This article helps to understand and implement the process of treatment with herbal medicines in liver diseases, which is safe and cost effective in comparison with the synthetic drugs.
This article tries to explore the safety and efficacy of the herbal drugs in the treatment of acute liver hepatitis and other liver disorders. The results revealed fewer side effects compared to synthetic drugs. It might be meaningful for the management and treatment of liver diseases in the clinic.
| 1. | Fernandez-Checa JC, Hirano T, Tsukamoto H, Kaplowitz N. Mitochondrial glutathione depletion in alcoholic liver disease. Alcohol. 1993;10:469-475. |
| 2. | Schuppan D, Atkinson J, Ruehl M, Riecken EO. Alcohol and liver fibrosis--pathobiochemistry and treatment. Z Gastroenterol. 1995;33:546-550. |
| 3. | Wang XD. Chronic alcohol intake interferes with retinoid metabolism and signaling. Nutr Rev. 1999;57:51-59. |
| 4. | Wu D, Cederbaum AI. Ethanol-induced apoptosis to stable HepG2 cell lines expressing human cytochrome P-4502E1. Alcohol Clin Exp Res. 1999;23:67-76. |
| 5. | Svensson S, Some M, Lundsjo A, Helander A, Cronholm T, Hoog JO. Activities of human alcohol dehydrogenases in the metabolic pathways of ethanol and serotonin. Eur J Biochem. 1999;262:324-329. |
| 6. | Lieber CS. Role of oxidative stress and antioxidant therapy in alcoholic and nonalcoholic liver diseases. Adv Pharmacol. 1997;38:601-628. |
| 7. | Torres-Duran PV, Miranda-Zamora R, Paredes-Carbajal MC, Mascher D, Ble-Castillo J, Diaz-Zagoya JC, Juarez-Oropeza MA. Studies on the preventive effect of Spirulina maxima on fatty liver development induced by carbon tetrachloride, in the rat. J Ethnopharmacol. 1999;64:141-147. |
| 8. | Saxena AK, Singh B, Anand KK. Hepatoprotective effects of Eclipta alba on subcellular levels in rats. J Ethnopharmacol. 1993;40:155-161. |
| 9. | Lin CC, Yen MH, Lo TS, Lin JM. Evaluation of the hepatoprotective and antioxidant activity of Boehmeria nivea var. nivea and B. nivea var. tenacissima. J Ethnopharmacol. 1998;60:9-17. |
| 10. | Zafar R, Mujahid Ali S. Anti-hepatotoxic effects of root and root callus extracts of Cichorium intybus L. J Ethnopharmacol. 1998;63:227-231. |
| 11. | Saraswat B, Visen PK, Patnaik GK, Dhawan BN. Ex vivo and in vivo investigations of picroliv from Picrorhiza kurroa in an alcohol intoxication model in rats. J Ethnopharmacol. 1999;66:263-269. |
| 12. | Doreswamy R, Sharma D. Plants drugs for liver disorders management. Indian drugs. 1995;32:139-144. |
| 13. | Evans DA, Subramoniam A, Rajashekaran S, Pushpangadan P. Effect of tricopuss eylanicus Gaertn, leaf extract on the energy metabolism in mice during excersize and at rest. Indian J Pharm. 2002;34:32-37. |
| 14. | Pandey G. XDravyaguna Vijanana; v I. Varanasi: Krishnadas Academy 1998; 191-196. |
| 15. | Kirtikar KR, Basu BD. Indian Medicinal Plants; v I. Allahabad. India: BLM Basu Publ 1991; 633-642. |
| 16. | The Useful Plants of India. Publication and Information Directorate. New Delhi: IR. 1986;547-553. |
| 17. | Rastogi RP, Mehrotra BN. Compendium of Indian Medicinal Plants; v 2. New Delhi: CDRI Publication 1999; 609-610. |
| 18. | Dwivedi AK, Chaudhary M, Sarine JPS. Standardisation of a new spermicidal agent sapindus saponin and its estimation in its formulation. Indian J Pharm Sci. 1990;52:165-167. |
| 19. | Castleman M. The Healing Herbs: The Ultimate guide to the curative powers of nature’s medicine. Emmaus PA: Rodale Press 1991; 305-307. |
| 20. | Peirce A. The American Pharmaceutical Association practical guide to natural medicines. New York: William Morrow and Company Inc 1999; 12. |
| 21. | Borgia M, Sepe N, Borgia R, Ori-Bellometti M. Pharmacological activity of an herbal extract: controlled clinical study. Curr Ther Res. 1981;29:525-536. |
| 22. | Duke JA. Green Pharmacy. Emmaus PA: Rodale Books 1997; 507. |
| 23. | Wang X, Lous Z, Mikage M, Namba T. Pharmacognostical studies on the Chinese crude drug da-huang rhubarb II. Botanical origin of three unofficial da-huang. Shoyakugaku Zasshi. 1988;42:302-309. |
| 24. | Vogel G. New natural products and Plant drugs with Pharma-cological, Biological and Therapeutical Activity. Springer Verlag: Berlin 1977; 249-265. |
| 25. | Kumar V, Cotran RS, Robbins SL. Cell injury and adaptation; 5th ed. Bangalore. India: Prime Books Publ 1992; 3-24. |
| 26. | Lee MK, Yeo H, Kim J, Kim YC. Protection of rat hepatocytes exposed to CCl4 in-vitro by cynandione A, a biacetophenone from Cynanchum wilfordii. J Pharm Pharmacol. 2000;52:341-345. |
| 27. | Kiso Y, Tohkin M, Hikino H. Assay method for antihepatotoxic activity using carbon tetrachloride induced cytotoxicity in primary cultured hepatocytes. Planta Med. 1983;49:222-225. |
| 28. | Johnston DE, Kroening C. Mechanism of early carbon tetrachloride toxicity in cultured rat hepatocytes. Pharmacol Toxicol. 1998;83:231-239. |
| 29. | Yasuda H, Izumi N, Shimada O, Kobayakawa T, Nakanishi M. The protective effect of tinoridine against carbon tetrachloride hepatotoxicity. Toxicol Appl Pharmacol. 1980;52:407-413. |