Published online Feb 14, 2007. doi: 10.3748/wjg.v13.i6.964
Revised: September 13, 2006
Accepted: December 29, 2006
Published online: February 14, 2007
We present a case of fetal liver failure caused by the activation of lamivudine-resistant hepatitis B virus (HBV) nine months after lamivudine treatment. A 57-year old man visited our hospital for the treatment of decompensated chronic hepatitis B. Lamivudine was started in December 2001. Subsequently, serum HBV was negative for HBV DNA with seroconversion from HBeAg to anti-HBe and improvement of liver function. However, HBV DNA and HBeAg were again detected in September 2002. He was complicated by breakthrough hepatitis and admitted to our hospital in November for severely impaired liver function. Vidarabine treatment was started and serum HBV DNA and alanine aminotransferase (ALT) decreased transiently. However, after the start of α-interferon treatment, HBV DNA level increased and liver function deteriorated. He died 1 mo after admission. An analysis of amino acid sequences in the polymerase region revealed that rtM204I/V with rtL80I/V occurred at the time of viral breakthrough. After the start of antiviral treatment, rtL180M was detected in addition to rtM204I/V and rtL80I/V, and became predominant in the terminal stage of the disease. HBV clone with a high replication capacity may be produced by antiviral treatment leading to the worsening of liver function. Antiviral therapy for patients with breakthrough hepatitis in advanced liver disease should be carefully performed.
- Citation: Suzuki Y, Yotsuyanagi H, Okuse C, Nagase Y, Takahashi H, Moriya K, Suzuki M, Koike K, Iino S, Itoh F. Fatal liver failure caused by reactivation of lamivudine-resistant hepatitis B virus: A case report. World J Gastroenterol 2007; 13(6): 964-969
- URL: https://www.wjgnet.com/1007-9327/full/v13/i6/964.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i6.964
Lamivudine is a nucleoside analogue that interrupts the reverse transcription of hepatitis B viral (HBV) pregenomic RNA. Lamivudine is effective for controlling chronic hepatitis B and currently recommended as the first line of treatment for chronic active hepatitis B[1,2]. Even for patients with decompensated liver cirrhosis, lamivudine improves liver function and extends transplantation free intervals[3-10]. Since more than 10% of patients with chronic HBV infection are estimated to develop liver cirrhosis and may eventually suffer from decompensated liver cirrhosis or hepatocellular carcinoma, the role of lamivudine in the treatment of advanced liver disease caused by chronic HBV infection is large[11-14].
The major problems concerning lamivudine treatment are the viral and biochemical breakthroughs caused by drug resistance. Amino acid mutation in the highly conserved tyrosine-methionine-aspartate-aspartate (YMDD) motif can occur six months after treatment and often increases alanine aminotransferase (ALT) level. Although the increase is usually mild, a marked increase in ALT level leading to fatal hepatic failure has been reported[15-17]. Factors other than the YMDD motif mutation that are associated with the worsening of liver function remain to be clarified.
Here, we report a case of fatal hepatic failure caused by lamivudine-resistant HBV. A serial analysis of viral amino acid sequences indicated that the acquisition of mutations outside the YMDD motif might be related to the deteriolation of the patient’s condition.
A 57-year old man visited our hospital in September 2001 for the treatment of decompensated chronic hepatitis B. In 1978, He was found to be positive for serum HBs antigen (HBsAg). In July 2001, he was admitted to a nearby hospital for ascites where he was diagnosed as having decompensated cirrhosis with exacerbated chronic hepatitis B. The symptomatic control of his ascites improved his general condition. For further treatment, he was referred to our hospital.
On his first visit, he showed no symptoms or signs of worsening hepatic failure or encephalopathy. No ascites or leg edema was observed. His bulbar conjunctiva was slightly jaundiced. Dilated vasculature was observed in his neck and chest. His ALT, total bilirubin and albumin were 50 IU/L, 3.1 mg/dL and 3.7 g/dL, and his prothrombin time was 76%. He was diagnosed as having liver cirrhosis with a Child-Pugh score of 8. He was negative for HBe antigen (HBeAg) and his HBV DNA level measured by transcription-mediated amplification and hybridization protection assay[18] was 106.5 genome copies/mL.
