Published online Feb 14, 2007. doi: 10.3748/wjg.v13.i6.882
Revised: November 23, 2006
Accepted: January 9, 2007
Published online: February 14, 2007
AIM: To investigate dynamic changes and significance of expression of NF-κBp65 in pancreatic tissues of rats with severe acute pancreatitis (SAP), as well as BN52021 effects.
METHODS: Wistar male rats were randomly divided into negative control group (NC group, n = 60), SAP-model group (SAP group, n = 60), and BN52021-treated group (BN group, n = 60), and each of the above groups was respectively divided into 6 subgroups at different time points after operation (1 h, 2 h, 3 h, 6 h, 12 h, and 24 h) (n = 10). By RT-PCR and Western blot, NF-κBp65 mRNA and its protein expression in pancreatic tissues of rats were detected respectively.
RESULTS: The expression of NF-κBp65 mRNA dynamically changed in both SAP groups and BN groups. The mRNA level was higher in SAP groups than NC groups at 2 h, 3 h, 12 h, and 24 h after operation (P < 0.05), higher in BN groups than NC groups at all time points (P < 0.05), and higher in BN groups than SAP group at 1 h (P < 0.05). The NF-κBp65 protein level was higher in SAP groups than NC groups at 1 h, 3 h, and 6 h (P < 0.01), and 2 h, 12 h, and 24 h (P < 0.05), higher in BN groups than NC groups at all time points (P < 0.05), and lower in BN groups than SAP groups at 1 h, 3 h, and 6 h (P < 0.05).
CONCLUSION: The expression of NF-κBp65 in pancreatic tissues is dynamically changed and the changes play an important role in pathogenesis of SAP. BN52021 exerts therapeutic effects through reducing the expression level of NF-κBp65 protein in the early stage of SAP.
- Citation: Xia SH, Fang DC, Hu CX, Bi HY, Yang YZ, Di Y. Effect of BN52021 on NFκ-Bp65 expression in pancreatic tissues of rats with severe acute pancreatitis. World J Gastroenterol 2007; 13(6): 882-888
- URL: https://www.wjgnet.com/1007-9327/full/v13/i6/882.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i6.882
Up to now, the precise pathogenesis of Severe acute pancreatitis (SAP) with a high mortality has not been completely elucidated although the theories of self-digestion, leukocyte over-activation, microcirculatory disorder, bacterial shifting and secondary infection, i.e. the second attack, immune functional change, cell apoptosis and oxygen free radicals etc. have explained the pathogenesis of SAP from different angles[1,2].
NF-κBp65 is a type of protein that can bind many kinds of cytokines and adhesion molecules at the κB site of their gene promoters to enhance transcription of the genes. It plays an important role in cellular signal transduction in different theories of SAP pathogenesis[3]. For example, the activation of NF-κBp65 is the decisive factor in many pathological states and it especially has a close relationship with the occurrence and pathophysiological process of severe infections[4]. In SAP pathogenesis, the clinical course of SAP is dependent on the over-activated inflammatory cells and inflammatory factors expressed, in which platelet activating factor (PAF) is a crucial transmitter for systematic inflammatory reaction of SAP. PAF was found to have the ability to activate NF-κB in 1994[5], and the studies since then have shown that PAF receptor (PAF-R) is a target gene of NF-κB[6]. All these findings have attracted researchers to further study the relationship between PAF and NF-κB.
BN52021 (ginkgolide B) is a specific antagonist to PAF-R. In recent years, studies at home and abroad have showed that it has significant physiological activities, such as platelet aggregration inhibition, anti-inflammation, and anti-shock, etc. BN52021 has significant effects in treatment of animals with SAP[7,8]. But the exact pathogenesis of BN52021 on SAP is unknown. This study was aimed at dynamically investigating the changes and significance of the expression of NF-κBp65 mRNA and its protein in pancreatic tissues and effects of BN52021 in rats with SAP.
