Published online Apr 21, 2008. doi: 10.3748/wjg.14.2338
Revised: February 22, 2008
Published online: April 21, 2008
AIM: To compare the preservation of non-heart-beating donor (NHBD) livers in cold histidine-trytophan-ketoglutarate (HTK) solution and extracorporeal liver perfusion (ECLP).
METHODS: Livers harvested from health pigs were stored for 10 h in cold HTK solution (group A, n = 4) or perfused with oxygenated autologous blood at body temperature (group B, n = 4). Both groups were then tested on the circuit for 4 h. Bile production, hemodynamic parameters, hepatocyte markers and reperfusion injury of extracorporeal livers were tested in each group. Liver tissues from each group were examined at the end of reperfusion.
RESULTS: At 1, 2, 3 and 4 h after reperfusion, bile production, hemodynamic parameters, hepatocyte markers and reperfusion injury of livers in group A were statistically different from those in group B (P < 0.05 or P < 0.01).
CONCLUSION: ECLP is better than HTK solution to preserve NHBD livers. ECLP can assess the graft viability before liver transplantation.
- Citation: Gong J, Lao XJ, Wang XM, Long G, Jiang T, Chen S. Preservation of non-heart-beating donor livers in extracorporeal liver perfusion and histidine-trytophan-ketoglutarate solution. World J Gastroenterol 2008; 14(15): 2338-2342
- URL: https://www.wjgnet.com/1007-9327/full/v14/i15/2338.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.2338
Standard liver preservation involves flushing the liver in situ with an organ preservation solution [University of Wisconsin (UW) solution or histidine-trytophan-ketoglutarate (HTK) solution] and its storage at 4°C for up to 15-18 h before transplantation[1]. During the process, pre-preservation injury, cold-preservation injury, re-warming injury, and reperfusion injury may occur. The occurrence rate of primary non-function (PNF) ranges 2%-23%, which is a major cause of death in transplantation[2]. Additionally, there is evidence that severe preservation injury is associated with increased liver graft rejection[3].
The system of extracorporeal liver perfusion (ECLP), which was established by our research team, can perfuse the liver with an oxygenated perfusate supplemented with nutritional support at body temperature during the preservation period[4]. Studies demonstrated that oxygenated perfusion is able to prevent adenosine tri-phosphate (ATP) loss during ischemia, and reset ATP levels after it[56].
In this preclinical study, we compared standard cold storage of livers in HTK solution to normothermic, sanguineous perfusion over a 10 h preservation period in the pig liver model.
Large white pigs weighing 20-25 kg were obtained from the Experimental Animal Center of Academy of Military Medical Sciences. All animals used in this study received humane care according to the National Guidelines for the Care of Animals in China. The pigs were housed at 23°C-25°C and fed with a standard food with free access to drinking water.
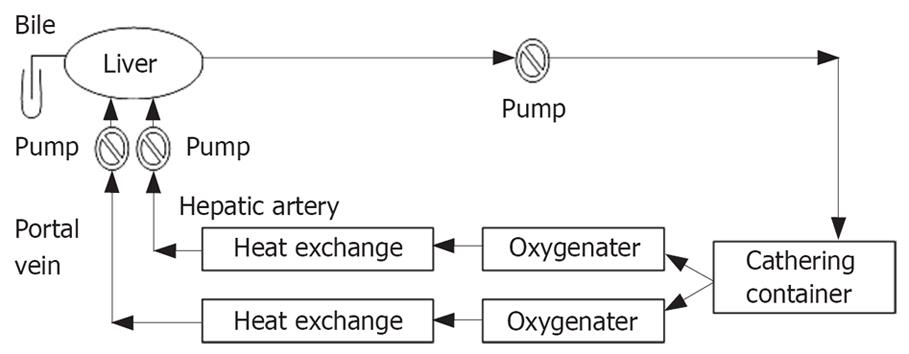
Blood pump (JHBP-2000B) was purchased from Guangzhou Jihua Medical Instruments Company. Hollow fiber oxygenater 3381 and heat exchanger were produced by Medtronic, Inc. USA. Digital measuring and controlling heating water box (GKX21Cr) was a product of Shanghai Jinping Instrument and Meter Company, Shanghai, China. Premature infant incubator (KXK-5G) was made by the 7th Shanghai Medical Instrument Factory, Shanghai, China. Gathering blood container 2000 was purchased from Dongguan Kewei Medical Instrument Company, Guangzhou, China. Euro-Collins solution and HTK solution were from Koehler Chemie Gmbh, Germany.
