Published online Feb 14, 2007. doi: 10.3748/wjg.v13.i6.975
Revised: December 21, 2006
Accepted: January 23, 2007
Published online: February 14, 2007
Amiodarone, a class III antiarrhythmic drug, is one of the most effective drugs used in the treatment of ventricular and paroxysmal supraventricular tachyarrhythmia. Adverse effects of amiodarone including pulmonary toxicity, hepatotoxicity, aggravation of arrhythmia, and thyroid diseases are well understood. A 66-year old woman with acute pancreatitis was admitted to our hospital with the complaint of epigastralgia radiating to both flanks for two months. Her symptoms and elevation of pancreatic enzymes did not respond to conventional medical treatment of pancreatitis for 18 d. No known causal factors for pancreatitis such as biliary tract stone, hypertriglyceridemia and alcohol consumption could be identified. Under the suspicion of amiodarone-induced acute pancreatitis, amiodarone was substituted by propafenone. Her symptoms soon alleviated and serum lipase level declined. Three months after hospital discharge, the abdominal pain did not recur. Amiodarone was approved to treat recurrent ventricular fibrillation or sustained ventricular tachyarrhythmia that has been resistant to other medications since 1986. Pancreatitis is a very rare adverse effect associated with the use of amiodarone, and only four cases of amiodarone-induced pancreatitis have been reported in literature. We report a patient who developed acute pancreatitis during amiodarone therapy.
- Citation: Chen YY, Chen CY, Leung KK. Acute pancreatitis and amiodarone: A case report. World J Gastroenterol 2007; 13(6): 975-977
- URL: https://www.wjgnet.com/1007-9327/full/v13/i6/975.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i6.975
Amiodarone has been approved to treat recurrent ventricular fibrillation or sustained ventricular tachyarrhythmia which has been resistant to other medications since 1986. A recent study showed that amiodarone is more effective than propafenone and sotalol against paroxysmal atrial fibrillation, suggesting that amiodarone is the first choice of treatment for maintaining sinus rhythm in patients with paroxysmal atrial fibrillation[1]. Although amiodarone is effective in treating atrial and ventricular arrhythmias, adverse effects are common and increase markedly after a year of treatment. Adverse effects may occur in many organ systems, such as pulmonary, cardiac, central nervous systems, as well as in thyroid, liver and gastrointestinal tract, and some of them can be very serious and even fatal[2]. Pancreatitis is a very rare adverse effect associated with the use of amiodarone, and only four cases of acute pancreatitis induced by amiodarone have been reported in the literature[3-6]. We report a patient who developed acute pancreatitis during amiodarone therapy.
A 66-year old woman was admitted to our hospital because of epigastrialgia and flank pain for two months. The patient had a long history of rheumatic heart disease involving mitral and aortic valves. She received aortic valve replacement in 1973 and mitral valve replacement in 1999. She had paroxysmal atrial fibrillation with rapid ventricular response controlled by quinidine and digoxin for many years. However, tachyarrhythmia attacked her very frequently in recent years, so she received a radiofrequency catheter ablation in 2000. At that time, electrophysiological study revealed sick sinus syndrome and a cardiac pacemaker (DDDR) was implanted. After ablation therapy, she experienced exertional dyspnea and orthopnea with the severity of New York Heart Association function class II. Coronary angiography revealed no sign of coronary artery stenosis. She visited the heart clinic regularly and was treated with warfarin, spirololactone, furosemide and digitalis.
She did not smoke cigarettes or drink alcoholic beverages. According to her medical records, she had no chronic diseases such as diabetes mellitus, hypertension, hyperlipidemia and chronic hepatitis. She did not have any exposure to toxic chemicals or travel overseas. Cholecystectomy was performed in 1981 due to gall bladder stones and acute cholecystitis. She did not experience any right upper abdominal discomfort after the operation. Total hysterectomy and bilateral salpingo-oophorectomy were done in 1984 due to uterine myoma and right ovarian cyst. The post-operation course was uneventful.
Approximately three months before this admission, amiodarone (200 mg per day) was given due to recurrent paroxysmal atrial fibrillation. The dosage was reduced to 100 mg daily one month before admission. About two months before this admission, she began to suffer from a constant pain at her epigastrium. It was a dull pain with radiation to her flanks. The pain did not change with body positions, but it slightly got worse after meals. She had no nausea, vomiting or diarrhea. She lost her appetite and 2 kilograms of her body weight within one month.
On admission, physical examination was unremarkable except for mild knocking pain on both flanks. The patient had no fever and jaundice. Laboratory examinations including urine analysis, complete blood count, liver and renal functions were within normal range. Serum amylase was 109 U/L (normal value < 220 U/L) and serum lipase was 395 U/L (normal value < 30 U/L). Ultrasound and triphase dynamic computer tomography of the abdomen demonstrated no dilatation of intrahepatic ducts or common bile duct, no stones, no pancreatic enlargement or necrosis, and no fluid accumulation. Because of the persisted elevation of serum lipase, endoscopic ultrasonography was done for a better visualization of the pancreas. The result revealed a reticular pattern over the whole pancreas that suggested the diagnosis of pancreatitis. Under the impression of pancreatitis, her oral intake was prohibited except for medications including amiodarone. Her abdominal symptoms persisted and serum lipase level was still high after three weeks of conventional medical treatments.
Because no causal factor for pancreatitis was found after the initial work-up, acute pancreatitis induced by amiodarone was suspected. So amiodarone was substituted by propafenone (400 mg) after a three-day overlapping of both drugs. After amiodarone was discontinued, her abdominal pain soon subsided and serum lipase gradually returned to nearly normal level at discharge. Three months after discharge, she was totally free of abdominal symptoms and her follow-up check of serum amylase and lipase level was normal.
Pancreatitis is usually presented with moderate to severe abdominal pain sometimes radiated to the flanks, accompanied with increased serum amylase and lipase and a variable degree of morphological changes in the pancreas. Gallstones and alcohol are the most common causes of acute pancreatitis. Other etiologies include hypertriglyceridemia, trauma, infection and drugs. Many medications have been reported in the literature to be related to the development of pancreatitis[7,8]. However, there is no clinical feature that can differentiate drug-induced pancreatitis from other factor caused-pancreatitis. Drug-induced pancreatitis is diagnosed by the following criteria: pancreatitis developed during treatments, resolved following the removal of the drug, and re-development following re-challenge of the offending drug[9]. A “definite” association can be made if the suspected drug meets all three criteria. If a relationship is supported by not all three criteria, the drug is classified as having a “possible” association. A drug is classified as “questionable” if it is supported by inadequate or contradictory evidence[9]. The summaries of four reported cases and our present case and the association between pancreatitis and amiodarone are shown in Table 1.
| Authors and year | Age/Sex | Serum amylase level(normal value) | Serum lipase level(normal value) | Pancreatitis developedduring the use ofamiodarone | Resolved followingthe removal ofamiodarone | Re-challengeof amiodarone | Association betweenamiodarone andpancreatitis |
| Sastri SV, et al 1990 | 67/M | 64 (0-45) | 38 (6-20) | Yes | No | No | Questionable |
| Munoz RAI, et al 1996 | 67/M | 387 (70-220) | 546 (not applicable) | Yes | Yes | No | Possible |
| Bosch X, et al 1997 | 46/F | 1480 (17-115) | 946 (0-190) | Yes | Yes | Yes | Definite |
| Famularo G, et al 2004 | 80/M | 732 (0-95) | 548 (10-140) | Yes | Yes | No | Possible |
| Present case 2006 | 66/F | 109 (0-220) | 395 (0-30) | Yes | Yes | No | Possible |
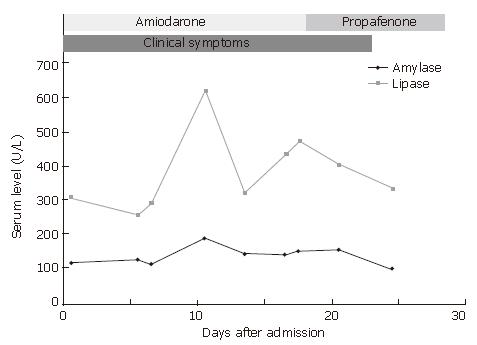
How do we explain the discrepancy between serum amylase and lipase in our patient? Although the elevation of amylase and lipase is an important indicator for acute pancreatitis, normoamylasemia has been reported in 19% of patients with acute pancreatitis[10]. Elevated lipase and normal amylase may occur in acute pancreatitis due to a longer time interval since onset of attack, pancreatitis caused by alcohol abuse or by hypertriglyceridemia[11]. Lipase level usually peaks at 24 h after the onset of acute pancreatitis and normalizes within 8 to 14 d from the onset of acute pancreatitis. Compared with serum amylase, serum lipase rises slightly later and remains elevated longer. Since our patient sought medical help two months after the onset of acute abdominal pain, her serum amylase might would have returned to a normal limit. Therefore, the possibility of acute pancreatitis could not be excluded due the normoamylasemia from the very beginning. Postmortem samples from patients who have received a long-term treatment with amiodarone reveal high concentrations of amiodarone and its metabolites in adipose tissue, liver, lung and pancreas[12]. Chronic deposition of amiodarone in pancreatic tissues may explain the prolonged elevation of lipase in our patient. The findings in ultrasound and computer tomography of the abdomen are required for the diagnosis of pancreatitis. However, in our patient, no obvious abnormality was found in abdominal ultrasonography and computer tomography. The only abnormal finding was a reticular pattern in endoscopic ultrasonography that highly suggested pancreatitis. Our finding is similar to Bosch’s patient whose abdominal ultrasonography and computer tomography were normal[5]. The temporal relationship between amiodarone therapy and the change of clinical symptoms and major laboratory data of this patient are shown in Figure 1.
Before reaching the conclusion that pancreatitis is induced by amiodarone, other etiological factors of pancreatitis should be ruled out. In our patient, no other etiological cause was found from her medical history, physical examination, laboratory results and image studies. Since we did not re-challenge with amiodarone due to ethical concerns, our patient fulfilled the “possible” diagnosis of amiodarone-induced pancreatitis. The possible mechanisms of drug-induced acute pancreatitis include pancreatic duct constriction, immunosuppression, direct cellular toxicity, osmotic or metabolic changes, and arteriolar thrombosis[13], but the exact mechanism of pancreatitis induced by amiodarone is still unknown.
Only four cases of acute pancreatitis induced by amiodarone have been reported in the literature[3-6]. Our case is the first Asian patient with acute pancreatitis induced by amiodarone. Although acute pancreatitis induced by amiodarone seems to be less severe than pancreatitis caused by gallstones or alcohol abuse, physicians and patients should completely understand the adverse effects of amiodarone including acute pancreatitis when they decide to use amiodarone.
S- Editor Liu Y L- Editor Wang XL E- Editor Lu W
| 1. | Roy D, Talajic M, Dorian P, Connolly S, Eisenberg MJ, Green M, Kus T, Lambert J, Dubuc M, Gagné P. Amiodarone to prevent recurrence of atrial fibrillation. Canadian Trial of Atrial Fibrillation Investigators. N Engl J Med. 2000;342:913-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 751] [Cited by in RCA: 688] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 2. | Connolly SJ. Evidence-based analysis of amiodarone efficacy and safety. Circulation. 1999;100:2025-2034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 190] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 3. | Sastri SV, Diaz-Arias AA, Marshall JB. Can pancreatitis be associated with amiodarone hepatotoxicity? J Clin Gastroenterol. 1990;12:70-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Muñoz Ruiz AI, Calvo Elipe A, Guerrero Vega E, Gorgojo Martínez JJ, Vera López E, Gilsanz Fernández C. Pancreatitis and inappropriate ADH secretion syndrome associated with amiodarone. An Med Interna. 1996;13:125-126. [PubMed] |
| 5. | Bosch X, Bernadich O. Acute pancreatitis during treatment with amiodarone. Lancet. 1997;350:1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Famularo G, Minisola G, Nicotra GC, De Simone C. Acute pancreatitis caused by amiodarone. Eur J Emerg Med. 2004;11:305-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Wilmink T, Frick TW. Drug-induced pancreatitis. Drug Saf. 1996;14:406-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 94] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 8. | Napier S, Thomas M. 36 year old man presenting with pancreatitis and a history of recent commencement of Orlistat case report. Nutr J. 2006;5:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Sura ME, Heinrich KA, Suseno M. Metronidazole-associated pancreatitis. Ann Pharmacother. 2000;34:1152-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Clavien PA, Robert J, Meyer P, Borst F, Hauser H, Herrmann F, Dunand V, Rohner A. Acute pancreatitis and normoamylasemia. Not an uncommon combination. Ann Surg. 1989;210:614-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 114] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 11. | Yadav D, Agarwal N, Pitchumoni CS. A critical evaluation of laboratory tests in acute pancreatitis. Am J Gastroenterol. 2002;97:1309-1318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 190] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 12. | Maggioni AP, Maggi A, Volpi A, D'Aranno V, Tognoni G, Giani P. Amiodarone distribution in human tissues after sudden death during Holter recording. Am J Cardiol. 1983;52:217-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Underwood TW, Frye CB. Drug-induced pancreatitis. Clin Pharm. 1993;12:440-448. [PubMed] |