BN52021 and sodium taurocholate were purchased from Sigma (USA), amylase kit was from Beijing Kemei Reagent Co. (Beijing, China), trizol and diethyl pyrocarbonate (DEPC) were from Invitrogen Company (USA), RT kit was from IBM Fermentas Company (Lithuania), dNTPs and the RNA enzyme inhibitor were from TaKaRa Company (Japan), DNA Tag enzyme was from Promega (USA), Primary antibody of NF-κBp65 rabbit-anti-rat serum and the enhanced chemiluminescence (ECL) system were from Santa Cruz Biotechnology (USA), secondary antibody of sheep-antirabbit was from Beijing Dingguo Biotechnology Co., Ltd. (Beijing, China), polyvinylidene fluoride (PVDF film) was from Millipore Corp. (USA), prestained marker was from Beijing Tianwei Time Biotechnology Co., Ltd. (Beijing, China), β-actin was from Beijing Zhongshan Biotechnology Co., Ltd. (Beijing, China), DYY-12 electrophoresis system and electric trans-blot SD were from Beijing Liuyi Instrument Factory (Beijing, China), type-2720 PCR apparatus was from ABI Company (USA), and gel scanning & imaging system and vertical electrophoresis system were from Bio-Rad (USA).
One hundred and eighty Wistar male rats (weighing 0.20-0.23 kg, 6-8 wk old, Grade II, Certificate SCXK 2002-001) were provided by Laboratory Animal Center of PLA Academy of Military Medical Sciences, China. All rats were maintained in an environment of controlled temperature (22°C-25°C), humidity (55%-58%), and lighting (12 h light/12 h dark), with free access to tap water and regular chow diet. They were randomly divided into the negative controlled group (NC group, n = 60), SAP-modeled group (SAP group, n = 60), and BN52021-treated group (BN group, n = 60), and each of the above groups was respectively divided into 6 subgroups at different time points after operation (1 h, 2 h, 3 h, 6 h, 12 h, and 24 h) (n = 10). SAP models were prepared according to the method by Aho et al[9]. Wistar male rats were weighed, marked and fasted for 24 h before the operation, with free access to water. Anaesthesia was conducted by abdominal cavity injection of 0.4% sodium pentobarbital (40 mg/kg). Rats were fixed in dorsal decubitus. The skin was prepared and sterilized. And a 2-cm incision was made along the middle line of the upper belly and the abdominal cavity was entered. The duodenum and pancreaticobiliary duct were searched, the hepatic end of the pancreaticobiliary duct was clipped with a non-invasive vascular clip, pancreaticobiliary duct retrograde centesis was conducted with an obtuse (pointless) needle through duodenum seromuscular layer, and then 5% sodium taurocholate (0.1 mL/100 g) was injected in the retrograde direction of pancreaticobiliary duct with a micro-syringe, at an injection rate of 0.20 mL/min. After the injection of the drug, the part of pancreaticobiliary duct entering the duodenum was clipped with a non-invasive vascular clip for 10 min, and then the vascular clip was removed. After making sure that there was no active bleeding in the abdominal cavity, the abdomen was closed in two layers, and the wound was covered with sterile gauze. For the rats in NC group, the duodenum was merely stirred and pancreas was touched several times after opening the abdomen, and then the abdomen was closed. For the BN group, BN52021 (5 mg/kg: dissolved with Me2SO) was injected intravenously within 15 min after the operation; and for the groups of NC and SAP, the same volume of physiological saline (0.9% NaCl) was injected through femoral vein.
The rats in each group received anaesthesia at respective time points after the operation (1 h, 2 h, 3 h, 6 h, 12 h, and 24 h), and venous blood was collected from the right atrium. After a 10 min water bath at 37°C, and then a centrifugation for 10 min at 3000 g/min, the supernatant of the blood was respectively placed into sterilized EP tubes, and stored in a refrigerator at -20°Cfor determination of serum amylase. Meanwhile, two portions of pancreatic tissues of each group were treated differently; one portion was placed in liquid nitrogen overnight, and then frozen in a refrigerator at -80°C for further use, and another portion was fixed with 40 g/L neutral buffer formaldehyde, embedded with paraffin wax, cut into slices, and then HE stained for pathological observation and scoring.