Livers were harvested from healthy pigs and preserved for 10 h in cold HTK solution (group A, n = 4) or perfused with oxygenated autologous blood at body temperature (group B, n = 4).
The function of all harvested livers was tested after preservation. The ECLP system was used to perfuse the livers with whole blood at body temperature as a substitute for transplantation. The blood used to perfuse livers in group B was changed between the preservation and reperfusion phases and their physiological parameters were compared.
Animals were anesthetized with 4 mg/kg propofol (intravenous injection, i.v.) and their ear veins were cannulated. The animals were intubated with an extended endotracheal tube. Anesthesia was maintained with i.v. propofol (1 mg/kg per hour) and halothane inhalation via an extended endotracheal tube throughout the procedure. The artery and vein of cervix were inserted into two catheters for transfusion and collecting blood. A midline incision was made to enter the peritoneal cavity. The bile duct was divided and catheterized for bile juice drainage. Hepatic vessels were identified and isolated in a standard fashion. After the collection of autoblood, the portal vein and hepatic artery were catheterized and the inferior vena cava (IVC) was divided with a suite of catheters, then the whole liver was removed. Approximately 1000 mL of autoblood was collected into a reservoir bottle.
During the preparation of liver, the perfusion apparatus was assembled and primed with 1000 mL of pig blood (Figure 1). The perfusion circuit consisting of blood pump, oxygenator, gathering blood container, perfusion tubing, gate clamp and heat exchanger was used to maintain the blood temperature at 39°C (normal temperature for a pig). Bottled oxygen was used and ratios were adjusted to maintain the physiological partial pressures. During priming of the circuit, pH, PaCO2, and Ca2+ were adjusted to the normal physiologic range. In general, the perfusion circuit was supplemented with 20 mL 50% glucose and 20 U/heparin.
The general conditions of liver were observed during perfusion. Bile production, hemodynamic parameters, hepatocyte markers and reperfusion injury of extracorporeal livers in each group were documented every hour during the reperfusion phase. At the end of each perfusion, the liver was sectioned and multiple random samples were assessed for histologic evaluation of reperfusion injury.
The data were analyzed using SPSS 11.0 statistical package. All values were presented as mean ± SD. For a single comparison, the significance of differences between means was determined by Student’s t-test. P < 0.05 was considered statistically significant.
At 1, 2, 3 and 4 h after perfusion, bile production of livers in group A was statistically different from that in group B (P = 0.0084, 0.0072, 0.0046, 0.0057, respectively; Table 1).
At 1, 2 and 3 h after perfusion, the pressure of hepatic artery in group A was statistically different from that in group B (P < 0.05), but at 4 h after perfusion, the pressure of hepatic artery in group A was not statistically different from that in group B. At 1 h after perfusion, the pressure of portal vein in group A was statistically different from that in group B (P < 0.05), but at 4 h after perfusion, the pressure of portal vein in group A was not statistically different from that in group C (Table 2).
A significant difference (P < 0.01) was found in alanine aminotransferase (ALT) between the two groups, which was increased to 115 IU/L in the cold-preserved livers. The corresponding value in the warm-preserved group never exceeded 55 IU/L. A similar change was seen in lactate dehydrogenase (LDH) release (P < 0.01), which was higher in cold-preserved livers (Table 3).