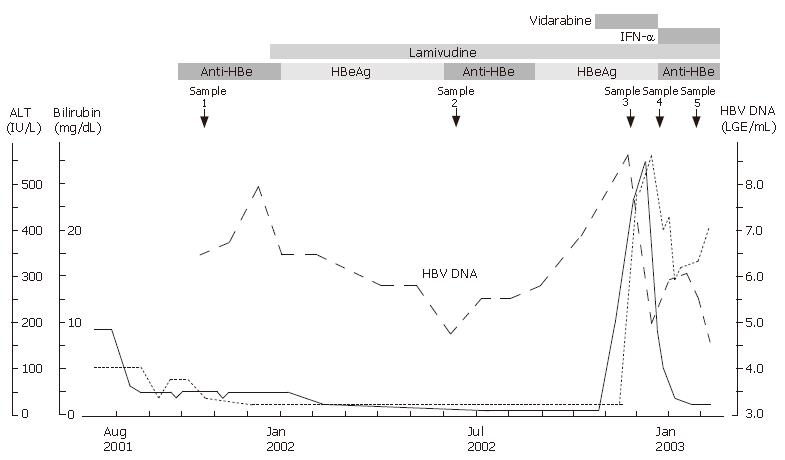
In November 2001, he was found to be positive for HBeAg and showed an increase in HBV DNA level. Because he had a history of decompensated chronic hepatitis B, lamivudine treatment (100 mg/d) was started in December. Figure 1 shows the clinical course. The high serum levels of bilirubin and ALT decreased and normalized within 6 mo after lamivudine treatment was started. The patient became negative for HBV DNA and HBeAg.
However, in September 2002, he was found to be positive for HBeAg again and showed an increase in HBV DNA level. In November 2002, he observed jaundice of his bulbar conjunctiva and was admitted to our hospital. Although he was alert, his bulbar conjunctiva and skin were jaundiced. His ALT, total bilirubin, were 474 IU/L, 11.4 mg/dL and 4.3 g/dL. His HBV DNA level was 108.6 genome copies/mL. He was diagnosed as having breakthrough hepatitis caused by lamivudine-resistant mutants of HBV. HBV with an amino acid substitution in the YMDD motif in the domain C of polymerase region was detected.
Because interferon is not indicated in patients with decompensated cirrhosis, vidarabine, which is effective for the control of active HBV infection[19-21], was administered together with lamivudine under informed consent. Liver function improved transiently with a decrease in HBV DNA within 2 wk. As prolonged vidarabine administration may induce several complications[22], vidarabine was switched to interferon-α. After the start of interferon-α treatment, HBV DNA level increased and liver function worsened. He died of hepatic failure and rupture of esophageal varices 1 mo after his admission.
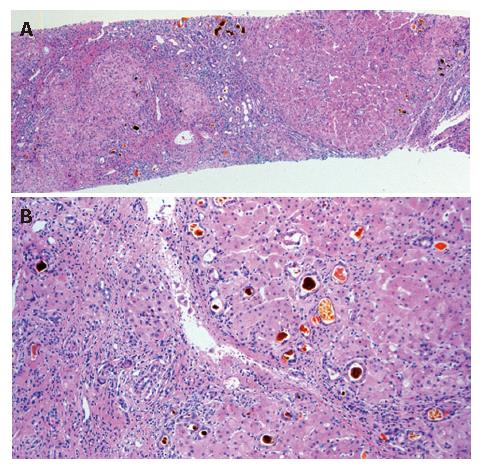
The histolopathology of the patient’s liver after necropsy showed cirrhosis with zonal necrosis. Hepatocyte regeneration was scarce (Figure 2).
To elucidate the viral factors affecting early viral breakthrough and fatal outcome, amino acid sequences of the upstream polymerase region (aa 1-250) of HBV DNA in serum were examined at 5 points as shown in Figure 1. The methods were as follows.