Determination of serum amylase was conducted using a fully automatic biochemical apparatus and an amylase kit.
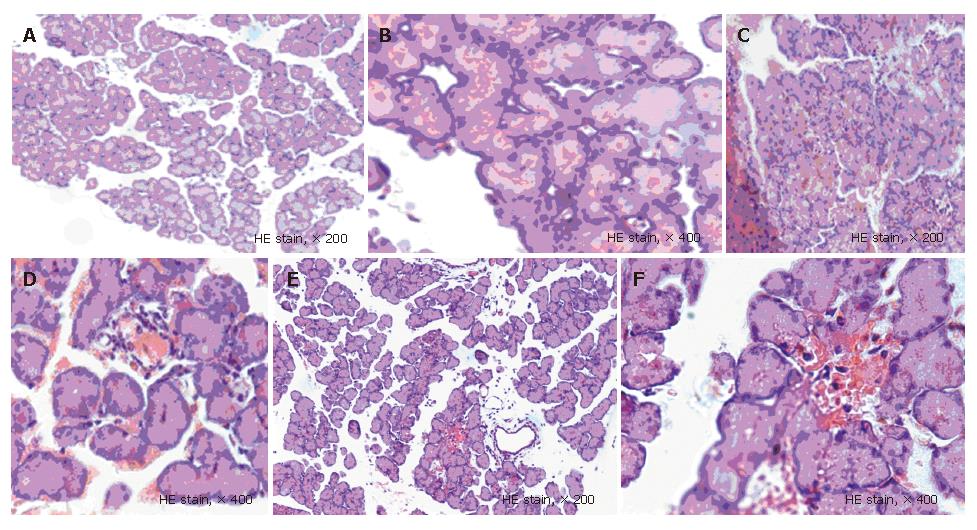
Pathological observation and scoring for pancreatic tissue samples (Table 1)[10]: 10 visual fields under a high-power microscope (HE stain, × 400) were randomly selected, and pathological changes of each item in the table were graded and scored, with a score of 0 for pathological changes of items not included in Table 1.
| Pathological grading | Pathological change | Scores |
| Edema | Inter-lobule local edema, widened pleura | 1 |
| Inter-lobule diffuse edema, widened intra-lobule clearance | 2 | |
| Increased intra-lobule clearance, alveolus swollen, and separated | 3 | |
| Inflammatory | White cells < 20/visual field under high-power microscope | 1 |
| Cell infiltration | White cells 20-50/visual field under high-power microscope | 2 |
| White cells > 30/visual field under high-power microscope, or micro-abscess occurs | 3 | |
| Hemorrhage | Parenchymal hemorrhage < 20% | 1 |
| Parenchymal hemorrhage 20%-50% | 2 | |
| Parenchymal hemorrhage > 50% | 3 | |
| Necrosis | Necrosis area < 20% | 1 |
| Necrosis area 20%-50% | 2 | |
| Necrosis area > 50% | 3 |
NF-κBp65 primer was prepared according to the previous method as reported[11]. The NF-κBp65 and β-actin primers were synthesized by Shanghai Yingjun Biotechnology Co. Ltd. (Table 2).
