Published online Feb 14, 2007. doi: 10.3748/wjg.v13.i6.934
Revised: December 10, 2006
Accepted: January 13, 2007
Published online: February 14, 2007
AIM: To observe the growth inhibitory effect of wild-type Kras2 gene on a colonic adenocarcinoma cell line Caco-2.
METHODS: Recombinant plasmid pCI-neo-Kras2 with wild type Kras2 open reading frame was constructed. The Caco-2 cells were transfected with either pCI-neo or pCI-neo-Kras2 using Lipofectamine 2000. The expression of wild type Kras2 was examined by Northern blot analysis. And the expression of wild type Kras2 protein was examined by Western blot analysis. The effects of wild-type Kras2 on cell proliferation were analyzed by monotetrazolium (MTT) assay, meanwhile analyses of cell cycle and spontaneous apoptosis rate were carried out by flow cytometry (FCM).
RESULTS: The plasmid of pCI-neo-Kras2 was successfully established. The growth rate of cells transfected with pCI-neo-Kras2 was significantly lower than the control cells transfected with the empty pCI-neo vector (P < 0.05). Cell cycle analysis revealed arrest of the pCI-neo-Kras2 transfected cells in G0/G1 phases, decreased DNA synthesis and decreased fractions of cells in S phase. The proliferative index of cells transfected with pCI-neo-Kras2 was decreased compared with the control cells (49.78% vs 64.21%),while the apoptotic rate of Caco-2 cells with stable Kras2 expression increased (0.30% vs 0.02%).
CONCLUSION: The wild-type Kras2 gene effectively inhibits the growth of the colonic adenocarcinoma cell line Caco-2.
-
Citation: Li H, Cao HF, Wan J, Li Y, Zhu ML, Zhao P. Growth inhibitory effect of wild-type
Kras2 gene on a colonic adenocarcinoma cell line. World J Gastroenterol 2007; 13(6): 934-938 - URL: https://www.wjgnet.com/1007-9327/full/v13/i6/934.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i6.934
A number of molecular studies have shown that colon carcinogenesis results from an accumulation of epigenetic and genetic alterations, including activation of the proto-oncogenes, and inactivation of the mutations of tumor suppressor genes or of DNA repair genes[1]. Previously extensive studies have been done on the activation of the K-ras2 proto-oncogene[2-4] and have revealed the association between colon carcinogenesis and the frequency of RAS mutations. Approximately 50% of colorectal tumors contain K-ras2 gene mutations, leading to activation of the K-ras2 oncogene, which is an early event in carcinogenesis. A recent study on Kras2, however, demonstrated that the wild-type Kras2 inhibited the growth, colony formation and in vivo tumor formation of lung cancer cells[5]. In addition, wild-type Kras2 has also been shown to inhibit colony formation and tumor development by transformed NIH/3T3 cells. These results indicated wild-type kras2 might exert its tumor suppressor effect in carcinogenesis. In the current study, we attempted to investigate the tumor suppressive effect of wild-type Kras2 on the proliferation of a colon cancer cell line. We transfected the wild-type Kras2 gene into Caco-2 cell, a human colon adenocarcinoma cell line, and established a stably transfected cell line by G418 selection. The effects of wild-type Kras2 on cell growth, cell cycle distribution and apoptosis were analyzed in Caco-2 cells.
Human colon tumor cell line Caco-2 was purchased from American Type Culture Center (ATCC) (Rockville, MD, USA) and was maintained in MEM-NEAA medium supplemented with 10% FCS and 100 U/mL penicillin-streptomycin in a humidified atmosphere with 5% CO2 at 37°C.
Appropriate institutional approval for human tissue studies and the informed consent from all subjects were both obtained. The wild-type Kras2 cDNA fragment was amplified by PCR. Briefly, total RNA from human peripheral blood was extracted by Trizol reagent (Invitrogen) and the Reverse Transcription System kit (Promega) was used to synthesize the cDNA. The primer sets Y1: 5’-ACCCACGCGTATGACTGAATATAAAC-3’ and Y2: 5’-AACGTCGACTTACATAATTACACACT-3’ (Shanghai Oke Corp.) with Mlu I and Sal I enzyme sites respectively were used to amplify the wild-type Kras2 cDNA. The PCR product was harvested from 1% agarose gel, digested by Mul I and Sal I enzymes and inserted into the pCI-neo vector at Mul I and Sal I cloning sites to construct the pCI-neo-Kras2 expression vector. All ligation products were transformed into DH5 competent cells. The constructs were confirmed by restriction enzyme mapping and DNA sequencing.