A significant difference (P < 0.01) was found in glucose concentrations of the perfusate between the two groups. At 2, 3 and 4 h after perfusion, the rate of oxygen consumption of livers in group A was statistically lower than that in group B (P < 0.01), but at 1 h after perfusion the rate of oxygen consumption of livers in group A was higher than that in group B (P < 0.05, Table 4).
| Group | Level of glucose in perfusate (mmol/L) | Oxygen consumption in livers | ||||||
| 1 h | 2 h | 3 h | 4 h | 1 h | 2 h | 3 h | 4 h | |
| A | 619.50 ± 19.54 | 787.00 ± 23.35 | 897.25 ± 17.78 | 974.25 ± 27.32 | 269.50 ± 12.45 | 234.50 ± 13.48 | 216.00 ± 7.75 | 192.25 ± 8.50 |
| B | 442.00 ± 19.58b | 535.00 ± 20.43b | 629.50 ± 45.89b | 687.25 ± 25.94b | 244.00 ± 8.44b | 267.00 ± 7.07a | 258.00 ± 4.97 | 251.25 ± 5.74 |
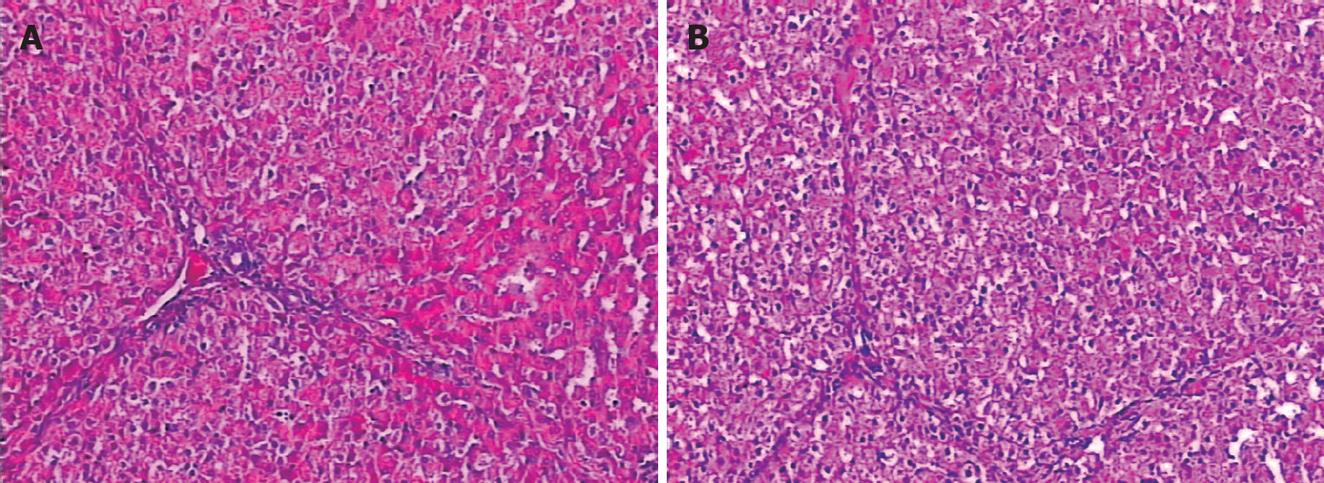
Changes in microstructure of hepatic cells: The livers in group A revealed edema of hepatic cells, sporadic acidophilic degeneration, mild vacuolization and sinusoidal dilatation. The livers in group B showed slight edema and necrosis of few hepatic cells (Figure 2).
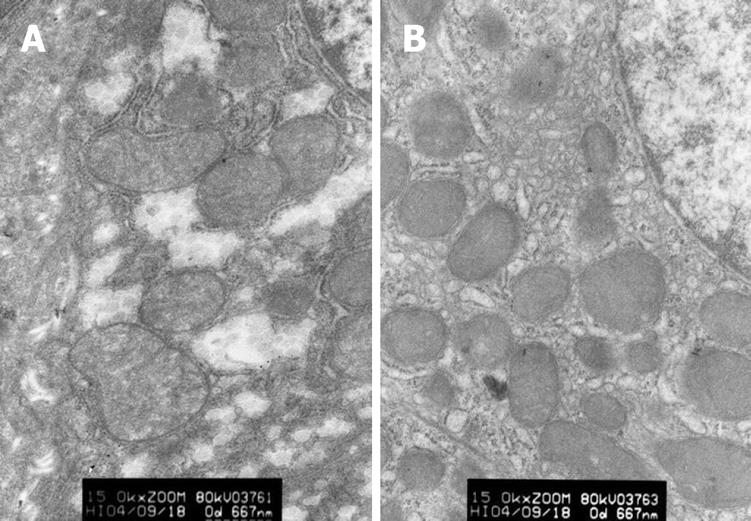
Changes in ultrastructure of hepatic cells: In group A, the karyotheca of hepatic cells was not clear, mitochondria swelled mildly, a few of crista vanished and endoplasmic reticulum expanded slightly. In group B, the karyotheca of hepatic cells was clear and regular, double nuclei were visible and endoplasmic reticulum was normal (Figure 3).