First, DNA was extracted from 100 μL of a serum sample using the QIAamp DNA blood mini kit (Qiagen Inc., Valencia, CA). Three fragments spanning the upper polymerase region of HBV DNA were amplified by nested PCR with the primers shown in Table 1. The first stage of amplification was carried out using a thermal cycler for 40 cycles (94°C for 1 min, 55°C for 1 min, 72°C for 1 min) in 100 μL of reaction mixture containing 200 mmol/L dNTPs, 1.0 mmol/L each of the primers and 1 × PCR buffer [50 mmol/L KCl, 10 mmol/L Tris-HCl (pH 8.3), 1.5 mmol/L MgCl2 and 0.001% (w/v) gelatin] and 2 units of Ampli-Taq polymerase gold (Perkin Elmer Cetus Corp., CT). Two microliters of the PCR products was subjected to the second stage of amplification under the same conditions as the first stage.
| Region 1 | ||
| Outer sense | nt 2222-2241 | CTTACTTTTGGAAGAGAAAC |
| Outer antisense | nt 2490-2509 | GGACAGTAGAAGAATAAAG |
| Inner sense | nt 2222-2241 | CTTACTTTTGGAAGAGAAAC |
| Inner antisense | nt 2478-2497 | GAATAAAGCCCAGTAAAGTT |
| Region 2 | ||
| Outer sense | nt 2413-2434 | GCGTCGCAGAAGATCTCAATC |
| Outer antisense | nt 2816-2835 | GTTCCCAAGAATATGGTGAC |
| Inner sense | nt 2434-2452 | CTCGGGAATCTCAATGTTAG |
| Inner antisense | nt 2816-2835 | GTTCCCAAGAATATGGTGAC |
| Region 3 | ||
| Outer sense | nt 2490-2509 | CTTTATTCTTCTACTGTACC |
| Outer antisense | nt 3121-3143 | CGATTGGTGGAGGCAGGAGGAGG |
| Inner sense | nt 2637-2656 | ATGCCTGCTAGGTTTTATCC |
| Inner antisense | nt 3121-3143 | CGATTGGTGGAGGCAGGAGGAGG |
Second, PCR products were purified using Wizard PCR preps DNA purification resin (Promega, WI) and cloned into a plasmid vector using the TA cloning kit (PCR cloning kit Qiagen, CA). Four clones were selected from each plate, from which recombinant plasmid DNA was purified using a commercially available kit (Plasmid midi kit, Qiagen, Valencia, CA). Nucleotide sequences were determined bidirectionally using the dye terminator cycle sequencing ready reaction kit (PE Applied Biosystems, CA) and the PCR primers. Sequencing was performed using an automated DNA sequencer (ABI 377: PE Applied Biosystems).
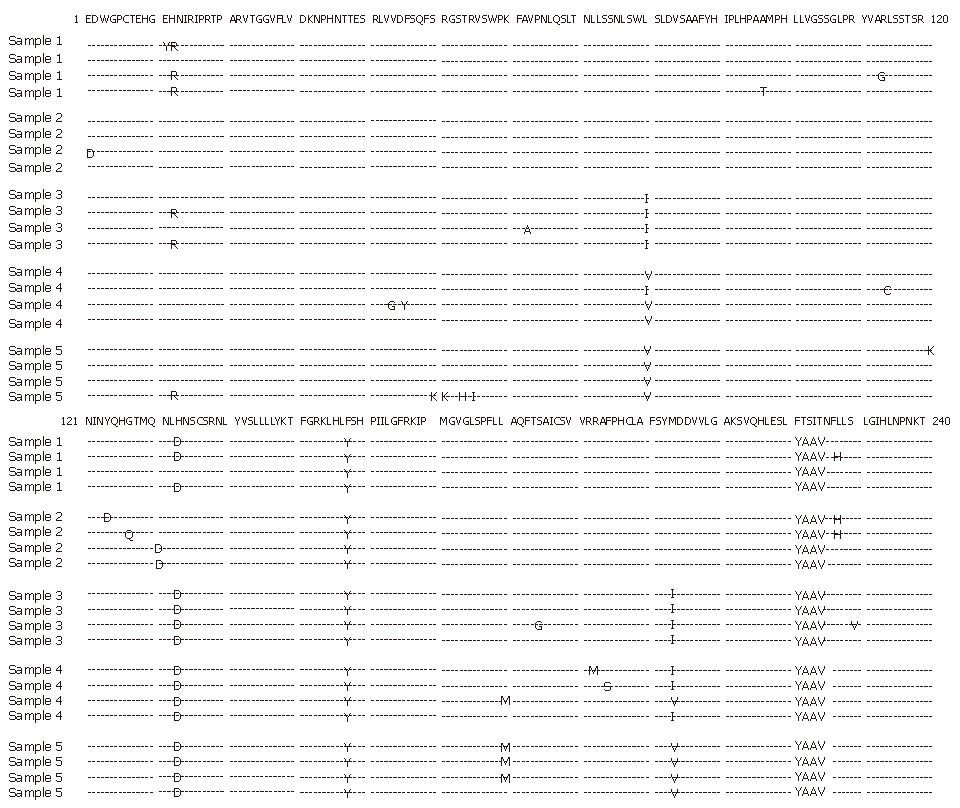
The determined amino acid sequences in the polymerase region are shown in Figure 3. No amino acid sequence changes were found at the start of lamivudine treatment. At the time of viral breakthrough, rtM204I with rtL80I became dominant. After the start of interferon treatment, rtM204I was replaced by rtM204V and rtL80I by rtL80V. At the final stage of the disease, mutation rtL180M appeared besides rtM204V and rtL80V.