For transfection experiment, Caco-2 cells were seeded into 100-mm culture dishes and grown until 80% confluence. The empty plasmid pCI-neo and the pCI-neo-Kras2 were stably transfected using Lipofectamine 2000 reagent (Invitrogen), according to the manufacturer’s instruction. Caco-2 cells were selected with 1.2 mg/mL G418 for 2 wk and maintained in normal MEM medium containing 0.6 mg/mL G418 (Amresco USA).
A total of 1.5 × 105 viable Caco-2 cells transfected with the pCI-neo empty vector or Kras2 stably expressing cells were plated into one well of six-well plates with 5 mL of culture medium and cultured until 90% cell confluence. Total RNA was isolated in 2 mL of TRIZOL reagent according to the manufacturer’s instruction. Twenty micrograms of total RNA were electrophoresed through a 1% agarose gel containing formaldehyde and were transferred to a Hybond N+ membrane (Amersham). The membrane was hybridized with a [32P]-labeled Kras2 cDNA probe in 5 mL hybridization buffer containing 50% deionized formamide, 0.5 × Denhardt’s solution, 0.5% sodium dodecyl sulfate (SDS), and 1 mg of salmon sperm DNA (Sigma). After hybridization overnight at 50°C, the membrane was washed according to the manufacturer’s instruction and exposed to BioMax-MS film (Eastman Kodak) for 2 d at -80°C. The autoradiographic bands were performed in quantitative analysis using Image software.
The cancer cells with stable expression of wild-type Kras2 and the control cells were separately seeded at the same time into 96-well culture plates and then routinely cultured for 6 d. MTT was added to the culture wells and the 570 nm wave-length absorption value (A value) was assayed at 5 h, 1, 2, 3, 4, 5 and 6 d after seeding of cells. The cell growth curves were protracted using the following formula, GI = Nc-Nt/Nc × 100%. Nc and Nt represent the A value of the control and the experimental group cells. All experiments were performed in triplicate and repeated 3 times.
Cell cycle analysis was performed by propidium iodide (PI) staining. Briefly, Caco-2 cells were firstly seeded into 10-cm dishes at 5 × 105. After further cultured for 2 d, the cells were trypsinized and harvested in PBS followed by two washes with PBS. After that the cells were resuspended in PBS at 1-2 × 106/mL and fixed with 70% cool ethanol for at least 1 h. Next, the cells were washed twice and centrifuged at 1000 ×g for 3 min. The pelleted cells were resuspended in 1 mL PBS and added with 50 μL of RNase A stock solution (10 g/mL) and mixed well, and then incubated for 3 h at 4°C. Finally, the cells were pelleted and added with 1 mL of PI staining solution (3.8 mmol/L sodium citrate, 50 μg/mL PI in PBS) and incubated for 1 h at room temperature. After that the cells were analyzed by flow cytometry on an FACSCalibur (Becton Dickinson Immunocytometry Systems, San Jose, CA) exciting at 488-nm, and the DNA-linked red fluorescence (PI) was measured through a 600-nm wavelength filter. This experiment was performed three times.
A total of 50 μg of proteins in whole cell lysates was separated by SDS-polyacrylamide gel electrophoresis and transferred to PVDF membranes (Millipore, Bedford, MA). After blocking with 5% (w/v) nonfat milk, the blots were incubated with the first anti-Kras2 antibody (F234, sc-30; SantaCruz Biotechnology, Santa Cruz, CA; 1:100 dilution in a blocking solution, 2 h at room temperature) or with the anti-GAPDH antibody (Sigma Chemical, St. Louis, MO; 1:2 000 dilution in a blocking solution, 1 h at room temperature). The blots were then washed with PBST and incubated with a secondary antibody (Amersham Biosciences, Buckinghamshire, UK). Positive signal bands were detected using ECL plus (Amersham Biosciences).
The results for MTT assay and cell cycle analysis were presented as the mean ± SD of three replicate culture wells. Analysis was performed using Statview software. All data were evaluated for paired variables to compare between two groups. P < 0.05 was considered to be statistically significant.
The plasmids including pCI-neo and pCI-neo-Kras2 were digested with Mul I and Sal I enzymes and the 576 bp and 5472 bp products were observed. The 576 bp Kras2 cDNA was inserted into the pCI-neo vector. And the inserted fragment was verified by sequencing.
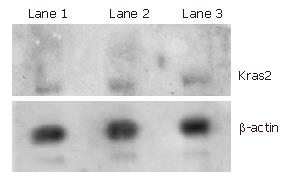
To measure the expression level of Kras2 gene in Caco-2 cells, northern blot analysis was used to detect the Kras2 mRNA. β-actin mRNA was also detected as an inner control. Consistent with the information provided by ATCC, the Kras2 transcript was not detected in Caco-2 cells (Figure 1).
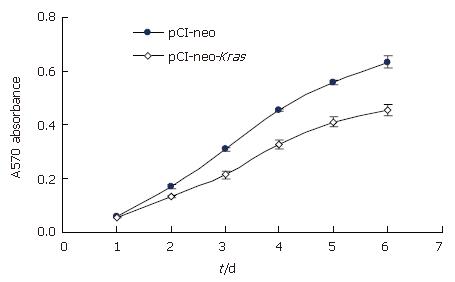
To determine the effect of wild-type Kras2 on the proliferative capacity of Caco-2 cells, a cell growth curve was generated by MTT assay. As shown in Figure 2, the growth rate of cells transfected with pCI-neo-Kras2 was significantly lower than the control cells transfected with the empty pCI-neo vector beginning from the third day after cell seeding.
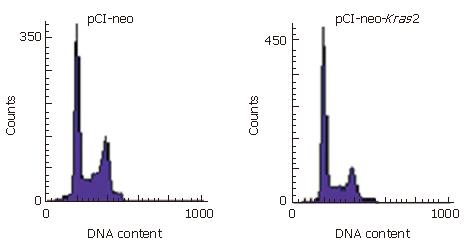
To detect the effect of wild-type Kras2 on cell differenti-ation and apoptosis of Caco-2 cells, cell cycle distribution and the apoptosis rate were analyzed by flow cytometry. The proliferative index of cells transfected with pCI-neo-Kras2 was decreased, compared with the control cells (49.78% vs 64.21%) (Table 1). As shown in Figure 3, the pCI-neo-Kras2 transfected cells were arrested in G0/G1 phases, compared with control cells, and the apoptotic rate of Caco-2 cells with stable Kras2 expression was increased (0.30% vs 0.02%).
| Cell lines | G0/G1phase | S phase | G2/M phase | Proliferativeindex |
| pCI-neo | 35.79 | 40.04 | 24.17 | 64.21 |
| PCI-neo-Kras2 | 50.22 | 32.88 | 16.9 | 49.78 |
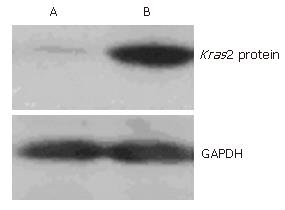
To determine whether the expression level of the Kras2 protein was different between the cells transfected with pCI-neo-Kras2 and the control cells transfected with the empty pCI-neo vector, we performed Western blot analysis on both cell lines using a monoclonal Kras2 antibody. The results showed that the Caco2 cell line without pCI-neo-Kras2 transfection expressed a faint protein product (Figure 4, lane A), and pCI-neo-Kras2 stably transfected cell line (lane B) expressed a 21 kDa product of Kras2. GAPDH was used as an internal control for loading volume.
Human colon carcinogenesis and tumor progression are considered to be the result of multigene alterations. Activating point mutations in ras gene are widely correlated with human colon carcinogenesis and progression, and have been detected in more human tumor types and at a higher frequency (25%-30% of all human tumors) than other oncogenes[6-9]. For example, ras mutations are detected in 40%-50% of colon carcinomas[10,11], 80% of pancreatic carcinomas[12-15], and 30%-50% of lung adenocarcinomas[16-20]. Kras2 mutations account for > 90% of the activating ras mutations observed in these tumor types. The oncogenic alleles of ras genes are generally perceived to be dominant because their transforming ability exists when normal alleles are also expressed[21]. However, recent evidence from both in vivo and in vitro studies suggested that the dominance of the ras oncogene might result from overexpression of the mutant ras allele or from deletion of the wild-type allele. Previous studies also suggested that the wild-type Kras2 allele could suppress the oncogenic potential of the mutant Kras2 allele[5]. Heterozygous Kras2 deficient mice were highly susceptible to chemically induced lung tumors compared to wild-type littermates. All tumors displayed an activated Kras2 because of a chemically induced mutation. Experimental evidence demonstrated that wild-type Kras2 inhibited cell growth, colony formation and tumor development in a murine lung tumor cell line containing an activated Kras2 allele. In addition, the loss of wild-type Kras2 was found in 67%-100% of chemically induced mouse lung adenocarcinomas harboring a mutant Kras2 allele[5]. Moreover, results from epidemiological investigations showed that loss of heterozygosity (LOH) of chromosome 12p was detected in nearly 50% of human lung adenocarcinomas and large cell carcinomas, and Kras2 mutations were detected at codon 12 in about 40% of human lung cancers[22]. We also observed that high frequent loss of heterozygosity in this domain occurred in carcinogenesis and progression of colon carcinoma[23]. These findings suggest that wild-type Kras2 is a tumor suppressor and is frequently lost during tumor progression.
Several mutational events common to tumor cells in colorectal cancer have been identified. It is not known whether the accumulation of these mutations occurs in a specific sequence. However, certain stages in the disease development do correlate with the presence of particular mutations[24]. In the genetic model of human colon tumorigenesis, activation of Kras2 mutation has been identified to promote colon carcinogenesis and progression, meanwhile, masking the potential tumor suppressor effect of the wild-type Kras2. To elucidate whether the wild-type Kras2 is a tumor suppressor in more tumor types, we hypothesized that the K-ras2 plays double roles of both “oncogenic” and “anti-oncogenic” effects. In physiological state, K-ras2 is a proto-oncogene, which is transformed to an oncogene by activating point mutation, whereas the wild-type K-ras can still maintain its tumor-suppressor function. To explore the biological role of the wild-type K-ras gene, it is necessary to exclude the interference of activated mutations of K-ras. Therefore, it is reasonable to select Caco-2 cells as the cell model in this study[25]. To our knowledge, this is the first report on the relationship between the presence of wild-type Kras2 and the growth and apoptotic properties of human colon cancer cells. Our results demonstrated that the wild-type Kras2 effectively inhibited the proliferation, and meanwhile promoted apoptosis of colon cancer cells. Ras is a GTP/GDP binding protein, affecting cell growth and proliferation via several signaling pathways[25]. In this study, wild-type Kras2 inhibited the growth of Caco-2 human colon cancer cells mainly through cell cycle arrest in G0/G1 phase, decreased DNA synthesis, and decreased fractions of cells in S phase.
Aberrant apoptosis mechanism also exists in colon cancer. Previous studies have demonstrated that the activating mutation of Ras inhibited apoptosis in colon cancer cells[26]. Here, we showed that the exogenous expression of wild-type Kras2 increased the apoptotic rate of Caco-2 cells (0.30% vs 0.02%), possibly via a mechanism involving Ras G-proteins and Raf-1 kinase, which needs to be further investigated[27].
Taken together, through exogenous expression of the wild-type Kras2 gene in Caco-2 colon cancer cells, we firstly demonstrate that the wild-type Kras2 gene is a potential tumor suppressor in human colon cancer. Our study also implies that increase of the expression level of wild-type Kras2 gene might be a gene therapeutic strategy for human colon cancer.
Conventional viewpoints suggest ras gene is closely related to carcinogenesis and progression of colon carcinoma. About 30 percent of tumors display mutation of ras gene, in which the most frequent one is Kras2, and a relatively high frequency of Kras2 mutation is seen in colon carcinoma, pancreas carcinoma and lung carcinoma. Recent studies raised doubt about the dominant oncogene roles of Kras2 gene, and showed the frequent loss of wild type Kras2 in human and mouse lung adenocarcinomas, and that loss of heterozygosity on chromosome 12p12-13 in Kras2 gene existed in non-small-cell lung cancer. In addition, wild-type Kras2 has also been shown to inhibit colony formation and tumor development by transformed NIH/3T3 cells. These results indicate that wild-type kras2 probably has a tumor suppressor effect in carcinogenesis.
We investigated the tumor suppressive effect of wild-type Kras2 on cell proliferation of a colon cancer cell line.
To our knowledge, this is the first report on the relationship between the presence of wild-type Kras2 and the growth and apoptosis properties of human colon cancer cells.
This study demonstrates that the wild-type Kras2 gene is a potential tumor suppressor in human colon cancer. Strikingly and of great potential clinical relevance, our results indicate that increase in the expression level of wild-type Kras2 gene might be a beneficial gene therapeutic strategy for human colon cancer.
Kras2, namely v-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog, is on chromosome 12p12-13, and is one of the three members of the ras gene: Hras1, Nras and K-ras2. Apoptosis, also called programmed cell death, or “cell suicide”: A form of cell death in which a programmed sequence of events leads to the elimination of cells without releasing harmful substances into the surrounding area. Apoptosis plays a crucial role in biological development and maintaining health by eliminating old cells, and unhealthy cells. Cell cycle, the sequence of stages that a cell passes through between one cell division and the next. A cell cycle can be divided into four main stages: the M phase, when nuclear and cytoplasmic division occurs; the G1 phase; the S phase, in which DNA replication occurs; and the G2 phase, a relatively quiescent period.
This is an interesting paper that challenges dogma of role of Kras2 in CRC. The study with appropriate methods investigating the effect of re-introduction of wild-type Kras2 in colorectal cancer cell lines on cell growth, proliferation and apoptosis. A major finding of the study was that re-expression of wild-type Kras2 inhibited cell growth and proliferation along with induction of the apoptosis in the studied cell line. It is worthy of publication in WJG.
S- Editor Liu Y L- Editor Zhu LH E- Editor Ma WH
| 1. | Halatsch ME, Hirsch-Ernst KI, Weinel RJ, Kahl GF. Differential activation of the c-Ki-ras-2 proto-oncogene in human colorectal carcinoma. Anticancer Res. 1998;18:2323-2325. [PubMed] |
| 2. | Rodenhuis S, Slebos RJ. Clinical significance of ras oncogene activation in human lung cancer. Cancer Res. 1992;52:2665s-2669s. [PubMed] |
| 3. | Perentesis JP, Bhatia S, Boyle E, Shao Y, Shu XO, Steinbuch M, Sather HN, Gaynon P, Kiffmeyer W, Envall-Fox J. RAS oncogene mutations and outcome of therapy for childhood acute lymphoblastic leukemia. Leukemia. 2004;18:685-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 85] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | Wang Y, Wang Y, Stoner G, You M. ras mutations in 2-acetylaminofluorene-induced lung and liver tumors from C3H/HeJ and (C3H x A/J)F1 mice. Cancer Res. 1993;53:1620-1624. [PubMed] |
| 5. | Zhang Z, Wang Y, Vikis HG, Johnson L, Liu G, Li J, Anderson MW, Sills RC, Hong HL, Devereux TR. Wildtype Kras2 can inhibit lung carcinogenesis in mice. Nat Genet. 2001;29:25-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 233] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 6. | Spandidos DA, Sourvinos G, Tsatsanis C, Zafiropoulos A. Normal ras genes: their onco-suppressor and pro-apoptotic functions (review). Int J Oncol. 2002;21:237-241. [PubMed] |
| 7. | Adjei AA. Blocking oncogenic Ras signaling for cancer therapy. J Natl Cancer Inst. 2001;93:1062-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 614] [Cited by in RCA: 618] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 8. | Bos JL. ras oncogenes in human cancer: a review. Cancer Res. 1989;49:4682-4689. [PubMed] |
| 9. | Anderson MW, Reynolds SH, You M, Maronpot RM. Role of proto-oncogene activation in carcinogenesis. Environ Health Perspect. 1992;98:13-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 75] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Perucho M, Goldfarb M, Shimizu K, Lama C, Fogh J, Wigler M. Human-tumor-derived cell lines contain common and different transforming genes. Cell. 1981;27:467-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 339] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 11. | Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, Nakamura Y, White R, Smits AM, Bos JL. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988;319:525-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4616] [Cited by in RCA: 4464] [Article Influence: 120.6] [Reference Citation Analysis (0)] |