The progressively increas of patients with end stage liver disease is extending the waiting-list for liver transplantation, which, unfortunately, is not followed by a suitable increase in the number of donors[7]. The shortage of donor organs has given rise to the interest in liver harvesting from NHBD, which can significantly reduce the large discrepancy between supply and demand for liver transplantation. However, NHBD livers suffer not only from warm ischemic injury but also from cold preservation and reperfusion injury[8]. In this pathophysiological process, a number of cytokines, such as TNF-α, release with the activation of Kupffer cells and the obstruction of microcirculation. When blood perfusion is terminated during ischemic preservation, the supply of oxygen and nutrients is eliminated along with the vehicle for disposal of waste and metabolic by-products. During anoxia, ineffective anaerobic metabolism leads to depletion in energy stores, with a concomitant build-up of acidic by-products, which finally results in loss of transcellular electrolyte gradients, cell swelling, influx of free calcium, and subsequent activation of phospholipases. The breakdown of ATP during ischemia creates a setting for the production of reactive oxygen intermediates during reperfusion and a cascade of ischemic injury[9–12].
During the first half of the twentieth century, Carrel[13] perfused organs with normothermic, oxygenated serum at supraphysiological volumes and demonstrated gross viability for several days. ECLP has too many external constraints and has thus been largely abandoned. Research of ECLP has largely centered on the treatment of acute liver failure as a bridge to transplantation[14–16]. The present study used the system of ECLP to perfuse NHBD livers using dual vessel normothermic sanguineous perfusion with oxygenated blood as the perfusate. The perfusate to the liver artery and portal vein can be oxygenated separately[4]. Compared to the storage in cold HTK solution, the ECLP circuit acts not only as a method to store NHBD livers but as a substitute for transplantation, thus, the effect of two preservation methods can be evaluated. The outcome of studies can be influenced by many factors in a large animal transplantation model. If these factors are not considered, any conclusions drawn from this study can be attributed directly to the preservation process.
The change in hemodynamic parameters reflects the condition of liver microcirculation[17]. After 1 h reperfusion with the total hepatic blood flow maintained, the difference in the pressure of liver artery and observed in two groups, showing the different grade obstruction of liver microcirculation. When the reperfusion was continued, the pressure of liver artery and portal vein decreased gradually, while the pressure of portal vein remained unchanged in two groups, indicating that the liver microcirculation is improved. However, the condition of liver microcirculation in group B was better than that in group A. The glucose level in the perfusate represents the balance between supply and uptake of hepatocytes[18], suggesting that the glucose level is an indirect measure of liver metabolism. It was reported that the functional state of an isolated liver can be measured from its ability to induce gluconeogenesis[19]. The estimated elimination capacity of glucose concentration seems to reflect the metabolic capabilities during reperfusion. In the study, the glucose level of perfusate in group A was higher than that in group B, suggesting that the ability of liver to take up glucose is different in two groups. Although oxygen consumption might be reasonably assumed to be a measure of hepatocyte function, it fell consistently during perfusion. This was in contrast to other markers of liver function, particularly synthetic activity. Greater oxygen consumption was seen in livers of group A after 1 h reperfusion, which may be explained by the respiratory burst and subsequent oxygen debt that follows prolonged periods of cold ischemia and subsequent reperfusion. This was in contrast to the livers of group B,in which a stable level of oxygen consumption was observed. Bile production increased with time, which may reflect the consumption of substrate and its synthesizing function[2021].
How to use NHBD livers reasonably and effectively is the key question, which can make the transplanted liver function well and give the recipient a realistic chance of survival. At present, the criteria to decide whether the organ should be utilized or discarded almost depend on an overall judgment made on macroscopic appearance, perhaps with a biopsy. The ECLP system offers an opportunity to assess the viability of a liver to be used[2223], which may lead not only to a more rational use of the current donor pool but also to an accurate assessment of marginal organs[24].
Although the technique of cold storage is effective and simple,it may have certain limitations in terms of ability to maintain a viable organ avoid of ischemic injury and preservation time. In addition, this technique does not allow for viability assessment, and therefore the use of marginal donor organs remains difficult. By simulating the natural physiological environment of the liver and providing oxygen and other substrates necessary for normal metabolism, ECLP may serve as an ideal organ preservation technique in the future[25–27].
In conclusion, it is better to preserve NHBD livers in ECLP than in cold HTK solution in terms of synthetic and metabolic serum markers, hemodynamic parameters, and histological appearances.
To minimize the ischemia-reperfusion injury in non-heart-beating donor (NHBD) livers, we compared the preservation of NHBD livers in cold histidine-trytophan-ketoglutarate (HTK) solution and extracorporeal liver perfusion (ECLP).
Extracorporeal liver perfusion of NHBD livers can promote cellular recovery from warm ischaemic injury. The present study describes a method of normothermic extracorporeal perfusion by which the viability of livers can be maintained for at least 72 h.
This is probably the first study using the ECLP system with oxygenating blood. The ECLP circuit can be used not only as a method to store NHBD livers but as a substitute for transplantation, and therefore the effect of two preservation methods can be evaluated.
The findings in this study support that normothermic, sanguineous and oxygenated ECLP is better than HTK solution to preserve NHBD liver.
The design of this study was rational and reliable. The statistical methods used were appropriate. The results were sufficient to draw the conclusions. The discussion was well organized and rational.
| 1. | Franco-Gou R, Mosbah IB, Serafin A, Abdennebi HB, Rosello-Catafau J, Peralta C. New preservation strategies for preventing liver grafts against cold ischemia reperfusion injury. J Gastroenterol Hepatol. 2007;22:1120-1126. |
| 2. | Schemmer P, Mehrabi A, Kraus T, Sauer P, Gutt C, Uhl W, Buchler MW. New aspects on reperfusion injury to liver--impact of organ harvest. Nephrol Dial Transplant. 2004;19 Suppl 4:iv26-iv35. |
| 3. | Nishida S, Nakamura N, Kadono J, Komokata T, Sakata R, Madariaga JR, Tzakis AG. Intrahepatic biliary strictures after liver transplantation. J Hepatobiliary Pancreat Surg. 2006;13:511-516. |
| 4. | Gong J, Wang XM, Long G, Guo ZT, Jiang T, Chen S. Establishment and evaluation of the system of extracorporeal liver perfusion in pigs. Hepatobiliary Pancreat Dis Int. 2005;4:94-97. |
| 5. | Iwane T, Akamatsu Y, Narita T, Nakamura A, Satomi S. The effect of perfusion prior to cold preservation and addition of biliverdin on the liver graft from non-heart-beating donors. Transplant Proc. 2006;38:3358-3361. |
| 6. | Vajdova K, Smrekova R, Mislanova C, Kukan M, Lutterova M. Cold-preservation-induced sensitivity of rat hepatocyte function to rewarming injury and its prevention by short-term reperfusion. Hepatology. 2000;32:289-296. |
| 7. | Scuderi V, Ceriello A, Maida P, Aragiusto G, Arenga G, Carfora T, Defez M, Giuliani A, Monti GN, Santaniello W. The marginal donor: a single-center experience in orthotopic liver transplantation. Transplant Proc. 2006;38:1069-1073. |
| 8. | Kaczmarek B, Manas MD, Jaques BC, Talbot D. Ischemic cholangiopathy after liver transplantation from controlled non-heart-beating donors-a single-center experience. Transplant Proc. 2007;39:2793-2795. |
| 9. | Bilzer M, Gerbes AL. Preservation injury of the liver: mechanisms and novel therapeutic strategies. J Hepatol. 2000;32:508-515. |
| 10. | Yuan GJ, Ma JC, Gong ZJ, Sun XM, Zheng SH, Li X. Modulation of liver oxidant-antioxidant system by ischemic preconditioning during ischemia/reperfusion injury in rats. World J Gastroenterol. 2005;11:1825-1828. |
| 11. | Centurion SA, Centurion LM, Souza ME, Gomes MC, Sankarankutty AK, Mente ED, Castro e Silva O. Effects of ischemic liver preconditioning on hepatic ischemia/reperfusion injury in the rat. Transplant Proc. 2007;39:361-364. |
| 12. | Kume M, Banafsche R, Yamamoto Y, Yamaoka Y, Nobiling R, Gebhard MM, Klar E. Dynamic changes of post-ischemic hepatic microcirculation improved by a pre-treatment of phosphodiesterase-3 inhibitor, milrinone. J Surg Res. 2006;136:209-218. |
| 14. | Pascher A, Sauer IM, Hammer C, Gerlach JC, Neuhaus P. Extracorporeal liver perfusion as hepatic assist in acute liver failure: a review of world experience. Xenotransplantation. 2002;9:309-324. |
| 16. | Bauer M, Paxian M, Kortgen A. Acute liver failure. Current aspects of diagnosis and therapy. Anaesthesist. 2004;53:511-530. |
| 17. | Monbaliu D, Crabbe T, Roskams T, Fevery J, Verwaest C, Pirenne J. Livers from non-heart-beating donors tolerate short periods of warm ischemia. Transplantation. 2005;79:1226-1230. |
| 18. | Bailey SM, Reinke LA. Effect of low flow ischemia-reperfusion injury on liver function. Life Sci. 2000;66:1033-1044. |
| 19. | Hems R, Ross BD, Berry MN, Krebs HA. Gluconeogenesis in the perfused rat liver. Biochem J. 1966;101:284-292. |
| 20. | Foley DP, Vittimberga FJ, Quarfordt SH, Donohue SE, Traylor AN, MacPhee J, McLaughlin T, Ricciardi R, Callery MP, Meyers WC. Biliary secretion of extracorporeal porcine livers with single and dual vessel perfusion. Transplantation. 1999;68:362-368. |
| 21. | Butler AJ, Rees MA, Wight DG, Casey ND, Alexander G, White DJ, Friend PJ. Successful extracorporeal porcine liver perfusion for 72 hr. Transplantation. 2002;73:1212-1218. |
| 22. | Neuhaus P, Blumhardt G. Extracorporeal liver perfusion: applications of an improved model for experimental studies of the liver. Int J Artif Organs. 1993;16:729-739. |
| 23. | Schon MR, Kollmar O, Wolf S, Schrem H, Matthes M, Akkoc N, Schnoy NC, Neuhaus P. Liver transplantation after organ preservation with normothermic extracorporeal perfusion. Ann Surg. 2001;233:114-123. |
| 24. | St Peter SD, Imber CJ, Lopez I, Hughes D, Friend PJ. Extended preservation of non-heart-beating donor livers with normothermic machine perfusion. Br J Surg. 2002;89:609-616. |
| 25. | Imber CJ, St Peter SD, Lopez de Cenarruzabeitia I, Pigott D, James T, Taylor R, McGuire J, Hughes D, Butler A, Rees M. Advantages of normothermic perfusion over cold storage in liver preservation. Transplantation. 2002;73:701-709. |
| 26. | Naruse K, Sakai Y, Guo L, Natori T, Shindoh J, Karasawa Y, Iida Y, Kojima K, Michishita K, Makuuchi M. Development of a new extracorporeal whole-liver perfusion system. J Artif Organs. 2003;6:211-217. |
| 27. | Rojas A, Chen L, Bartlett RH, Arenas JD. Assessment of liver function during extracorporeal membrane oxygenation in the non-heart beating donor swine. Transplant Proc. 2004;36:1268-1270. |