| Gene | Primer series (5'-3') | Gene No. | Position | Length |
| NF-κBp65 | GAAGAAGCGAGACCTGGAG | NM_199267 | 424-442 | 398 bp |
| TCCGGAACACAATGGCCAC | 821-803 | |||
| β-actin | TCCTAGCACCATGAA GATC | NM_031144 | 1044-1062 | 190 bp |
| AAACGCAGCTCAGTAACAG | 1233-1215 |
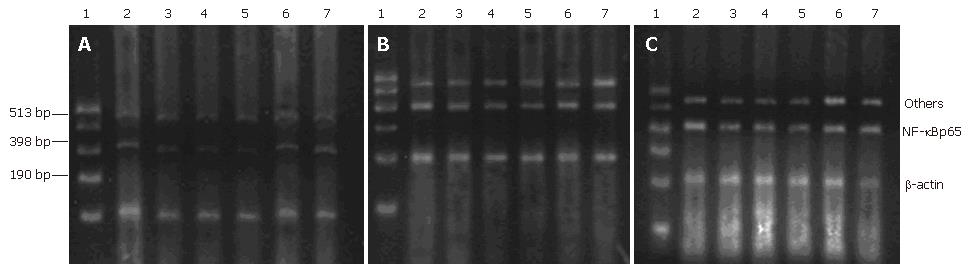
First, the total RNA of pancreatic tissues was extracted with Trizol Reagent for each group. Then, the integraty of the total RNA was examined by agarose electrophoresis, its concentration and purity were determined by a UV spectrophotometer, and the total RNA concentration of the sample was calculated. According to instructions of the RT reagent kit, 1 μg of the total RNA was used, and OligdT18 was used as a primer to produce 20 μL of the reaction system. PCR reaction was conducted by using 4 μL cDNA-RT product, 3 μL 10 × PCR buffer, 3 μL dNTPs of 200 μmol/L, 3 μL upstream primer of 10 pmol/μL, 3 μL downstream primer of 10 pmol/μL, 1μL Taq enzyme, and 13 μL deionized double-distilled water. Thermal cycle conditions were as follows: Pre-degeneration for 4 min at 94°C, 45 s at 94°C, 1 min at 72°C; after 35 cycles, an extension was conducted for another 5 min at 72°C. For each PCR reaction, a negative control with the same volume of deionized double-distilled water instead of cDNA was used, and meanwhile, amplification was conducted to control DNA contamination. The housekeeping gene was amplified, i.e. β-actin, as internal reference for gene quantitative expression. The sequence of PCR product was determined by Shanghai Yingjun Biotechnology Co., Ltd., and the results were in accordance with the corresponding mRNA. To 5 μL of RT-PCR product, 1 μL of supernatant buffer (containing 0.2 mg/mL ethidium bromde) was added, electrophoresis was conducted on gels containing 1.5% agarose at 80 V for 45 min, and photographed with Quantity One Gel Imaging System. A semiquantitative analysis of the experimental results was conducted.
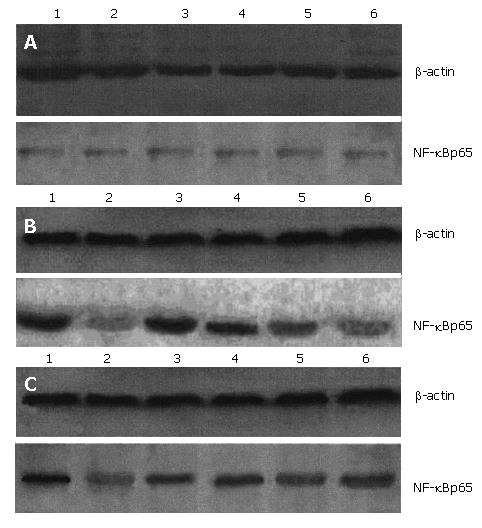
The prepared total protein was added to gel-added buffer at a ratio of 1:2 and then heated in water at 100°C for 5 min. For its vertical plating electrophoresis, the added volume was 15 μL. Each sample was palced on 2 parallel glue plates, one of which was used for staining and the other for membrane transferring. The voltage and time of electrophoresis were 80 v, 10 min and 120 v, 60 min for concentrating glue and isolating glue, respectively. The gels and membranes were placed among 6 sheets of filter paper and then between 2 foamy pads and put into electrical transfer tank containing buffer. After precooling for 10 min in icy water, the gel and membrane were galvanized for trans-printing for 4 h at a current of 1 mA/cm2. The trans-printed gel was stained to determine whether the trans-printing was complete. The trans-printed product was put into a solution containing 0.5% bovine serum albumin, slightly shaken and enclosed for 1 h. NF-κBp65 antibody (diluted in 1:400) was added and placed at 4°C overnight. After the labeled PVDF membrane was washed with 0.05% Tween-20 buffer (TBST) for 5 min three times, it was incubated with IgG labeled with HRP (diluted in 1:200) at 4°C overnight. The membrane was washed with 0.05% TBST again for 5 min three times. The liquid of ECL A and that of ECL B were mixed at an equal volume (0.125 mL liquid/cm2 membrane). The PVDF membrane was immerged in the mixed liquid for 1 min. Then the membrane was put on the freshness-retaining membrane. The trans-printing membrane was fixed in the developing tray and pictured using medical film for 30 s to 1 min, developed for 1-3 min and fixed for 1 min. Finally, the membrane was cleaned with water and dried up. The PVDF membrane was put into the desorption liquid, shaken at 50°C for 30 min, enclosed, added with β-actin antibody (diluted in 1:200) and placed at 4°C overnight. Then the above-mentioned process was repeated again for imaging. The results were photographed, saved in a computer, analysis was conducted by using Image-Pro Plus 4.5 image-analyzing software, and the integrated optical density (IOD) was calculated. The optical density ratio of target protein and β -actin in the same membrane was taken as the value of the fi nal experimental results.
The above experiments were repeated 3 times, the average values were calculated as the final data, and expressed as mean ± SEM. Data were processed with SPSS11.5 statistical software, normal test was conducted using t test and single-factor analysis of variance. Results were considered statistically significant when P < 0.05 or P < 0.01.
It showed that the serum amylase in the SAP groups and the BN groups significantly increased at each time point than those in the NC groups (P < 0.05); however, the values in the BN groups significantly decreased at 3 h, 6 h, and 24 h than those in the SAP groups (P < 0.05) (Table 3).
The pathological results showed that there was no obvious abnormality in abdominal cavity at all time points, and the pancreatic structure was almost normal in NC groups; in the SAP groups, hemorrhagic ascites occurred, necrosis foci were present in the pancreas, a number of saponifying spots occurred in the mesentery and greater omentum, and inflammatory cells infiltrated in pancreatic stroma and glandular lobule, and diffuse bleeding and piecemeal necrosis occurred and over time, the pathological changes were exacerbated; in the BN groups, the pathological changes were less serious than those in the SAP groups. It was shown that the scores in the SAP groups and the BN groups significantly increased at all time points than those in the NC groups (P < 0.05); however, the scores in the BN groups markedly decreased at 3 h, 6 h, and 24 h than those in the SAP groups (P < 0.05) (Figure 1, Table 4).
The expression of NF-κBp65 mRNA was dynamically changed in both SAP groups and BN groups in a dual-peak manner. The two peaks of the expression appeared at 1 h and 24 h in the SAP groups and at 1 h and 12 h in the BN groups and then the expression reached its lowest level at 6 h. The mRNA level was higher in SAP groups than NC groups at 2 h, 3 h, 12 h, and 24 h after operation (P < 0.05), higher in BN groups than NC groups at all time points (P < 0.05), and higher in BN group than SAP group at 1 h (P < 0.05) (Figure 2, Table 5).
| Groups | Time points | |||||
| 1 h | 2 h | 3 h | 6 h | 12 h | 24 h | |
| NC | 220.01 ± 94.51 | 186.30 ± 51.52 | 167.19 ± 51.55 | 114.91 ± 55.96 | 123.91 ± 54.78 | 131.08 ± 66.84 |
| SAP | 340.47 ± 119.14 | 269.03 ± 66.03a | 229.45 ± 50.34a | 197.07 ± 96.02 | 307.42 ± 100.62a | 376.97 ± 0.22a |
| BN | 470.80 ± 122.78bc | 268.70 ± 87.64b | 267.95 ± 49.66b | 277.64 ± 83.37b | 337.21 ± 103.02b | 340.24 ± 0.44b |
The NF-κBp65 protein level was markedly higher in SAP groups than NC groups at 1 h, 3 h, and 6 h (P < 0.01), and 2 h, 12 h, and 24 h (P < 0.05), also higher in BN groups than NC groups at all time points (P < 0.05), but lower in BN group than SAP groups at 1 h, 3 h, and 6 h (P < 0.05) (Figure 3, Table 6).
Studies in recent years confirmed that cytokines and adhesion molecules play an important role in pathogenesis of SAP. NF-κBp65, which is a type of protein that can bind many kinds of cytokines and adhesion molecules at the κB site of their gene promoters to enhance transcription of the genes, plays an important role in the occurrence and development of SAP[4,11]. Meanwhile, it is one member of the family of transcription-regulating protein and a homologous or heterogenous dimmer that contains 5 subunits of p50, p52, p65, cRel and RelB. Under normal condition, it binds to its inhibiting protein single IκB (including IκBα, IVBβ and IκBε) and has no activity. Upon stimulation of cells by such activating signals as endotoxin or TNF-α, it separates from IκB and enters the cellular nucleus and binds with specific κB sequence of DNA to enhance transcription of genes of infl ammatory media and cytokines. In 1997, Dunn et al, for the first time, found that activation of NF-κBp65 is an important early event in the occurrence of acute pancreatitis (AP) through establishing bile-originated AP by ligation of pancreatic duct. Blinman et al showed that the activity of NF-κBp65 is significantly enhanced and the expression of IL-6 and KC increases in the pancreatic tissues in the early stage of edematous pancreatitis in rats induced by litorin. Therefore, they believe that the activation of NF-κBp65 plays an important role in pathogenesis of AP; IL-1, IL-6, IL-8, IL-10 and TNF are associated with AP. Since the production of these inflammatory cytokines is regulated by NF-κBp65 and activator protein 1, it is believed that NF-κBp65 plays the major role in the infl ammatory media regulating network[12,13].
In this study, we found that the expression of NF-κBp65 mRNA and protein were significantly higher in SAP groups than in NC groups at all time points. Moreover, the expression markedly increased in the early stage, dynamically changed in dual-peak and time-dependent manners. These findings confirm that NF-κBp65 plays an important role in the occurrence and development of SAP. The mechanisms include: SAP causes severe endotoxemia, and LPS and endotoxin component bind with their respective binding protein and CD14 molecule on cell membrane to form an LPS-LBP-CD14 complex to activate the signal transduction of Toll-like receptor 4, and the signal transduction further activates gene transcription of cytokines (such as IL-1, IL-6 and IL-8)[14]. In SAP, the pancreatic elastase is activated to activate NF-κBp65 to exert its inflammation-inducing effects through the TLR4/NF-κB pathway. Consequently, damage of pancreatic self-digestion function and injuries of other organs outside of pancreas occur[15]. In SAP, PLA2 is activated to activate expression of NF-κBp65 of target cells in tissues[16]. NF-κBp65 is firstly activated in the pancreatic tissue to release cytokines. The cytokines start the “waterfall reaction” of inflammatory media through the “triggering role”. The inflammatory media such as PAF are released into the blood. Though the activation of NF-κB in the pancreatic tissues can be partly inhibited by intra- and extracellular pathways of negative feedback of NF-κBp65, the inflammatory media can again activate NF-κBp65 in other organs outside of pancreas (neutrophils, Kuffer cells, liver, lung and intestines, etc.) to produce more inflammatory media. As a result, they participate in the development of SAP and aggravate the injuries of pancreas and other organs or even cause SIRS, ARDS and MOF[4].
BN52021, one of the effective components of Chinese medicine ginkgo biloba leaf and a strong antagonist against the inflammatory medium of PAF, can not only block the signal transduction of PAF but also decrease blood concentration of PAF to exert its biological effect[17]. This study showed that BN52021 could decrease serum amylase level and alleviate pathological changes in SAP, which is consistent with that reported by other researchers[18,19]. The expression of NF-κBp65 mRNA was significantly higher in SAP and BN groups than in NC group at all time points after operation. However, it was markedly higher in BN groups than in SAP group at 1 h after the operation. The reason for the latter might be that the Me2SO used for dissolution of BN52021 can aggravate inflammatory reaction in the early stage of SAP, resulting in enhancement of NF-κBp65 mRNA expression, however, the mechanism needs to be further elucidated. Our results suggest that BN52021 does not significantly affect the expression of NF-κBp65 mRNA. The expression of NF-κBp65 protein was significantly lower in BN groups than in SAP groups at 1, 3 and 6 h after operation. However, it was still markedly higher in BN groups than in NC groups at all time points after operation. These findings suggest that BN52021 can inhibit the expression of NF-κBp65 protein in the early stage, to some extent, which might be one mechanism of its therapeutic effects on SAP. However, there was difference in the effect of BN52021 on NF-κBp65 mRNA expression and its protein expression. We believe that BN52021 causes downstream changes in signal transduction, leading to the difference, the mechanism of which also needs to be further studied.
In summary, the increase of NF-κBp65 expression plays an important role in pathogenesis of SAP. BN52021 can decrease serum level of amylase and alleviate pathological changes in SAP. Meanwhile, it can decrease expression of NF-κBp65 protein in pancreatic tissues in the early stage of SAP to exert its therapeutic effects.
BN52021 (ginkgolide B) is a specific antagonist to platelet activating factor receptor (PAF-R). In recent years, studies at home and abroad have shown that it has significant physiological activities, such as platelet aggregration inhibition, anti-inflammation, and anti-shock. BN52021 has significant effects in treatment of animals with severe acute pancreatitis (SAP). But the exact pathogenesis of BN52021 on SAP is unknown. This study was aimed to dynamically investigate the changes and significance of the expression of NF-κBp65 mRNA and its protein in pancreatic tissues and effects of BN52021 in rats with SAP.
To explore molecule mechanism of BN52021 on severe acute pancreatitis.
BN52021 has remarkable curative effects in acute pancreatitis. But the mechanism of BN52021 hasn’t been researched extensively. The study explored the significance of NF-κBp65 in platelet activating factor receptor signal transduction.
The results may provide theortic and experimental evidence for study, and application of BN52021, and new approaches for therapy of severe acute pancreatitis.
NF-κBp65 is a type of protein that can bind many kinds of cytokines and adhesion molecules at the κB site of their gene promoters to enhance transcription of the genes. It plays an important role in cellular signal transduction in different theories of pathogenesis of severe acute pancreatitis. BN52021, namely code of ginkgolide B, one of the effective components of Chinese medicine ginkgo biloba leaf and a strong antagonist against the inflammatory medium of platelet activating factor (PAF), can not only block the signal transduction of PAF but also decrease blood content of PAF to exert its biological effect. It has significant physiological activities, such as platelet aggregration inhibition, anti-inflammation, and anti-shock.
The results show that the expression of NF-κBp65 in pancreatic tissues dynamically changed and the changes played an important role in pathogenesis of severe acute pancreatitis (SAP). BN52021 exerted some kind of therapeutic effect through reducing the expression level of NF-κBp65 proteins in the early stage of SAP.
S- Editor Liu Y L- Editor Zhu LH E- Editor Lu W
| 1. | Tenner S, Banks PA. Acute pancreatitis: nonsurgical management. World J Surg. 1997;21:143-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 2. | Klar E, Messmer K, Warshaw AL, Herfarth C. Pancreatic ischaemia in experimental acute pancreatitis: mechanism, significance and therapy. Br J Surg. 1990;77:1205-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 172] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 3. | Tak PP, Firestein GS. NF-kappaB: a key role in inflammatory diseases. J Clin Invest. 2001;107:7-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3011] [Cited by in RCA: 3178] [Article Influence: 132.4] [Reference Citation Analysis (0)] |
| 4. | Gray KD, Simovic MO, Chapman WC, Blackwell TS, Christman JW, Washington MK, Yull FE, Jaffal N, Jansen ED, Gautman S. Systemic nf-kappaB activation in a transgenic mouse model of acute pancreatitis. J Surg Res. 2003;110:310-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Siebenlist U, Franzoso G, Brown K. Structure, regulation and function of NF-kappa B. Annu Rev Cell Biol. 1994;10:405-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1634] [Cited by in RCA: 1655] [Article Influence: 53.4] [Reference Citation Analysis (0)] |
| 6. | Chaqour B, Howard PS, Richards CF, Macarak EJ. Mechanical stretch induces platelet-activating factor receptor gene expression through the NF-kappaB transcription factor. J Mol Cell Cardiol. 1999;31:1345-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | McKenna DJ, Jones K, Hughes K. Efficacy, safety, and use of ginkgo biloba in clinical and preclinical applications. Altern Ther Health Med. 2001;7:70-86, 88-90. [PubMed] |
| 8. | Liu LR, Xia SH. Role of platelet-activating factor in the pathogenesis of acute pancreatitis. World J Gastroenterol. 2006;12:539-545. [PubMed] |
| 9. | Aho HJ, Koskensalo SM, Nevalainen TJ. Experimental pancreatitis in the rat. Sodium taurocholate-induced acute haemorrhagic pancreatitis. Scand J Gastroenterol. 1980;15:411-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 258] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 10. | Wu JX, Yuan YZ, Xu JY, Qin LF, Li DG, Lu HM. Pathological Characteristics of Acute Necrotizing Pancreatitis in Rats and the Methods of Evaluation. Zhongguo Shiyan Dongwu Xuebao. 2002;10:210-213. |
| 11. | Butcher HL, Kennette WA, Collins O, Zalups RK, Koropatnick J. Metallothionein mediates the level and activity of nuclear factor kappa B in murine fibroblasts. J Pharmacol Exp Ther. 2004;310:589-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 41] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Bi HY, Xia SH. Role of NF-κB in systemic inflammatory response syndrome. Shijie Huaren Xiahua Zazhi. 2004;12:2873-2841. |
| 13. | Shi C, Zhao X, Lagergren A, Sigvardsson M, Wang X, Andersson R. Immune status and inflammatory response differ locally and systemically in severe acute pancreatitis. Scand J Gastroenterol. 2006;41:472-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Nishimune H, Vasseur S, Wiese S, Birling MC, Holtmann B, Sendtner M, Iovanna JL, Henderson CE. Reg-2 is a motoneuron neurotrophic factor and a signalling intermediate in the CNTF survival pathway. Nat Cell Biol. 2000;2:906-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 120] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 15. | Hietaranta A, Mustonen H, Puolakkainen P, Haapiainen R, Kemppainen E. Proinflammatory effects of pancreatic elastase are mediated through TLR4 and NF-kappaB. Biochem Biophys Res Commun. 2004;323:192-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 56] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Luo SF, Lin WN, Yang CM, Lee CW, Liao CH, Leu YL, Hsiao LD. Induction of cytosolic phospholipase A2 by lipopolysaccharide in canine tracheal smooth muscle cells: involvement of MAPKs and NF-kappaB pathways. Cell Signal. 2006;18:1201-1211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Vogensen SB, Strømgaard K, Shindou H, Jaracz S, Suehiro M, Ishii S, Shimizu T, Nakanishi K. Preparation of 7-substituted ginkgolide derivatives: potent platelet activating factor (PAF) receptor antagonists. J Med Chem. 2003;46:601-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Wang XP, Yu YZ, Xu JY. The therapeutic effect of Ginkgolides on acute pancreatitis in rats and its mechanisms. Zhongguo Yaolixue Tongbao. 1995;11:199-201. |
| 19. | Ji Z, Wang B, Li S. The role of platelet activating factor in pathogenesis of acute pancreatitis in dogs. Zhonghua WaiKe ZaZhi. 1997;35:108-110. [PubMed] |