Lamivudine monotherapy is effective in suppressing HBV replication and ameliorating liver disease in chronic hepatitis B patients regardless of HBeAg positivity. A one-year study of HBeAg-positive chronic hepatitis B patients showed that 16% of these patients become seroconverted to anti-HBe and 72% of these patients showed normalization of their ALT levels[23]. Furthermore, treatment with lamivudine is associated with histologic improvement not only in terms of necroinflammatory score but also in terms of fibrosis score after long-term treatment[24].
One advantage of lamivudine is that it can be used safely in patients with decompensated cirrhosis[3-10]. In contrast to IFN-α, lamivudine is well tolerated without any significant side effects even in patients with decompensated cirrhosis. Furthermore, lamivudine can improve liver function and survival prognosis.
However, the emergence of a drug-resistant mutant is a big problem in lamivudine treatment. A large-scale Asian study showed that lamivudine resistant HBV infection occurred in 23% of patients in year one and 65% of patients in year five. Hepatitis flares, which occurred more commonly in patients with lamivudine resistant mutations, occurred in 10% of patients in year one, and in 18% to 21% of patients in years two to five. Among patients with lamivudine resistant HBV infection, occurrence of hepatic decompensation increased significantly in patients with lamivudine resistant HBV infection for more than 4 years (from 0% to 6%)[25]. In this large-scale Asian study, liver-disease-related death occurred in two patients.
The prognosis of patients with lamivudine-resistant HBV infection, particularly those with advanced liver disease, may be determined by the timing and severity of breakthrough hepatitis. However, the viral factors that may influence the severity of this hepatitis remain to be clarified. A recent study indicated that patients with a normal ALT level even after the emergence of a YMDD motif mutant are characterized by HBeAg negativity during pretreatment, HBeAg loss during therapy, a longer duration from the commencement of therapy until the emergence of YMDD mutant, and lack of mixed-type YMDD mutants[26]. In contrast, patients with severely exacerbated hepatitis after the emergence of a YMDD mutant tend to have more substitutions in the reverse transcriptase (rt) region within the polymerase gene at the time of hepatitis exacerbation than those without hepatitis exacerbation[26].
Our patient acquired amino acid mutations in the polymerase region one after the other. Amino acid changes in rtM204/I appeared at the time of viral breakthrough. After the initial treatment with vidarabine, rtM204/V substituted for rtM204/I in one of the four clones. During the interferon treatment, rtM204/V became predominant.
Another mutation observed in our patient was rtL80I/V. Ogata et al[27] showed that rtL180M is accompanied with rtM204I in some patients with resistance to lamivudine. Because the mutation at aa position 80 was found at the same time as that at aa position 204 in our patient, it is not clear whether the mutation at aa position 80 affects the clinical course.
At the final stage of the disease with deterioration of the condition of the patient, rtL180M, rtM204I/V and rtL80I/V became predominant. Natsuizaka et al[28] showed that rtL180M and rtM204V are related to the exacerbation of hepatitis. Interestingly, a patient with a marked elevation in HBV DNA level in Ogata’s report had rtL180M in addition to rtL80I and rtM204I. Therefore, the acquisitions of rtL80I/V and rt180M in addition to rtM204I/V may be associated with a severe exacerbation of hepatitis. Large-scale studies are necessary to elucidate this hypothesis.
