INTRODUCTION
Gardner syndrome (GS) is a rare autosomal dominant inherited disorder with a high degree of penetrance characterized by intestinal polyposis, bone and soft-tissue tumours, including osteoma, epidermal inclusion cyst, lipoma, fibroma, gastric and duodenal polyposis, desmoid fibromatosis. It is regarded as a clinical subgroup of familial adenomatous polyposis (FAP). Since GC was first reported by Gardner and his colleague in early of 1950s, the association of hereditary colonic polyposis and osteomatosis with multiple cutaneous and subcutaneous tumours in GS has been extensively studied[1–3].
Compared with FAP, the extracolonic manifestations of GC may be explained by the variable penetrance of a common mutation. The disorder is linked to the binding of 5q21-q22 (the adenomatous polyposis coli locus, APC gene). More than 1400 different mutations of this gene have been identified[4]. The mutated specific area of the APC gene determines the extracolonic manifestations as well as the number, time frame and malignant potential of adenomatous polyps. It was reported that the mutation of MYH gene (1p34.3-p32.1) and environmental factors, such as diet, exercise and smoking, also play an important role in the pathogenesis of GS[5]. Although most GS cases show familial clustering, one-third of cases occur due to spontaneous mutations.
The clinical presentation of GS is variable and its diagnosis is often delayed, despite the presence of clues for a significant amount of time. Since GS may involve different organs, it is usually very difficult to treat it, and the therapeutical effect is also uncertain. We present a rare case of a 23-year-old girl with GS who underwent a series of operations and medications which achieved satisfactory therapeutic effects.
CASE REPORT
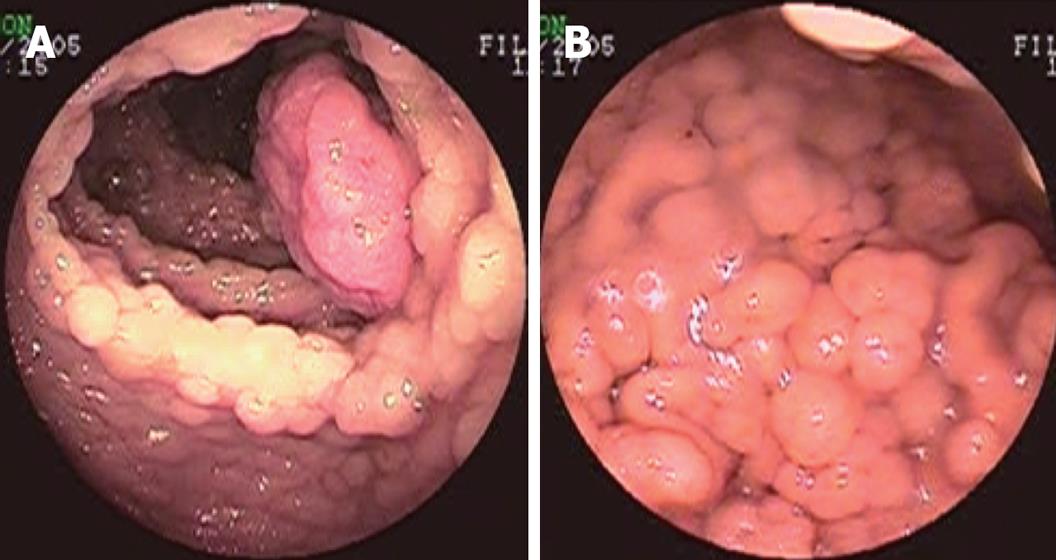
A 23-year-old female presented with nausea, vomiting and mucous diarrhoea, occasionally with blood in the stool, for 1 mo. Colonoscopy revealed numerous polyps covering the entire colon and rectum, mostly sigmoid colon and rectum, which were consistent with the diagnosis of FAP (Figure 1A). Gastroscopy showed numerous polyps covering the fundus and corpus ventriculi, mostly fundus ventriculi (Figure 1B). Biopsy of colon and stomach polyps showed moderate differentiation. Hemoglobin was 72 g/L. The patient was finally diagnosed as GS with innutrition and anemia.
Figure 1 Preoperative endoscopy examination.
A: Colonoscopy showing multiple polyps covering the entire colon and rectum; B: Gastroscopy showing multiple polyps covering the fundus and corpus ventriculi.
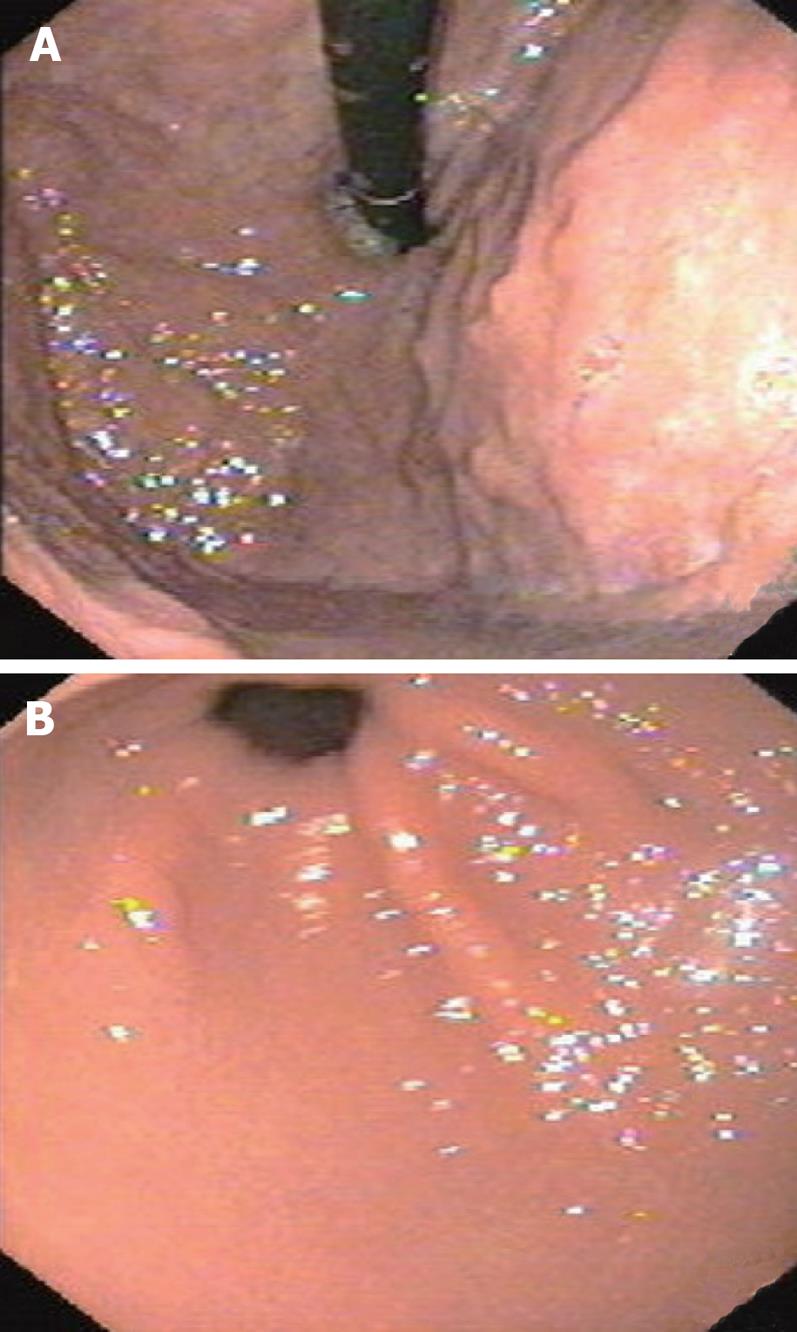
This patient was first treated with restorative proctoco-lectomy in combination with ileal pouch anal anastomosis (RPC/IPAA) and ileostomy. Pathological examination of the specimen showed that the colorectal polypi were tubular adenomas with moderate differentiation. Then, an ileostomy closure operation and two times of snare polypectomy were performed. After operation, the patient took oral traditional Chinese medicine pills twice per day for about 6 mo. The traditional Chinese medicine pills were made of 3.0 g Fructus mume (smoked plum) and 3.0 g Bombyx batryticatu (stiff silkworm). Innutrition and anaemia gradually recovered. Gastroscopy showed that the remnant gastric polypi gradually decreased and finally disappeared (Figure 2A and B). The patient had 2-3 times of solid stool per day at the time when we wrote this report.
Figure 2 Postoperative gastroscopy examination shows disappearance of polyps of the fundus and corpus ventriculi (A) and smooth mucous membrane of the corpus ventriculi and pars pylorica (B).
DISCUSSION
GS is considered a variant of FAP with certain extraco-lonic manifestations (such as osteoma, gastric or duodenal polyposis and desmoid fibromatosis). It was reported that GS is caused by truncating mutations of the APC gene (codons 1403 and 1578) differing from classic FAP (codons 169-1600), attenuated FAP (amino terminal to codon 157), and congenital hypertrophy of the retinal pigmented epithelium (codons 463-1387)[4]. However, there is evidence that patients with identical mutations may have different phenotypic expressions because of unclear reasons. The majority of GS patients may have a family history, but about 25% of GS patients can present with a new dominant mutation and are the first affected member of the family. These patients are generally not under medical surveillance before they have bowel symptoms, and 67% of them may have developed colorectal cancer. In 100% of all untreated patients, cancer develops in the large intestine before the age of 40 years. Hence, prophylactic colectomy is indicated, although desmoid tumors of the mesenteric and abdominal wall may develop after surgery. We think that RPC/IPAA is the best operation for FAP (including GS) patients because it not only resects all large intestine mucous membranes to avoid carcinogenesis, but also preserves the intestine function and sex ability and avoids colostomy, thus improving the life quality of patients. In order to insure the concrescence of ileal pouch anal anastomosis, temporary ileostomy is occasionally necessary. After 1 to 3 mo when the anastomosis reaches its concrescence, we can perform an ileostomy closure operation. The distal ileum would play the role of colon in absorbing water from dejecta if the colon is resected, so we should take care to preserve the distal ileum for self-control defecation. The patient were underwent to a series of operations with satisfactory therapeutic effects, showing that such operations can cure similar patients.
It was reported that the carcinogenesis of gastric polypi is obviously lower than that of colorectal polypi and its carcinogenesis time may be about 10 years later than colorectal polypi[2]. The gastric polypi in this patient gradually decreased and finally disappeared after two times of snare polypectomy and treatment with traditional Chinese medicine. Although the idiographic reason remains unclear, the prognosis of gastric polypi is well, suggesting that excessive radical operation cannot be performed for gastric polypi. It was reported that non-steroidal anti-inflammatory drugs (NSAID) can maintain the regression of colorectal adenoma in patients with FAP and hereditary nonpolyposis colorectal cancer (HNPCC)[6–11]. Use of NSAID may pave a new secure path for the treatment FAP (including GS) and HNPCC. The component of traditional Chinese medicine is complex, the correlation of between traditional Chinese medicine and disappearance of gastric polypi is unclear. However, reports showed that there are many organic acids and other helpful components in Fructus mume and Bombyx batryticatus, such as oxalic acid (OA), tartaric acid (TA), malic acid (MA), vitamin C (VC), lactic acid (LA), acetic acid (AA), citric acid (CA), succinic acid (SA), β-sitosterol, uracil, meso-erythritol and palmitic acid, etc[1213]. More studies are needed to make sure whether these organic acids and helpful components have the effects of NSAID.
It was reported that about 25% of patients with GS have no family history and the miniaturization of family also makes the hereditary behavior unobvious[14–16]. It is very easy to misdiagnose these patients who have no obvious family history or colorectal polypi. We should examine the stomach, thyroid, tooth, skull and eyeground of such patients with colorectal polypi. The examination of APC and MYH mutation is helpful to differentiate patients with GS and FAP, but APC and MYH mutation examination is uncertain, thus not widely applied.