| 12. | Mariyama M, Kishi K, Nakamura K, Obata H, Nishimura S. Frequency and types of point mutation at the 12th codon of the c-Ki-ras gene found in pancreatic cancers from Japanese patients. Jpn J Cancer Res. 1989;80:622-626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 94] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Shibata D, Almoguera C, Forrester K, Dunitz J, Martin SE, Cosgrove MM, Perucho M, Arnheim N. Detection of c-K-ras mutations in fine needle aspirates from human pancreatic adenocarcinomas. Cancer Res. 1990;50:1279-1283. [PubMed] |
| 14. | Bremner R, Balmain A. Genetic changes in skin tumor progression: correlation between presence of a mutant ras gene and loss of heterozygosity on mouse chromosome 7. Cell. 1990;61:407-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 186] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Pinto MM, Emanuel JR, Chaturvedi V, Costa J. Ki-ras mutations and the carcinoembryonic antigen level in fine needle aspirates of the pancreas. Acta Cytol. 1997;41:427-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Mills NE, Fishman CL, Rom WN, Dubin N, Jacobson DR. Increased prevalence of K-ras oncogene mutations in lung adenocarcinoma. Cancer Res. 1995;55:1444-1447. [PubMed] |
| 17. | Rodenhuis S, Slebos RJ, Boot AJ, Evers SG, Mooi WJ, Wagenaar SS, van Bodegom PC, Bos JL. Incidence and possible clinical significance of K-ras oncogene activation in adenocarcinoma of the human lung. Cancer Res. 1988;48:5738-5741. [PubMed] |
| 18. | Suzuki Y, Orita M, Shiraishi M, Hayashi K, Sekiya T. Detection of ras gene mutations in human lung cancers by single-strand conformation polymorphism analysis of polymerase chain reaction products. Oncogene. 1990;5:1037-1043. [PubMed] |
| 19. | Reynolds SH, Anna CK, Brown KC, Wiest JS, Beattie EJ, Pero RW, Iglehart JD, Anderson MW. Activated protooncogenes in human lung tumors from smokers. Proc Natl Acad Sci USA. 1991;88:1085-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 43] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Li S, Rosell R, Urban A, Font A, Ariza A, Armengol P, Abad A, Navas JJ, Monzo M. K-ras gene point mutation: a stable tumor marker in non-small cell lung carcinoma. Lung Cancer. 1994;11:19-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Li J, Zhang Z, Dai Z, Plass C, Morrison C, Wang Y, Wiest JS, Anderson MW, You M. LOH of chromosome 12p correlates with Kras2 mutation in non-small cell lung cancer. Oncogene. 2003;22:1243-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 40] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Diaz R, Ahn D, Lopez-Barcons L, Malumbres M, Perez de Castro I, Lue J, Ferrer-Miralles N, Mangues R, Tsong J, Garcia R. The N-ras proto-oncogene can suppress the malignant phenotype in the presence or absence of its oncogene. Cancer Res. 2002;62:4514-4518. [PubMed] |
| 23. | Li H, Wan J, Li Y, Zhu ML, Zhao P. Loss of heterozygosity on chromosome 12p12-13 region in Chinese patients with colon carcinoma. Zhonghua YiXue YiChuanXue ZaZhi. 2005;22:694-697. [PubMed] |
| 24. | Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8087] [Cited by in RCA: 8006] [Article Influence: 228.7] [Reference Citation Analysis (1)] |
| 25. | Shields JM, Pruitt K, McFall A, Shaub A, Der CJ. Understanding Ras: 'it ain't over 'til it's over'. Trends Cell Biol. 2000;10:147-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 598] [Cited by in RCA: 587] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 26. | Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7459] [Cited by in RCA: 7637] [Article Influence: 332.0] [Reference Citation Analysis (0)] |
| 27. | Raines KW, Cao GL, Lee EK, Rosen GM, Shapiro P. Neuronal nitric oxide synthase-induced S-nitrosylation of H-Ras inhibits calcium ionophore-mediated extracellular-signal-regulated kinase activity. Biochem J. 2006;397:329-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |