Published online Apr 21, 2008. doi: 10.3748/wjg.14.2308
Revised: February 25, 2008
Published online: April 21, 2008
AIM: To investigate the effect and mechanism of blockade of the CXC chemokine receptor-4 (CXCR4) signaling pathway by AMD3100, a small non-peptide CXCR4 inhibitor, on invasion and metastasis of colorectal cancer cells in vitro.
METHODS: Human colorectal cancer cell line SW480 was treated with AMD3100 at different final concentrations. 3-(4,5-dimethylthiazol-2-yl)-2.5-dipheny-tetrazolium bromide (MTT) assay was used to detect the effect of AMD3100 on cell proliferation. The invasion ability of SW480 cells was determined by cell invasion assay kit. In the presence of AMD3100, the CXCL12-mediated migratory response of SW480 cells was tested by classical chemotaxis assays. RT-PCR analysis and Western blotting were used to detect the expression of vascular endothelial growth factor (VEGF), matrix metalloproteinase-2 (MMP-2) and -9 (MMP-9) in SW480 cells.
RESULTS: Cell viability was significantly suppressed by AMD3100 in a dose-dependent manner. AMD3100 (100 and 1000 ng/mL) significantly inhibited the invasion ability of SW480 cells. Treatment with AMD3100 markedly reduced the expression of VEGF and MMP-9 but not MMP-2 in SW480 cells.
CONCLUSION: The CXCL12/CXCR4 system is an important mediator of proliferation and invasion of CXCR4-expressing colorectal cancer cells. AMD3100 inhibited invasion and metastasis activity of the colorectal cancer cell line SW480 through down-regulation of VEGF and MMP-9 expression.
-
Citation: Li JK, Yu L, Shen Y, Zhou LS, Wang YC, Zhang JH. Inhibition of CXCR4 activity with AMD3100 decreases invasion of human colorectal cancer cells
in vitro . World J Gastroenterol 2008; 14(15): 2308-2313 - URL: https://www.wjgnet.com/1007-9327/full/v14/i15/2308.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.2308
Stromal cell-derived factor-1 (SDF-1 or CXCL12) and its unique receptor CXC chemokine receptor-4 (CXCR4) have prominent roles in invasion and metastasis of a diverse number of cancers. The interaction between SDF-1 and CXCR4 has been shown to direct tumor cells to organ sites with high levels of SDF-1 expression, which suggests this molecular pair plays a key role in chemotaxis and homing of metastatic cells. Convincing evidence indicates elevated CXCR4 expression in primary tumors is associated with lymph node metastasis in breasts[1–4], head and neck[5], and colon[6]. Furthermore, CXCR4 expression is associated with intraperitoneal carcinomatosis of ovarian[7] and gastric[8] cancer. High or persistent expression of CXCR4 has been associated with poor prognosis in osteosarcoma[9], epithelial ovarian cancer[10], colon cancer[61112], esophageal carcinoma[13], and melanoma[14]. Recently, intensive research has shown that binding of CXCL12 to CXCR4 plays a role in tumor angiogenesis by influencing the secretion of vascular endothelial growth factor (VEGF)[15] and increasing invasion associated with matrix metalloproteinase (MMP)-9 activation[16]. Therefore, interruption of the interaction between CXCR4 and SDF-1 has received considerable attention since it may provide a means of inhibiting the metastatic process.
AMD3100, a bicyclam molecule, has been identified as a specific inhibitor of CXCR4. It had originally been developed as an inhibitor of T-tropic human immunodeficiency virus (HIV) infectivity and has been used to block HIV infection of T-tropic, X4-using, virus and induce X4 transformation to R5, which validate CXCR4 as a target for HIV therapy in clinical trials. Furthermore, AMD3100 has also been demonstrated to be an effective mobilizer of hematopoietic stem cells in both healthy volunteers and multiple myeloma and non-Hodgkin's lymphoma patients[1718]. In recent studies, anti-CXCR4 treatment has suppressed primary tumor growth by inhibiting tumor angiogenesis, and has prevented lung metastasis of squamous cell carcinoma of the head and neck (SCCHN) in animal models[19]. Treatment of animals with AMD3100 resulted in decreased activation of the mitogen-activated protein kinase and AKT pathways in xenograft brain tumors, which are pathways downstream of CXCR4 that promote survival, proliferation and migration[20].
However, the influence of various concentrations of AMD3100 on invasion and proliferation in human colorectal cancer cell lines has not been reported. The present study showed that inhibition of CXCR4, through using non-peptide small molecule AMD3100, had significant antitumor activity, which represents a novel strategy for targeting highly metastatic colorectal cancer cells.
Human colorectal cancer (CRC) cell lines were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). Tumor cells were maintained in RPMI 1640, supplemented with 10% fetal calf serum, 100 U/mL penicillin, and 100 &mgr;g/mL streptomycin in a humidified incubator with an atmosphere of 5% CO2, 95% air at 37°C. The specific chemokine receptor CXCR4 antagonist, AMD3100 (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in sterile PBS (GIBCO, Carlsbad, CA, USA) as a × 1000 stock solution, and then diluted with the culture medium for experiments.
Cell viability was determined by MTT assay. Briefly, exponentially growing CRC cells were seeded in 96-well culture plates in serum-free medium at an optimal density. After 24 h incubation, either PBS or the indicated dose of AMD3100 was added for 2 h incubation in eight parallel holes. Then, CXCL12 (Peprotech, Rocky Hill, NJ, USA) was added daily at 20 ng/mL. MTT assays (Beyotime, Haimen, China) were performed after 24, 48 and 72 h of AMD3100 treatment. Absorbance was measured at 590 nm. The results were calculated as mean values of eight wells per treatment group.
Cell lines were assessed for migration utilizing a Boyden chamber chemotaxis assay. Chambers with 8 mm pore filters (HTS Transwell-24 System; Corning, Acton, MA, USA) were used. CXCL12 was added to the lower wells with 0.5% fetal bovine serum medium. Selected cells were treated with PBS or AMD3100 15 min prior to assay performance. After 3 h incubation at 37°C, cells within the inserts were removed from the upper surface of the membrane using a moist cotton-tipped swab. Migratory cells on the lower surface of the membrane, which had migrated through the polycarbonate membrane, were fixed in 100% ethanol, washed with phosphate buffer solution, air dried and stained with crystal violet for 30 min, then rinsed several times with distilled water. Migration was quantitated by dissolving stained cells in 10% acetic acid, and an equal amount of the dye/solution mixture was transferred onto a 96-well plate for colorimetric reading of A590. Invasion assay was performed using Cell Invasion Kit (Chemicon, Temecula, CA, USA). The inserts had an 8-&mgr;m pore size polycarbonate membrane with a precoated thin layer of basement membrane matrix (ECMatrix, Temecula, CA, USA). After 24 h incubation, invasive cells on the lower surface of the membrane were stained and calculated as previous described. Assays were performed in triplicate.
Whole-cell protein and nuclear protein extracts from SW480 CRC cells were prepared with Cell lysis buffer for Western and IP (Beyotime, Haimen, China) and Nuclear Extract Kit (Active Motif, Carlsbad, CA, USA), respectively, according to the manufacturers' instructions. Protein concentrations were determined using an assay kit (Bio-Rad, Hercules, CA, USA). Lysates containing 100 &mgr;g protein were mixed with loading buffer with 5% β-mercaptoethanol, and heated for 5 min at 100°C. Samples were separated by SDS-PAGE and transferred onto nitrocellulose membranes by semidry blotting. Membranes were incubated in TBS buffer for 1 h at room temperature, followed by hybridization with anti-CXCR4 antibody (Chemicon, Temecula, CA, USA); 1:1000 dilution), anti-MMP-9, anti-MMP-2 antibody, anti-VEGF antibody (Boster, Wuhan, China; 1:500 dilution) at 4°C overnight. After three washes in TBS/0.1% Tween 20, the membranes underwent hybridization with a horseradish peroxidase-conjugated secondary antibody, rabbit IgG (Santa Cruz Biotechnology, Santa Cruz, CA, USA; 1:5000 dilution) for 1 h at room temperature. After three washes in TBS/0.1% Tween 20, signals were detected by chemiluminescence using the Western blotting luminol reagent (Santa Cruz Biotechnology).
Total RNA extraction from CRC cells was performed with Trizol Reagent (Invitrogen, Carlsbad, CA, USA). Then, 2 &mgr;g total RNA was reverse transcribed with the First Strand cDNA Synthesis Kit (Takara, Japan). Subsequently, 2 &mgr;L cDNA product was subjected to PCR amplification with Taq DNA polymerase (Takara, Japan) on a thermal cycler using the following primers. The Random 9 mers primers for CXCR4, MMP-2, MMP-9, VEGF and β-actin were constructed on the basis of published sequences. The PCR primers used to detect each factor were as follows: CXCR4, sense strand 5’-GGAGGGGATCAGTATATACA-3’; antisense strand 5’-GAAGATGATGGAGTAGATGG-3’ (145 bp)[12]; MMP-2, sense strand 5’-GTGCTGAA GGACACACTAAAGA-AGA-3’; antisense strand 5’-TTGCCATCCTTCTCAAAGTTGTAGG-3’, (605 bp)[21]; VEGF, sense strand 5’-CCTGGTGGACATCTTCCAGGAGTACC-3’; antisense strand 5’-GAAGCTCATCTCTCCTATGTGCTGGC-3’, (196 bp)[22]; MMP-9, sense strand 5’-TCCCT-GGAGACCTGAGAACC-3’; antisense strand 5’-GTCGTCGGTGTCGTAGT-TGG-3’ (704 bp)[23] and β-actin, sense strand 5’-ATCTGGCACCA CACCTTCTACAA-TGAGCTGCG-3’; antisense strand 5’-CGTCATACTCCTGCTTGCTGATCCACATCTG-C-3’ (838 bp)[24]. The PCR conditions were as follows: One cycle of denaturing at 94°C for 2 min, followed by 30-35 cycles amplification, 56°C for 45 s and 72°C for 2 min. The PCR products were loaded onto 2% agarose gels and visualized with ethidium bromide under UV light. The ratio value of each group and the GAPDH group was taken as the relative value.
Numeric data are presented as mean ± SD of three experiments. The paired student's t test or one-way ANOVA was used for comparing the differences between groups. Statistical significance was assigned if P < 0.05. Analyses were performed using SPSS 13.0 (SPSS, Chicago, IL, USA).
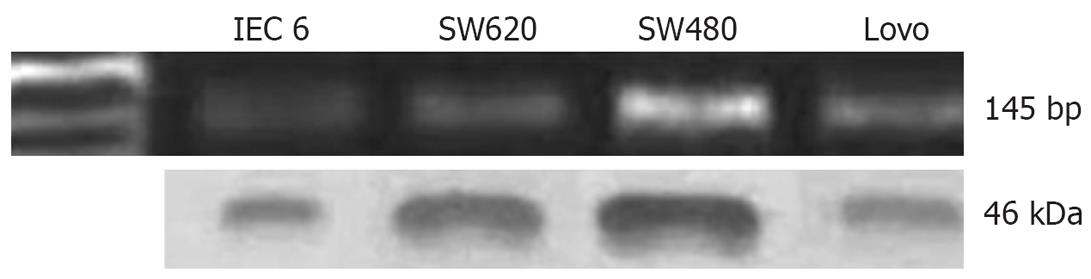
In our previous study, we analyzed CXCR4 expression in several cell lines (SW480, SW620, Lovo and IEC-6). In particular, our data showed lymph-node-metastasis-derived cell line SW480 expressed CXCR4 at a high level, by using RT-PCR and Western blotting (Figure 1). We also examined mRNA expression of CXCL12, the ligand of CXCR4, by RT-PCR. We found no expression of CXCL12 mRNA in any of the colorectal cancer cell lines (data not shown).
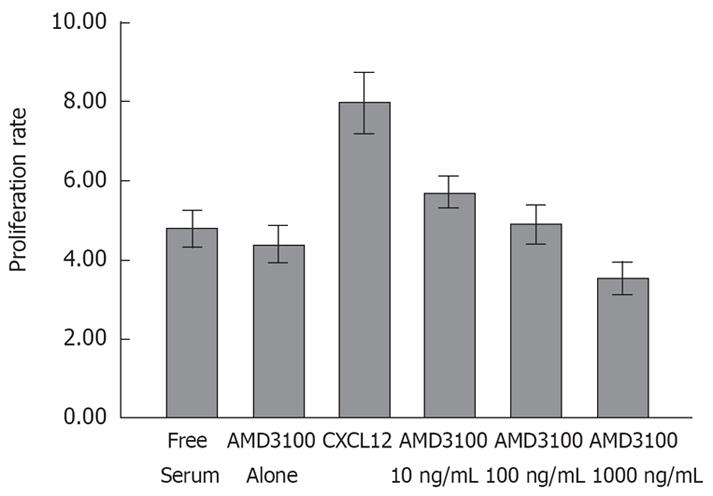
In our previous studies, we found no expression of CXCL12 mRNA in any of the CRC cancer cell lines. After 3 d incubation, CXCL12 greatly enhanced SW480 cells viability in the absence of serum (Figure 2). The enhancing effect of CXCL12 on cell proliferation was strongly inhibited by treatment with different doses of AMD3100. In a dose-dependent fashion, the proliferation rate was reduced to 6.10 ± 0.13, 4.49 ± 0.22, 3.58 ± 0.13 respectively (P < 0.05). The effect of 100 and 1000 ng/mL AMD3100 was statistically significant (P < 0.01, n = 8) compared to that of the CXCL12 group (7.97 ± 0.811). Although a decrease in proliferation was also observed in the AMD3100 alone group compared to the serum-free cells (vehicle-treated cells), the inhibition rate was not significantly different, probably due to a specific effect of blocking CXCL12-CXCR4 interaction. The assay also revealed that, in 24 h, there was no significant difference in viability in any of the groups. Therefore, the cell invasion assay was performed at 24 h to remove its influence on cell viability.
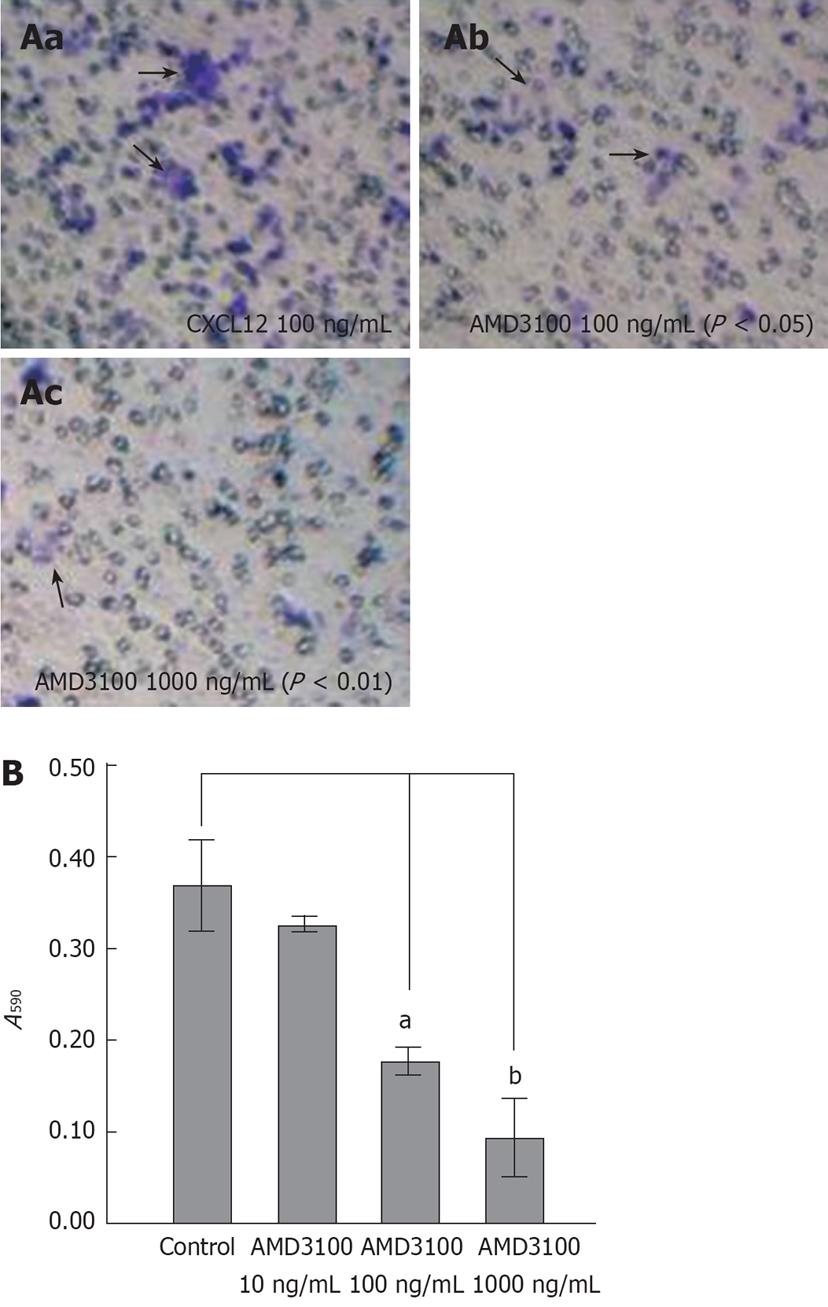
To evaluate the effects of inhibition of CXCL12-CXCR4 interaction on CRC invasion, we performed an in vitro invasion assay using AMD3100. After 24 h incubation, AMD3100 markedly reduced invasion of SW480 cells at concentrations of 100 and 1000 ng/mL (Table 1), by 28.43% (P < 0.05) and 77.23% (P < 0.01), respectively.
The effect of AMD3100 on inhibiting CXCL12-induced migration of CRC cells was estimated by a classical chemotaxis assay. The selected CXCR4-positive cell line, SW480, did migrate in response to CXCL12 in a classical chemotaxis assay, with an optimal response at 100 ng/mL. After AMD3100 treatment, chemotactic activity of SW480 cells was reduced in a dose-dependent manner (Figure 3B). The inhibition rate with AMD3100 at 10, 100 and 1000 ng/mL was 5.24%, 47.27% and 62.37%, respectively. The latter two achieved a significant difference compare to the control group (a, b and c in Figure 3A).
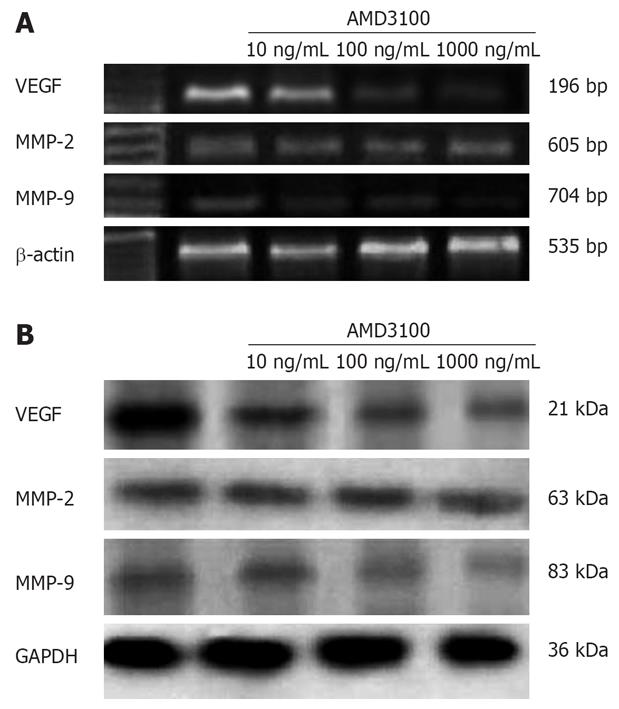
The CXCL12-CXCR4 axis contributes to invasion and specific organ metastasis through regulation of its target genes, which have recently been shown to be VEGF and MMPs[7162526]. Therefore, we detected the effect of AMD3100 on expression of VEGF, MMP-2 and MMP-9. As shown in Figure 4, 24 h incubation with AMD3100 reduced MMP-9 and VEGF protein expression in a dose-dependent manner in SW480 cells. RT-PCR demonstrated that the expression of MMP-9 but not MMP-2 and VEGF mRNAs in SW480 cells was significantly downregulated by 100 and 1000 ng/mL AMD3100. Densitometric analysis revealed the relative expression decreased to 17.58% ± 3.79% for MMP-9, and 39.44% ± 3.07% for VEGF, as compared to the controls (P < 0.05).
A growing body of literature has indicated CXCR4 is important in a variety of cancers, and more specifically, that this receptor can be a propitious target in treating cancer. In experimental systems, convincing evidence has shown that selective inhibition of CXCR4 suppresses CXCL12-induced migration of cancer cells, invasion, neoangiogenesis and metastases. Neutralizing the interactions of CXCL12 and CXCR4 by monoclonal antibody significantly impairs metastasis of breast cancer cells to regional lymph nodes and lungs[27]. Human breast tumor growth can be delayed by inhibiting CXCR4 with siRNA[28]. Similarly, CXCR4 antagonists, T140 analogs, inhibit SDF-1-induced migration of human breast cancer, leukemia and endothelial cells in vitro, in a manner relevant to tumor spread and angiogenesis[29]. The CXCR4 inhibitor RCP168 partially abrogates the protective effect of stromal co-culture system and greatly enhances chemotherapy-induced apoptosis in chronic lymphocytic leukemia cells[30]. Redjal et al have demonstrated the ability of AMD3100 to reduce the activation of extracellular signal-regulated kinases 1 and 2 and Akt, all of which are pathways downstream of CXCR4 that promote cell survival, proliferation and migration. Moreover, in vivo, combining sub-therapeutic doses of AMD3100 with a conventional cytotoxic agent in an order-dependent manner produces synergistic effects, which result from both a decrease in proliferation and an increase in apoptosis of tumor cells[31].
Our previous studies have shown a significant association between CXCR4 expression and lymph nodal status and increased risk for metastasis in colorectal carcinoma. These initial observations prompted us to further question whether modulation of CXCR4 affects the invasive ability of CRC cells. In the present study, we demonstrated that AMD3100, a CXCR4-specific inhibitor, strongly suppressed CXCL12-induced activity and invasion of the SW480 cell line, which expressed CXCR4 at a high level. Recently, researchers have found that CXCL12-CXCR4 signaling directly regulates MMP-9 expression in CRC cell lines[16]. In our present study, the use of AMD3100 markedly reduced the mRNA and protein expression of MMP-9 but not MMP-2 in SW480 cells, through blocking the CXCL12-CXCR4 signaling pathway. This suggests that inhibiting SDF-1/CXCR4 interaction may have a variety of therapeutic benefits in CRC patients. Meanwhile, we also observed a directional migration of CRC cells depending on the presence of extracellular matrix (ECM) proteins. AMD3100 significantly inhibited CXCL12-induced migratory activity and ECM-dependent direct invasive ability.
Recent studies have also shown VEGF expression is associated with poor survival and prognosis in CRC, and that increased VEGF expression is correlated with increased microvessel density, local invasion, liver metastasis, and early recurrence after curative resection[2032]. In the present study, we also found that AMD3100 significantly decreased mRNA and protein expression of VEGF in SW480 cells, which suggests that drug treatment may not only block CXCL12-induced angiogenesis, but also impair formation of microvasculature in metastasis stimulated by VEGF.
In serum-free medium, adding CXCL12 may significantly increase proliferation activity of tumor cells. This kind of protective microenvironment generally exists in various solid tumors, such as CXCR4-positive renal cancer and intracranial glioblastoma, which functionally improves cancer-cell viability, anti-apoptotic survival and chemoresistance[29]. Production of CXCL12 in tumor stroma may keep cancer cells tolerant to hypoxic or poor nutritional conditions. On the other hand, hypoxia-induced CXCR4 expression may activate downstream signal transduction, and subsequently regulate cancer cell biological behavior to adapt to hypoxia. In our study, we tested in the presence of CXCL12, the effect of various concentrations of AMD3100 on cell viability. Interestingly, although the treatment failed to decrease the number of cancer cells in serum-free medium, AMD3100 abrogated CXCL12 stimulation during cell proliferation in a dose-depended manner. Additionally, AMD3100 alone did not significantly affect cell proliferation compared with the unstimulated group, which suggests no autocrine growth stimulatory loops exist in this cell line.
In conclusion, CXCL12-CXCR4 signals are responsible for the invasion and metastasis of human CRC via regulation of cell viability and the expression of genes related to proteolysis and angiogenesis. Blocking the CXCL12-CXCR4 pathway may provide a novel means of preventing the invasion and metastasis of CRC.
CXC chemokine receptor-4 (CXCR4) is by far the most common chemokine receptor overexpressed in human cancer cells. High-level expression of CXCR4 in various primary cancers is significantly associated with poor prognosis and extent of metastasis. Targeting CXCR4, therefore, is now regarded as a novel and efficient strategy for treating human cancer metastases. There are two categories of therapeutic strategy for targeting CXCR4, anti-CXCR4 monoclonal antibody and specific small molecular CXCR4 antagonists such as AMD3100, T140, ALX40-4C and CTCE-9908. Each of these inhibits CXCR4 via different mechanisms.
Specific small-molecule CXCR4 antagonists such as AMD3100 may play an important role in the treatment of HIV infections, and many other pathological processes that are dependent on CXCL12-CXCR4 interactions (e.g. rheumatoid arthritis, asthma and cancer metastasis). Treatment of animals with AMD3100 results in decreased activation of the mitogen-activated protein kinase and AKT pathways in xenograft brain tumors. Administration of AMD3100 and 1, 3-bis (2-chloroethyl)-1-nitrosourea (BCNU) in an order-dependent manner produces synergistic cytotoxicity, which results from both a decrease in proliferation and an increase in apoptosis of glioblastoma cells.
We have demonstrated that CXCL12-induced activity and invasion of CRC cells were markedly suppressed by blocking the CXCL12-CXCR4 signaling pathway with AMD3100, a small non-peptide inhibitors of CXCR4. These findings strongly suggest CXCR4 plays an important role in CRC progression and may provide a novel and effective molecular target for treatment of CRC.
Our study showed that blockade of the CXCL12-CXCR4 axis affects the viability, invasion and migration of CRC cells by using various doses of AMD3100. The data also demonstrated the inhibitory effect was dose-dependent. It may be a good start to move from elucidation of the mechanism of action of chemokine receptors in cancer metastasis to development of novel therapeutic targets based on the CXCL12-CXCR4 axis.
Chemokines are small secreted peptides that control adhesion and transendothelial migration of leukocytes, especially during immune and inflammatory reactions. They are divided into four subfamilies: CC, CXC, C and CX3C based on the position of their NH2-terminal cysteine residues, and bind to seven transmembrane domain G protein-coupled receptors. CXCL12, also known as SDF-1, belongs to the CXC chemokine family and CXCR4 is the only known physiological receptor for SDF-1.
This is a very interesting study. The authors showed inhibition of CRC cell viability and metastasis by blocking CXCL12-CXCR4 interaction. Although this does not necessarily predict a positive result in human trials, the accumulation of these data may provide additional clues regarding some unanswered questions.
| 1. | Cabioglu N, Yazici MS, Arun B, Broglio KR, Hortobagyi GN, Price JE, Sahin A. CCR7 and CXCR4 as novel biomarkers predicting axillary lymph node metastasis in T1 breast cancer. Clin Cancer Res. 2005;11:5686-5693. |
| 2. | Andre F, Cabioglu N, Assi H, Sabourin JC, Delaloge S, Sahin A, Broglio K, Spano JP, Combadiere C, Bucana C. Expression of chemokine receptors predicts the site of metastatic relapse in patients with axillary node positive primary breast cancer. Ann Oncol. 2006;17:945-951. |
| 3. | Kang H, Watkins G, Douglas-Jones A, Mansel RE, Jiang WG. The elevated level of CXCR4 is correlated with nodal metastasis of human breast cancer. Breast. 2005;14:360-367. |
| 4. | Su YC, Wu MT, Huang CJ, Hou MF, Yang SF, Chai CY. Expression of CXCR4 is associated with axillary lymph node status in patients with early breast cancer. Breast. 2006;15:533-539. |
| 5. | Uchida D, Begum NM, Almofti A, Nakashiro K, Kawamata H, Tateishi Y, Hamakawa H, Yoshida H, Sato M. Possible role of stromal-cell-derived factor-1/CXCR4 signaling on lymph node metastasis of oral squamous cell carcinoma. Exp Cell Res. 2003;290:289-302. |
| 6. | Schimanski CC, Schwald S, Simiantonaki N, Jayasinghe C, Gonner U, Wilsberg V, Junginger T, Berger MR, Galle PR, Moehler M. Effect of chemokine receptors CXCR4 and CCR7 on the metastatic behavior of human colorectal cancer. Clin Cancer Res. 2005;11:1743-1750. |
| 7. | Scotton CJ, Wilson JL, Scott K, Stamp G, Wilbanks GD, Fricker S, Bridger G, Balkwill FR. Multiple actions of the chemokine CXCL12 on epithelial tumor cells in human ovarian cancer. Cancer Res. 2002;62:5930-5938. |
| 8. | Yasumoto K, Koizumi K, Kawashima A, Saitoh Y, Arita Y, Shinohara K, Minami T, Nakayama T, Sakurai H, Takahashi Y. Role of the CXCL12/CXCR4 axis in peritoneal carcinomatosis of gastric cancer. Cancer Res. 2006;66:2181-2187. |
| 9. | Laverdiere C, Hoang BH, Yang R, Sowers R, Qin J, Meyers PA, Huvos AG, Healey JH, Gorlick R. Messenger RNA expression levels of CXCR4 correlate with metastatic behavior and outcome in patients with osteosarcoma. Clin Cancer Res. 2005;11:2561-2567. |
| 10. | Jiang YP, Wu XH, Shi B, Wu WX, Yin GR. Expression of chemokine CXCL12 and its receptor CXCR4 in human epithelial ovarian cancer: an independent prognostic factor for tumor progression. Gynecol Oncol. 2006;103:226-233. |
| 11. | Ottaiano A, Franco R, Aiello Talamanca A, Liguori G, Tatangelo F, Delrio P, Nasti G, Barletta E, Facchini G, Daniele B. Overexpression of both CXC chemokine receptor 4 and vascular endothelial growth factor proteins predicts early distant relapse in stage II-III colorectal cancer patients. Clin Cancer Res. 2006;12:2795-2803. |
| 12. | Kim J, Takeuchi H, Lam ST, Turner RR, Wang HJ, Kuo C, Foshag L, Bilchik AJ, Hoon DS. Chemokine receptor CXCR4 expression in colorectal cancer patients increases the risk for recurrence and for poor survival. J Clin Oncol. 2005;23:2744-2753. |
| 13. | Koishi K, Yoshikawa R, Tsujimura T, Hashimoto-Tamaoki T, Kojima S, Yanagi H, Yamamura T, Fujiwara Y. Persistent CXCR4 expression after preoperative chemoradiotherapy predicts early recurrence and poor prognosis in esophageal cancer. World J Gastroenterol. 2006;12:7585-7590. |
| 14. | Scala S, Ottaiano A, Ascierto PA, Cavalli M, Simeone E, Giuliano P, Napolitano M, Franco R, Botti G, Castello G. Expression of CXCR4 predicts poor prognosis in patients with malignant melanoma. Clin Cancer Res. 2005;11:1835-1841. |
| 15. | Guleng B, Tateishi K, Ohta M, Kanai F, Jazag A, Ijichi H, Tanaka Y, Washida M, Morikane K, Fukushima Y. Blockade of the stromal cell-derived factor-1/CXCR4 axis attenuates in vivo tumor growth by inhibiting angiogenesis in a vascular endothelial growth factor-independent manner. Cancer Res. 2005;65:5864-5871. |
| 16. | Brand S, Dambacher J, Beigel F, Olszak T, Diebold J, Otte JM, Goke B, Eichhorst ST. CXCR4 and CXCL12 are inversely expressed in colorectal cancer cells and modulate cancer cell migration, invasion and MMP-9 activation. Exp Cell Res. 2005;310:117-130. |
| 17. | Fricker SP, Anastassov V, Cox J, Darkes MC, Grujic O, Idzan SR, Labrecque J, Lau G, Mosi RM, Nelson KL. Characterization of the molecular pharmacology of AMD3100: a specific antagonist of the G-protein coupled chemokine receptor, CXCR4. Biochem Pharmacol. 2006;72:588-596. |
| 18. | Khan A, Greenman J, Archibald SJ. Small molecule CXCR4 chemokine receptor antagonists: developing drug candidates. Curr Med Chem. 2007;14:2257-2277. |
| 19. | Yoon Y, Liang Z, Zhang X, Choe M, Zhu A, Cho HT, Shin DM, Goodman MM, Chen ZG, Shim H. CXC chemokine receptor-4 antagonist blocks both growth of primary tumor and metastasis of head and neck cancer in xenograft mouse models. Cancer Res. 2007;67:7518-7524. |
| 20. | Rubin JB, Kung AL, Klein RS, Chan JA, Sun Y, Schmidt K, Kieran MW, Luster AD, Segal RA. A small-molecule antagonist of CXCR4 inhibits intracranial growth of primary brain tumors. Proc Natl Acad Sci USA. 2003;100:13513-13518. |
| 21. | Uchima Y, Sawada T, Nishihara T, Maeda K, Ohira M, Hirakawa K. Inhibition and mechanism of action of a protease inhibitor in human pancreatic cancer cells. Pancreas. 2004;29:123-131. |
| 22. | Brown KJ, Maynes SF, Bezos A, Maguire DJ, Ford MD, Parish CR. A novel in vitro assay for human angiogenesis. Lab Invest. 1996;75:539-555. |
| 23. | Gao Y, Wang JJ, Wang GF, Xu Q, Guo J. Effect of hypoxia on production and secretion of matrix metalloproteinases in tumor cells. Ai Zheng. 2005;24:180-183. |
| 24. | Zhu Z, Yao J, Wang F, Xu Q. TNF-alpha and the phenotypic transformation of human peritoneal mesothelial cell. Chin Med J (Engl). 2002;115:513-517. |
| 25. | Neuhaus T, Stier S, Totzke G, Gruenewald E, Fronhoffs S, Sachinidis A, Vetter H, Ko YD. Stromal cell-derived factor 1alpha (SDF-1alpha) induces gene-expression of early growth response-1 (Egr-1) and VEGF in human arterial endothelial cells and enhances VEGF induced cell proliferation. Cell Prolif. 2003;36:75-86. |
| 26. | Samara GJ, Lawrence DM, Chiarelli CJ, Valentino MD, Lyubsky S, Zucker S, Vaday GG. CXCR4-mediated adhesion and MMP-9 secretion in head and neck squamous cell carcinoma. Cancer Lett. 2004;214:231-241. |
| 27. | Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50-56. |
| 28. | Lapteva N, Yang AG, Sanders DE, Strube RW, Chen SY. CXCR4 knockdown by small interfering RNA abrogates breast tumor growth in vivo. Cancer Gene Ther. 2005;12:84-89. |
| 29. | Burger M, Hartmann T, Krome M, Rawluk J, Tamamura H, Fujii N, Kipps TJ, Burger JA. Small peptide inhibitors of the CXCR4 chemokine receptor (CD184) antagonize the activation, migration, and antiapoptotic responses of CXCL12 in chronic lymphocytic leukemia B cells. Blood. 2005;106:1824-1830. |
| 30. | Zeng Z, Samudio IJ, Munsell M, An J, Huang Z, Estey E, Andreeff M, Konopleva M. Inhibition of CXCR4 with the novel RCP168 peptide overcomes stroma-mediated chemoresistance in chronic and acute leukemias. Mol Cancer Ther. 2006;5:3113-3121. |
| 31. | Redjal N, Chan JA, Segal RA, Kung AL. CXCR4 inhibition synergizes with cytotoxic chemotherapy in gliomas. Clin Cancer Res. 2006;12:6765-6771. |
| 32. | Rodriguez J, Zarate R, Bandres E, Viudez A, Chopitea A, Garcia-Foncillas J, Gil-Bazo I. Combining chemotherapy and targeted therapies in metastatic colorectal cancer. World J Gastroenterol. 2007;13:5867-5876. |