In our patient, vidarabine decreased HBV DNA levels and improved liver function. Although the long-term use of vidarabine is contraindicated because of its possible side effects including irreversible neurotoxicity[22], its short-term use is effective for controlling active HBV infection and herpes simplex viral infection.
Vidarabine was replaced by interferon-α because adefovir dipivoxil was not available in 2002. Serum HBV DNA and bilirubin levels increased again, which led to a fatal outcome, and HBV clones that have rtL180M became predominant, indicating that withdrawal of vidarabine and administration of interferon may be dangerous for the treatment of severe breakthrough hepatitis. Since interferon may potentially precipitate immunological flares and liver failure[29], nucleotide analogues that are effective for lamivudine-resistant HBV such as adefovir dipivoxil[30-32], entecavir[33-35] and tenofovir[36,37], should be used for the treatment of severe breakthrough hepatitis instead of vidarabine or interferon.
In conclusion, antiviral therapy should be considered in the treatment of patients with hepatic failure after breakthrough hepatitis caused by HBV mutants to lamivudine. The serial acquisition of amino acid mutations outside the YMDD motif in the polymerase region may be associated with severe hepatitis.
S- Editor Wang GP L- Editor Wang XL E- Editor Lu W
| 1. | Lok AS, McMahon BJ. Chronic hepatitis B: update of recommendations. Hepatology. 2004;39:857-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 365] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 2. | Liaw YF, Leung N, Guan R, Lau GK, Merican I, McCaughan G, Gane E, Kao JH, Omata M. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2005 update. Liver Int. 2005;25:472-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 271] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 3. | Villeneuve JP, Condreay LD, Willems B, Pomier-Layrargues G, Fenyves D, Bilodeau M, Leduc R, Peltekian K, Wong F, Margulies M. Lamivudine treatment for decompensated cirrhosis resulting from chronic hepatitis B. Hepatology. 2000;31:207-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 295] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 4. | Yao FY, Bass NM. Lamivudine treatment in patients with severely decompensated cirrhosis due to replicating hepatitis B infection. J Hepatol. 2000;33:301-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 138] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 5. | Kapoor D, Guptan RC, Wakil SM, Kazim SN, Kaul R, Agarwal SR, Raisuddin S, Hasnain SE, Sarin SK. Beneficial effects of lamivudine in hepatitis B virus-related decompensated cirrhosis. J Hepatol. 2000;33:308-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 113] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | Sponseller CA, Bacon BR, Di Bisceglie AM. Clinical improvement in patients with decompensated liver disease caused by hepatitis B after treatment with lamivudine. Liver Transpl. 2000;6:715-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Fontana RJ, Keeffe EB, Carey W, Fried M, Reddy R, Kowdley KV, Soldevila-Pico C, McClure LA, Lok AS. Effect of lamivudine treatment on survival of 309 North American patients awaiting liver transplantation for chronic hepatitis B. Liver Transpl. 2002;8:433-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 98] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | Yao FY, Terrault NA, Freise C, Maslow L, Bass NM. Lamivudine treatment is beneficial in patients with severely decompensated cirrhosis and actively replicating hepatitis B infection awaiting liver transplantation: a comparative study using a matched, untreated cohort. Hepatology. 2001;34:411-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 195] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 9. | Hann HW, Fontana RJ, Wright T, Everson G, Baker A, Schiff ER, Riely C, Anschuetz G, Gardner SD, Brown N. A United States compassionate use study of lamivudine treatment in nontransplantation candidates with decompensated hepatitis B virus-related cirrhosis. Liver Transpl. 2003;9:49-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 46] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Manolakopoulos S, Karatapanis S, Elefsiniotis J, Mathou N, Vlachogiannakos J, Iliadou E, Kougioumtzan A, Economou M, Triantos C, Tzourmakliotis D. Clinical course of lamivudine monotherapy in patients with decompensated cirrhosis due to HBeAg negative chronic HBV infection. Am J Gastroenterol. 2004;99:57-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Fattovich G, Brollo L, Giustina G, Noventa F, Pontisso P, Alberti A, Realdi G, Ruol A. Natural history and prognostic factors for chronic hepatitis type B. Gut. 1991;32:294-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 291] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 12. | Liaw YF, Tai DI, Chu CM, Chen TJ. The development of cirrhosis in patients with chronic type B hepatitis: a prospective study. Hepatology. 1988;8:493-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 452] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 13. | Liaw YF, Lin DY, Chen TJ, Chu CM. Natural course after the development of cirrhosis in patients with chronic type B hepatitis: a prospective study. Liver. 1989;9:235-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 110] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Fattovich G, Giustina G, Schalm SW, Hadziyannis S, Sanchez-Tapias J, Almasio P, Christensen E, Krogsgaard K, Degos F, Carneiro de Moura M. Occurrence of hepatocellular carcinoma and decompensation in western European patients with cirrhosis type B. The EUROHEP Study Group on Hepatitis B Virus and Cirrhosis. Hepatology. 1995;21:77-82. [PubMed] |
| 15. | Kim JW, Lee HS, Woo GH, Yoon JH, Jang JJ, Chi JG, Kim CY. Fatal submassive hepatic necrosis associated with tyrosine-methionine-aspartate-aspartate-motif mutation of hepatitis B virus after long-term lamivudine therapy. Clin Infect Dis. 2001;33:403-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Wang JH, Lu SN, Lee CM, Lee JF, Chou YP. Fatal hepatic failure after emergence of the hepatitis B virus mutant during lamivudine therapy in a patient with liver cirrhosis. Scand J Gastroenterol. 2002;37:366-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Kagawa T, Watanabe N, Kanouda H, Takayama I, Shiba T, Kanai T, Kawazoe K, Takashimizu S, Kumaki N, Shimamura K. Fatal liver failure due to reactivation of lamivudine-resistant HBV mutant. World J Gastroenterol. 2004;10:1686-1687. [PubMed] |
| 18. | Kamisango K, Kamogawa C, Sumi M, Goto S, Hirao A, Gonzales F, Yasuda K, Iino S. Quantitative detection of hepatitis B virus by transcription-mediated amplification and hybridization protection assay. J Clin Microbiol. 1999;37:310-314. [PubMed] |
| 19. | Hoofnagle JH, Minuk GY, Dusheiko GM, Schafer DF, Johnson R, Straus S, Jones EA, Gerin JL, Ishak K. Adenine arabinoside 5'-monophosphate treatment of chronic type B hepatitis. Hepatology. 1982;2:784-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 29] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Weller IV, Lok AS, Mindel A, Karayiannis P, Galpin S, Monjardino J, Sherlock S, Thomas HC. Randomised controlled trial of adenine arabinoside 5'-monophosphate (ARA-AMP) in chronic hepatitis B virus infection. Gut. 1985;26:745-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Marcellin P, Ouzan D, Degos F, Brechot C, Metman EH, Degott C, Chevalier M, Berthelot P, Trepo C, Benhamou JP. Randomized controlled trial of adenine arabinoside 5'-monophosphate in chronic active hepatitis B: comparison of the efficacy in heterosexual and homosexual patients. Hepatology. 1989;10:328-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Hoofnagle JH, Hanson RG, Minuk GY, Pappas SC, Schafer DF, Dusheiko GM, Straus SE, Popper H, Jones EA. Randomized controlled trial of adenine arabinoside monophosphate for chronic type B hepatitis. Gastroenterology. 1984;86:150-157. [PubMed] |
| 23. | Lai CL, Chien RN, Leung NW, Chang TT, Guan R, Tai DI, Ng KY, Wu PC, Dent JC, Barber J. A one-year trial of lamivudine for chronic hepatitis B. Asia Hepatitis Lamivudine Study Group. N Engl J Med. 1998;339:61-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1381] [Cited by in RCA: 1348] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 24. | Dienstag JL, Goldin RD, Heathcote EJ, Hann HW, Woessner M, Stephenson SL, Gardner S, Gray DF, Schiff ER. Histological outcome during long-term lamivudine therapy. Gastroenterology. 2003;124:105-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 602] [Cited by in RCA: 574] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 25. | Lok AS, Lai CL, Leung N, Yao GB, Cui ZY, Schiff ER, Dienstag JL, Heathcote EJ, Little NR, Griffiths DA. Long-term safety of lamivudine treatment in patients with chronic hepatitis B. Gastroenterology. 2003;125:1714-1722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 584] [Cited by in RCA: 590] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 26. | Suzuki F, Akuta N, Suzuki Y, Sezaki H, Arase Y, Hosaka T, Someya T, Kobayashi M, Saitoh S, Ikeda K. Clinical and virological features of non-breakthrough and severe exacerbation due to lamivudine-resistant hepatitis B virus mutants. J Med Virol. 2006;78:341-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Ogata N, Fujii K, Takigawa S, Nomoto M, Ichida T, Asakura H. Novel patterns of amino acid mutations in the hepatitis B virus polymerase in association with resistance to lamivudine therapy in japanese patients with chronic hepatitis B. J Med Virol. 1999;59:270-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 28. | Natsuizaka M, Hige S, Ono Y, Ogawa K, Nakanishi M, Chuma M, Yoshida S, Asaka M. Long-term follow-up of chronic hepatitis B after the emergence of mutations in the hepatitis B virus polymerase region. J Viral Hepat. 2005;12:154-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Janssen HL, Brouwer JT, Nevens F, Sanchez-Tapias JM, Craxi A, Hadziyannis S. Fatal hepatic decompensation associated with interferon alfa. European concerted action on viral hepatitis (Eurohep). BMJ. 1993;306:107-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 41] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Perrillo R, Schiff E, Yoshida E, Statler A, Hirsch K, Wright T, Gutfreund K, Lamy P, Murray A. Adefovir dipivoxil for the treatment of lamivudine-resistant hepatitis B mutants. Hepatology. 2000;32:129-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 308] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 31. | Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, Chang TT, Kitis G, Rizzetto M, Marcellin P, Lim SG, Goodman Z, Wulfsohn MS. Adefovir dipivoxil for the treatment of hepatitis B e antigen-negative chronic hepatitis B. N Engl J Med. 2003;348:800-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 780] [Cited by in RCA: 735] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 32. | Marcellin P, Chang TT, Lim SG, Tong MJ, Sievert W, Shiffman ML, Jeffers L, Goodman Z, Wulfsohn MS, Xiong S. Adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B. N Engl J Med. 2003;348:808-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1060] [Cited by in RCA: 1019] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 33. | Chang TT, Gish RG, Hadziyannis SJ, Cianciara J, Rizzetto M, Schiff ER, Pastore G, Bacon BR, Poynard T, Joshi S. A dose-ranging study of the efficacy and tolerability of entecavir in Lamivudine-refractory chronic hepatitis B patients. Gastroenterology. 2005;129:1198-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 197] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 34. | Chang TT, Gish RG, de Man R, Gadano A, Sollano J, Chao YC, Lok AS, Han KH, Goodman Z, Zhu J. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N Engl J Med. 2006;354:1001-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1107] [Cited by in RCA: 1090] [Article Influence: 57.4] [Reference Citation Analysis (0)] |
| 35. | Lai CL, Shouval D, Lok AS, Chang TT, Cheinquer H, Goodman Z, DeHertogh D, Wilber R, Zink RC, Cross A. Entecavir versus lamivudine for patients with HBeAg-negative chronic hepatitis B. N Engl J Med. 2006;354:1011-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 915] [Cited by in RCA: 910] [Article Influence: 47.9] [Reference Citation Analysis (0)] |
| 36. | van Bömmel F, Wünsche T, Mauss S, Reinke P, Bergk A, Schürmann D, Wiedenmann B, Berg T. Comparison of adefovir and tenofovir in the treatment of lamivudine-resistant hepatitis B virus infection. Hepatology. 2004;40:1421-1425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 279] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 37. | van der Eijk AA, Hansen BE, Niesters HG, Janssen HL, van de Ende M, Schalm SW, de Man RA. Viral dynamics during tenofovir therapy in patients infected with lamivudine-resistant hepatitis B virus mutants. J Viral Hepat. 2005;12:364-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |