Published online Nov 28, 2007. doi: 10.3748/wjg.v13.i44.5944
Revised: August 31, 2007
Accepted: October 23, 2007
Published online: November 28, 2007
AIM: To investigate the in-vitro activation of cytotoxic T lymphocytes (CTLs) by fusion of mouse hepatocellular carcinoma (HCC) cells and lymphotactin gene-modified dendritic cells (DCs).
METHODS: Lymphotactin gene modified DCs (DCLptn) were prepared by lymphotactin recombinant adenovirus transduction of mature DCs which differentiated from mouse bone marrow cells by stimulation with granulocyte/macrophage colony-stimulating factor (GM-CSF), interleukin-4 (IL-4) and tumor necrosis factor alpha (TNF-α). DCLptn and H22 fusion was prepared using 50% PEG. Lymphotactin gene and protein expression levels were measured by RT-PCR and ELISA, respectively. Lymphotactin chemotactic responses were examined by in-vitro chemotaxis assay. In-vitro activation of CTLs by DCLptn/H22 fusion was measured by detecting CD25 expression and cytokine production after autologous T cell stimulation. Cytotoxic function of activated T lymphocytes stimulated with DCLptn/H22 cells was determined by LDH cytotoxicity assay.
RESULTS: Lymphotactin gene could be efficiently transduced to DCs by adenovirus vector and showed an effective biological activity. After fusion, the hybrid DCLptn/H22 cells acquired the phenotypes of both DCLptn and H22 cells. In T cell proliferation assay, flow cytometry showed a very high CD25 expression, and cytokine release assay showed a significantly higher concentration of IFN-γ and IL-2 in DCLptn/H22 group than in DCLptn, DCLptn+H22, DC/H22 or H22 groups. Cytotoxicity assay revealed that T cells derived from DCLptn/H22 group had much higher anti-tumor activity than those derived from DCLptn, H22, DCLptn+H22, DC/H22 groups.
CONCLUSION: Lymphotactin gene-modified dendritoma induces T-cell proliferation and strong CTL reaction against allogenic HCC cells. Immunization-engineered fusion hybrid vaccine is an attractive strategy in prevention and treatment of HCC metastases.
-
Citation: Sheng XL, Zhang H.
In-vitro activation of cytotoxic T lymphocytes by fusion of mouse hepatocellular carcinoma cells and lymphotactin gene-modified dendritic cells. World J Gastroenterol 2007; 13(44): 5944-5950 - URL: https://www.wjgnet.com/1007-9327/full/v13/i44/5944.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i44.5944
Dendritic cells (DCs) are the most important antigen-presenting cells (APCs)[1-3]. DCs-based vaccinations have been demonstrated to be effective in inducing antigen-specific cytotoxic T lymphocyte (CTL) responses[4-9]. Previous studies in mouse tumor models or cancer patients demonstrated that vaccination with hybridomas from tumor cells and DCs induces regression of established carcinomas, lymphomas and myeloma[10-16]. This study was to investigate the in-vitro immune effects of fusion of mouse hepatocellular carcinoma (HCC) cells and lymphotactin (Lptn) gene-modified DCs and its antitumor activity.
Five- to six-week old Female BALB/c (H-2Kd) mice were obtained from the Animal Resource Center of Shanghai Institute for Biological Sciences, Chinese Academy of Sciences, and maintained in specific pathogen-free conditions for use at the age of 6-8 wk. Recombinant Ad5 adenoviruses harbouring mouse lymphotactin (AdLptn) or LaZ gene (AdLacZ) were kindly provided by Dr. Cao Xue-Tao. The recombinant adenoviruses were propagated in human embryonic kidney 293 (HEK293) cells, and purified by cesium chloride (CsCL) density gradient centrifugation. Titers of AdLptn and AdLacZ determined by plaque assay on HEK293 cells were 3.6 × 109 plaque-forming units (PFU)/mL and 4.5 × 109 PFU/mL, respectively. H22 cells, established as a BALB/c mouse origin HCC cell line, were purchased from China Center for Type Culture Collection. All the cells were cultured in RPMI-1640 (H22 cells) or DMEM (HEK293 cells) medium supplemented with 10% heat-inactivated fetal calf serum (FCS), 2 mmol/L glutamine, 100 U/mL penicillin and 100 μg/mL streptomycin.
DCs were prepared as previously described[17] with certain modifications. Briefly, bone marrow cells prepared from femora and tibias of normal BALB/c mice were depleted of red blood cells with ammonium chloride and plated in RPMI-1640 plus 10% FCS and 10 ng/mL granulocyte/macrophage colony-stimulating factor (GM-CSF; R&D) with conjunction of 10 ng/mL interleukin-4 (IL-4; R&D) on d 1. On d 3, nonadherent granulocytes, T and B cells were gently removed and fresh media were added. On d 5, loosely adherent proliferating DC aggregates were dislodged and re-plated in the fresh media, and supplemented with 50 ng/mL tumor necrosis factor-α (TNF-α; R&D). On d 7, the released nonadherent mature DCs were harvested. CD11c-positive DCs accounted for more than 80% of the harvested cells as measured by flow cytometry.
Cultured DCs were pelleted and washed with PBS prior to the addition of virus. Virus stock (stored at -80°C) was thawed at room temperature and diluted in serum-free RPMI-1640 medium. The pellets of DCs were resuspended in serum-free RPMI-1640 and virus was added. After 2 h
incubation with virus, cells were washed once in PBS. DCs were resuspended in a cytokine-supplemented medium which was retained after DC culture. Twenty-four hours after gene modification, LacZ gene-modified DCs (DCLacZ) were collected for X-gal staining to evaluate the gene transfer efficiency. Lymphotactin gene-modified DCs (DCLptn) were collected for phenotypic analysis and fused with H22 cells in vitro.
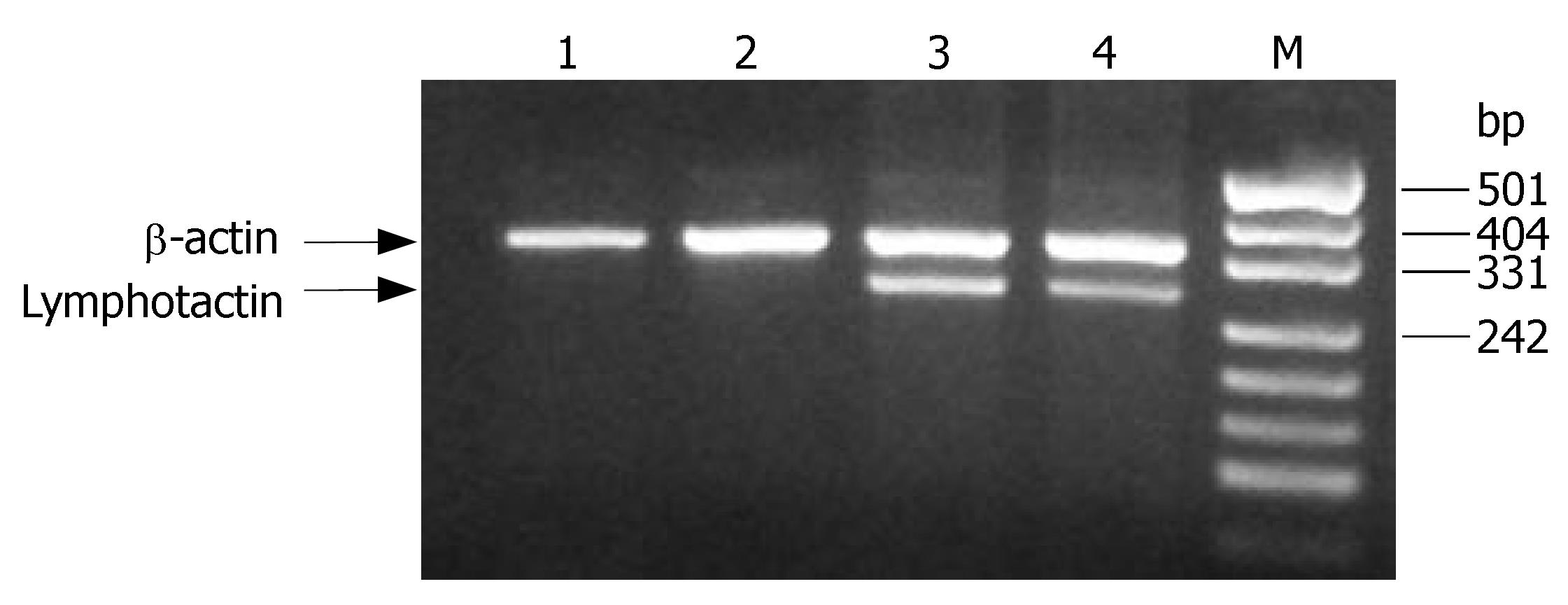
Total cellular RNA was isolated from cells using the TRIzol reagent (Life Technologies) according to the manufacturer’s instructions. cDNA was prepared from total RNA using a hexanucleotide random primer and SuperScrip Moloney murine leukemia virus reverse transcriptase (Life Technologies). PCR primers for the amplification of mouse lymphotactin and beta-actin used are as follows (lymphotactin forward primer: 5'TGGGGACTGAAGTCCTAGAAG3'; reverse primer: 5'TTACCCAGTCAGGGTTACTGCTGCTGTG3', with the product size of 300 bp. Beta-actin forward primer: 5'TGGAATCCTGTGGCATCCATGAAAC3'; reverse primer: 5'TAAAAGCCAGCTCAGTAACAGTCCG3', with the expected size of 359 bp.). PCR was performed in a Perkin Elmer Cetus DNA thermal cycler using Taq DNA polymerase (Life Technologies). The program consisted of 25 cycles of template denaturation at 94°C for 1 min, annealing of primers at 60°C for 1 min and synthesis at 72°C for 2 min, followed by a final extension at 72°C for 10 min. The PCR products were analyzed by agarose gel electrophoresis. Controls without reverse transcriptase were used to confirm that the RT-PCR products obtained were not the result of contamination with genomic DNA.
Lymphotactin protein in the supernatants from DCLptn was quantitatively determined with a commercial “sandwich” enzyme immunoassay kit (R&D) according to the manufacture’s instructions. Briefly, Costar EIA microplates were coated with 100 μL of 2 μg/mL rat-anti-mouse lymphotactin as a capture antibody, incubated overnight at room temperature, and blocked with 1% bovine serum albumin (BSA) in PBS. Then, 100 μL of serially diluted standards or culture supernatant samples was added in triplicate and incubated at room temperature for 2 h. The plates were washed and incubated at room temperature for 2 h with 100 μL of 400 ng/mL biotinylated goat anti-mouse lymphotactin as a detection antibody. After washing, wells were incubated for 20 min in 100 μL of streptavidin- horseradish peroxidase (HRP) solution, and developed with substrate solution.
DCLptn were fused with tumor cells at a 3:1 (DC: tumor) ratio using 50% polyethylene glycol (PEG, 50% PEG/10% DMSO in PBS, Sigma). In brief, H22 cells were inactivated by 30 μg/mL mitomycin, washed and mixed with DCLptn. After centrifugation, 1 mL of 50% PEG was added to the cell pellets for 2 min at 37°C. Then, an additional 10 mL of warm serum-free medium was added to dilute PEG over the next 3 min with continuous stirring. PEG-treated cells were centrifuged at 400 ×g for 5 min, resuspended with RPMI-1640 medium supplemented with 20% FCS, 10 ng/mL GM-CSF and 10 ng/mL IL-4, and cultured overnight.
To determine the efficiency of cell fusion, H22 cells were stained with PKH-26 (red fluorescence, Sigma) and DCLptn were stained with PKH-2 (green fluorescence, Sigma). The cells stained with the fluorescence dyes were treated with PEG and cultured overnight as described above. On the next day, the stained cells were analyzed using a FACScan flow cytometer (Becton Dickinson) under a confocal microscope.
After washing, cells were resuspended in PBS containing 1% BSA, and stained with fluorescence-conjugated monoclonal antibody (H-2Kd, I-Ad, CD80, CD86, CD40, CD54) or isotype control antibody for 30 min at 4°C. The stained cells were washed and analyzed using FACS
Chemotactic responses of lymphotactin to T cells were examined using modified boyden microchemotaxis chambers (Neuro Probe, Gaithersburg) and polyvinyl pyrrolidone-free 5 μm pore size polycarbonate membranesy. Briefly, spleen cells from naïve BALB/C mice were used as effector cells. The bottom wells of the chamber were loaded with supernants of H22, DC, DCLptn, DCLacZ or RPMI-1640 alone, and the upper wells contained 1 × 105 effector cells. After 1 h incubation and staining, data were obtained by counting five nonoverlapping high power microscopic fields from each well. Cells were considered chemoattracted if the chemotactic index (number of cells migrating in experimental well/number of cells migrating in RPMI-1640 medium only) was greater than 2.
To determine the proliferation and differentiation of lymphocytes, CD25 expression and cytokine production after autologous T cell stimulation were assayed. Briefly, spleen cells from naïve BALB/C mice were passed over nylon wool with their purity determined by FACS (percentage of CD3+ cells near 90%) and used as responder cells at 1 × 105/well in 96-well U-bottom plates. Syngeneic H22, DCLptn, H22+ DCLptn (H22 cells co-cultured with DCLptn at a ratio of 3:1), DC/H22 (H22 cells fused with DC at a ratio of 3:1) and DCLptn/H22 (H22 cells fused with DCLptn at a ratio of 3:1) cells were inactivated with 30 μg/mL mitomycin for 30 min and added to responder cells in varying cell numbers. Cells were cultured at 37°C in RPMI-1640 medium containing 10% FCS and 5% CO2 for 2 d. Control wells contained T cells alone. At the end of experiment, supernatants were harvested for cytokine production assay by ELISA and co-cultured T-cells were collected for analyzing CD25 expression by FACS.
Cytotoxic function of the activated T lymphocytes stimulated with DCLptn/H22 was determined by cytotoxicity test. Inactivated cells were co-cultured with spleen T cells separated from naïve BALB/C mice at a 1:10 ratio in the presence of 20 U/mL mouse IL-2 for 7 d. The stimulated T cells were isolated and used as effector cells in lactate dehydrogenase (LDH, Roche) cytotoxicity assay. H22 cells were used as target cells. All steps were performed following the manufacturer’s instructions. Briefly, after washed with assay medium (RPMI1640 with 1%BSA), the effector cells were co-cultured at 37°C with target cells in a 96-well round bottom plate for 6 h, then the plate was centrifuged and the supernatants were transferred to another flat-bottom ELISA plate. One hundred μL of LDH detection mixture was added to each well and incubated at room temperature in the dark for 30 min. Absorbance was measured with an ELISA reader at 490 nm. The spontaneous release of LDH by target cells or effector cells was assayed by incubation of target cells in the absence of effector cells and vice versa, the maximum release of LDH was determined by incubation of the target cells in 1% Triton X-100 in assay medium. The percentage of cell-mediated cytotoxicity was determined by the following equation: cytotoxicity (%) = [(mixture of effectors and targets-effector control)/(maximum-spontaneous)] × 100.
Data were expressed as mean ± SD. Experiment results were analyzed using SPSS 10.0 statistical package. Differences among groups were assessed by the Student’s t test. P < 0.05 was considered statistically significant.
DC and H22 did not express any detectable Lptn, which was detected in DCLptn and H22Lptn (Figure 1). The results indicate that adenovirus vector could effectively transducer the Lptn gene.
In order to quantitatively determine Lptn protein in supernatants from gene-modified DCs, culture supernatants were harvested and determined for Lptn production by ELISA. The results showed that about 0.35 ± 0.04 ng/mL Lptn could be detected in the supernatants of DCLptn, while nearly no Lptn could be detected in the supernatants from untransfected DC, DCLacZ and H22 cells.
Consistent with ELISA results, only the supernatant from DCLptn was positive for chemotaxis assay (chemotaxis index = 3.2 ± 0.15), but from DC, DCLacZ, H22 groups was negative. The results indicate that recombinant Lptn secreted from DCLptn had an effective biological activity.
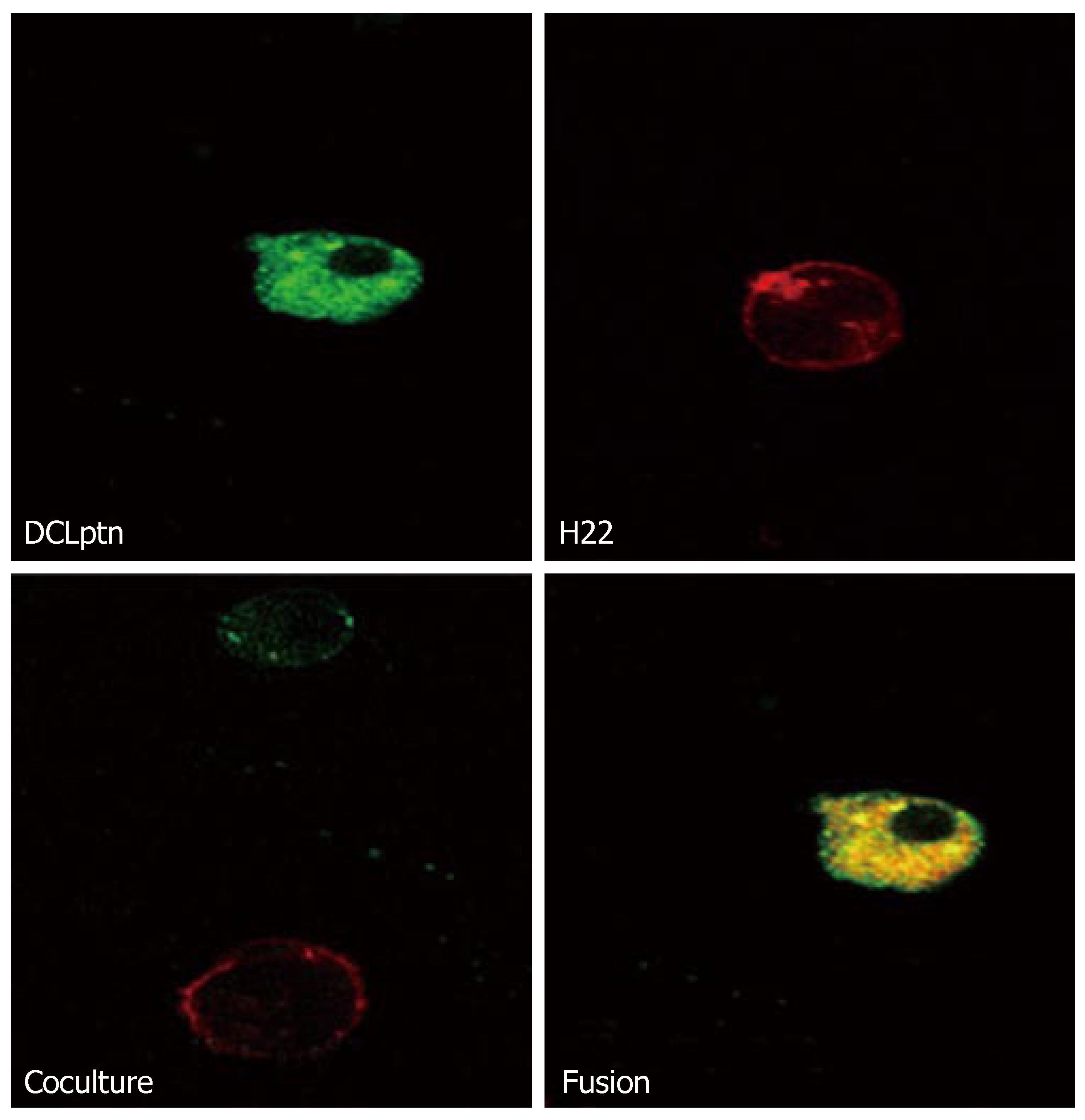
Fusion was examined by confocal microscopy (Figure 2) and flow cytometry (Figure 3). The fusion cells were yellow under confocal microscope. The fusion efficiency assayed by FACS was 15%-22%.
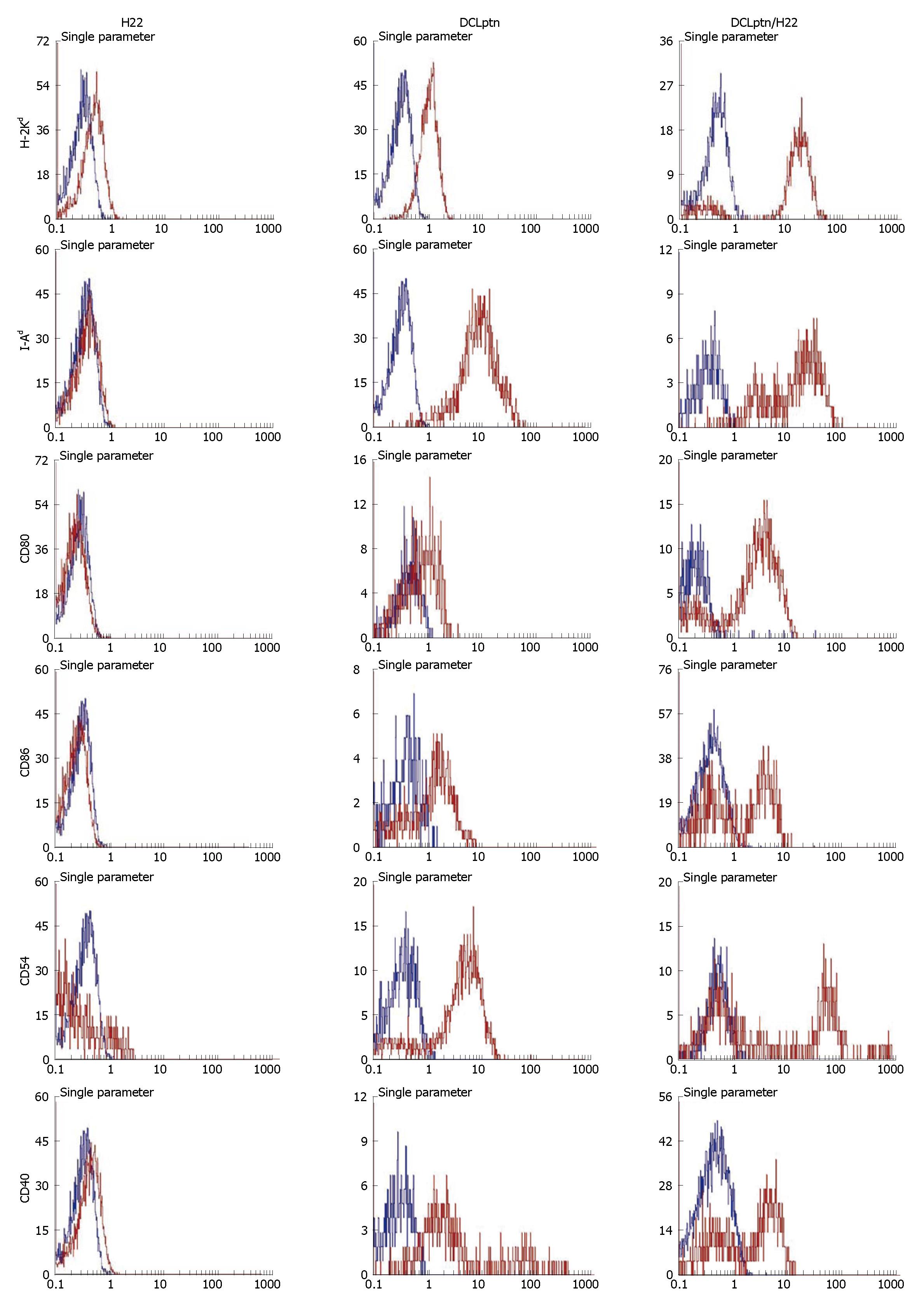
FACS analysis showed that DCs encoding lymphotactin were positive for H2-Kd, I-Ad, CD80, CD86, CD40, CD54. However, H22 cells expressed a moderate level of I-Ad. The expression levels of H-2Kd, CD80, CD86, CD40 and CD54 were almost negative. Hybrid DCLptn/H22 cells acquired the phenotypes of both DCLptn and H22 cells.
Flow cytometry showed that a very high CD25 expression was observed in T lymphocytes generated in autologous mixed lymphocyte reaction with DCLptn/H22 fusions (58.23% ± 11.65%) when compared to T cells either cultured with DCLptn cells (39.12% ± 12. 35%), H22 (10.78% ± 5. 46%), DC/H22 cells (41.55% ± 12.82%), or DCLptn+H22 cells (43.03% ± 10.52%). By in vitro cytokine release assay, significantly higher concentrations of IFN-γ and IL-2 were noted in supernatants of DCLptn/H22 co-cultured with T cells compared to those of DCLptn, DCLptn + H22, DC/H22 or H22 co-cultured with T cells. No difference was noted between concentrations of IL-4 or IL-10 in supernatants of all groups (Table 1).
| IFN-γ | IL-2 | IL-10 | IL-4 | |
| A (H22) | 510.3 ± 9.32 | 39.7 ± 2.72 | 91.48 ± 1.59 | 58.64 ± 0.4 |
| B (DCLptn) | 1015.51 ± 7.2f | 88.47 ± 3.17f | 96.20 ± 1.27 | 55.89 ± 2.95 |
| C (DCLptn+H22) | 999.64 ± 11.86f | 82.39 ± 3.02f | 97.33 ± 2.23 | 59.78 ± 1.21 |
| D (DC/H22) | 992.45 ± 10.16df | 93.28 ± 0.91df | 131.94 ± 0.32d | 98.71 ± 2.14d |
| E (DCLptn/H22) | 1886.08 ± 56.75b | 170.12 ± 2.11b | 217.13 ± 1.91b | 167.58 ± 0.94b |
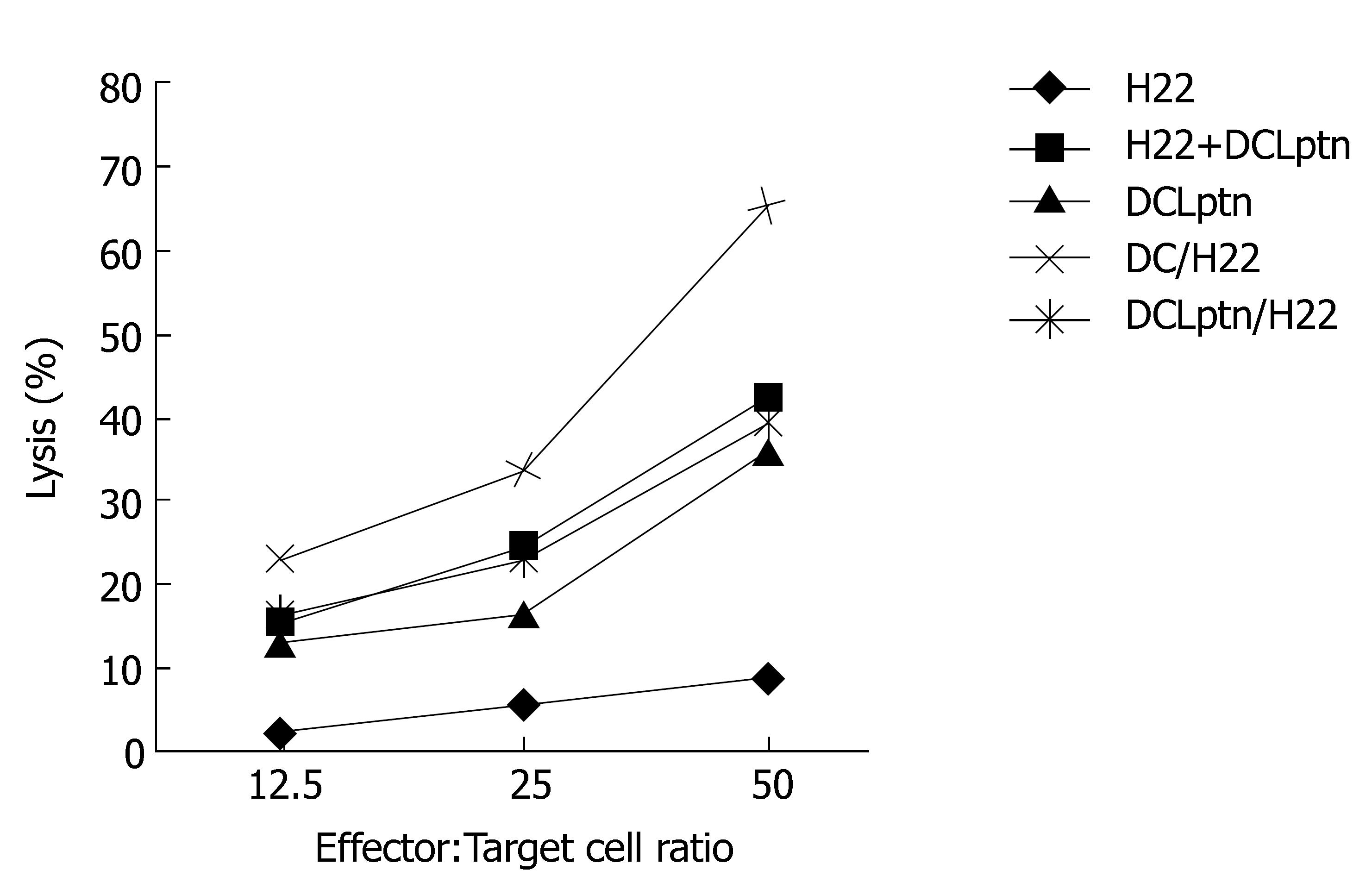
Cytotoxic assay revealed that T cells derived from DCLptn/H22 group possessed an extremely higher anti-tumor activity than those derived from DCLptn, H22, DCLptn + H22, DC/H22 groups. Although there were no differences among DCLptn, DCLptn + H22 and DC/H22 groups, the anti-tumor activity of DCLptn, DCLptn + H22 and DC/H22 groups was remarkably higher than that of H22 groups (Figure 4).
CD8+ T cells are critical components in immune responses to tumors and can differentiate into cytotoxic T lymphocytes and acquire the ability to lyse tumor antigen expressing cells. Activation of CD8+ T cells requires two steps[18-20]: presentation of antigenic peptides on professional antigen presenting cells and helper function provided by CD4+ T cells via Th1/Th2 cytokines. When DCs and HCC cells are fused, antigens are processed and displayed on the cell surface through MHC classIpathway which stimulates CD8+ T cells, and some antigens may be displayed by MHC class II molecules, which stimulate CD4+ T cells. On the other hand, mature DCs express MHCI, MHC II and co-stimulatory molecules that provide necessary signals for the stimulation of naïve T cells[21,22]. Upon stimulation, proliferating CD4+ T lymphocytes differentiate along the Th1 pathway, resulting in increased IFN-γ and IL-2 production, contributing to the activation of tumor-specific CTLs and enhancing the cytotoxic effect. Evidence from cytokine release assays indicates that in cultures with proliferating lymphocytes, the production and secretion of Th1-associated cytokines (IFN-γ, IL-2) but not Th2-associated cytokines (IL-4, IL-10) are increased. In our study, the fusion groups had a higher CTL activity than H22 group.
Activation of lymphocytes is a dynamic, multistep process. Although MHC and costimulatory molecules are critical for successful T-cell activation, signals that regulate this process have not been fully elucidated. It is believed that chemokines are an essential mediator. Migration of DCs to the sites of inflammation where they capture antigens and subsequently migrate to the local lymph nodes is regulated by the expression of different chemokines and their receptors[23,24]. Lymphotactin as a C chemokine produced mainly by T and nature killer (NK) cells, is a chemoattractant both in vitro and in vivo[25-28]. In our study, DCs and H22 cells did not express Lptn, and the Lptn gene-modified hybridima had a stronger CTL activity and a higher Th1 cytokine production, suggesting that Lptn modification can improve preferential chemotaxis of hybridoma on T cells and consequently optimize the microenvironment of antigen presentation to T cells.
CD25, α-chain of the IL-2 receptor, is expressed in the early to moderate phase after T-cell activation, the clonal proliferation of activated T cells depends on the expression of this receptor and resting lymphocytes do not express CD25[29,30]. Therefore, CD25 expression is commonly used as a marker for T cell activation. Quantification of surface IL-2 receptor expression on activated lymphocytes by flow cytometry after in vitro stimulation with specific antigens is useful in measuring cellular immunity. In the present study, we used this method to assess the lymphotactin gene- modified hybridoma’s stimulation on co-cultured T cells. By using this method, we were able to study the effect of stimulation on a heterogeneous cell population without the risk of selective depletion of cells, to exclude non-specific stimulation due to the separation, and to express CD25 at the early to moderate (24-48 h) phase of mixed lymphocyte reaction, thus shortening the co-culture time and keeping the viability of T cells.
In conclusion, lymphotactin gene-modified dendritoma induces potent T-cell proliferation and strong CTL reaction against allogenic HCC cells. Immunization-engineered fusion hybrid vaccine is an attractive strategy in prevention and treatment of cancer metastases.
Despite recent advances in surgical technique and radio- and chemotherapy, the prognosis of patients with malignant tumors remains dismal. The resistance of these tumors to conventional treatment may stem from their well-documented ability to exert local and systemic immunosuppressive effects. Therefore, alternative treatments are required.
Dendritic cells are the most potent APC for inducing an antigen-specific CTL response. This property, coupled with the fact that it is now possible to generate, ex vivo, a large number of functional dendritic cells from a patient’s peripheral blood monocytes or CD34 haemopoietic stem cells, have led to a considerable interest in use of dendritic cell vaccines as a means to induce antitumour immunity. Various strategies have been developed to introduce tumor specific antigens into DCs and thereby to generate cytotoxic T lymphocyte (CTL) responses against malignant cells. One of the important approaches to the induction of primary antitumor immunity is through the generation of tumor cell and DC fusion.
Although some effective results have been obtained by vaccinating mice with fusion of DCs and other tumor-cell types, it still remains a challenge. Several parameters must be optimized in order to maximize the efficacy of immunotherapy for dendritoma. In the present study, the authors have found that after Lptn gene modification, activated T cells can acquire more tumor antigens from DCLptn/H22 and have a stronger cytotoxicity to target cells.
This may be an attractive strategy in prevention and treatment of cancer metastases.
Dendritoma: fusion formed by dendritic cells and carcinoma cells.
This paper investigated the in vitro activation of cytotoxic T lymphocytes by fusion of mouse hepatocellular carcinoma (HCC) cells and dendritic cells modified by transfection of the lymphotactin gene. The authors conclude that lymphotactin modifies dendritoma and induces T cell proliferation and strong reaction of cytotoxic lymphocytes against allogenic HCC cells. These results are of certain interest.
S- Editor Zhu LH L- Editor Wang XL E- Editor Lu W
| 1. | Steinman RM. The dendritic cell advantage: New focus for immune-based therapies. Drug News Perspect. 2000;13:581-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 2. | Mosca PJ, Lyerly HK, Clay TM, Morse MA, Lyerly HK. Dendritic cell vaccines. Front Biosci. 2007;12:4050-4060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4895] [Cited by in RCA: 4820] [Article Influence: 192.8] [Reference Citation Analysis (0)] |
| 4. | Osada T, Clay TM, Woo CY, Morse MA, Lyerly HK. Dendritic cell-based immunotherapy. Int Rev Immunol. 2006;25:377-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 5. | Parajuli P, Mathupala S, Mittal S, Sloan AE. Dendritic cell-based active specific immunotherapy for malignant glioma. Expert Opin Biol Ther. 2007;7:439-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Velten FW, Rambow F, Metharom P, Goerdt S. Enhanced T-cell activation and T-cell-dependent IL-2 production by CD83+, CD25high, CD43high human monocyte-derived dendritic cells. Mol Immunol. 2007;44:1544-1550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Sabbatini P, Odunsi K. Immunologic approaches to ovarian cancer treatment. J Clin Oncol. 2007;25:2884-2893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Tuettenberg A, Schmitt E, Knop J, Jonuleit H. Dendritic cell-based immunotherapy of malignant melanoma: success and limitations. J Dtsch Dermatol Ges. 2007;5:190-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Qiu J, Li GW, Sui YF, Song HP, Si SY, Ge W. Heat-shocked tumor cell lysate-pulsed dendritic cells induce effective anti-tumor immune response in vivo. World J Gastroenterol. 2006;12:473-478. [PubMed] |
| 10. | Hao S, Bi X, Xu S, Wei Y, Wu X, Guo X, Carlsen S, Xiang J. Enhanced antitumor immunity derived from a novel vaccine of fusion hybrid between dendritic and engineered myeloma cells. Exp Oncol. 2004;26:300-306. [PubMed] |
| 11. | Yasuda T, Kamigaki T, Kawasaki K, Nakamura T, Yamamoto M, Kanemitsu K, Takase S, Kuroda D, Kim Y, Ajiki T. Superior anti-tumor protection and therapeutic efficacy of vaccination with allogeneic and semiallogeneic dendritic cell/tumor cell fusion hybrids for murine colon adenocarcinoma. Cancer Immunol Immunother. 2007;56:1025-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Rosenblatt J, Kufe D, Avigan D. Dendritic cell fusion vaccines for cancer immunotherapy. Expert Opin Biol Ther. 2005;5:703-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Kim GY, Chae HJ, Kim KH, Yoon MS, Lee KS, Lee CM, Moon DO, Lee JS, Jeong YI, Choi YH. Dendritic cell-tumor fusion vaccine prevents tumor growth in vivo. Biosci Biotechnol Biochem. 2007;71:215-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Gong J, Koido S, Chen D, Tanaka Y, Huang L, Avigan D, Anderson K, Ohno T, Kufe D. Immunization against murine multiple myeloma with fusions of dendritic and plasmacytoma cells is potentiated by interleukin 12. Blood. 2002;99:2512-2517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 84] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Li J, Theofanous L, Stickel S, Bouton-Verville H, Burgin KE, Jakubchak S, Wagner TE, Wei Y. Transfer of in vitro expanded T lymphocytes after activation with dendritomas prolonged survival of mice challenged with EL4 tumor cells. Int J Oncol. 2007;31:193-197. [PubMed] |
| 16. | Deng YJ, Xia JC, Zhou J, Wang QJ, Zhang PY, Zhang LJ, Rong TH. Antitumor efficacy of fusion cells from esophageal carcinoma cells and dendritic cells as a vaccine in vitro. Ai Zheng. 2007;26:137-141. [PubMed] |
| 17. | Lambert LA, Gibson GR, Maloney M, Barth Jr RJ. Equipotent Generation of Protective Antitumor Immunity by Various Methods of Dendritic Cell Loading With Whole Cell Tumor Antigens. J Immunother (1991). 2001;24:232-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Santana MA, Esquivel-Guadarrama F. Cell biology of T cell activation and differentiation. Int Rev Cytol. 2006;250:217-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Shen L, Rock KL. Priming of T cells by exogenous antigen cross-presented on MHC class I molecules. Curr Opin Immunol. 2006;18:85-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 125] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 20. | Bryant P, Ploegh H. Class II MHC peptide loading by the professionals. Curr Opin Immunol. 2004;16:96-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 102] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 21. | Gong J, Koido S, Kato Y, Tanaka Y, Chen D, Jonas A, Galinsky I, DeAngelo D, Avigan D, Kufe D. Induction of anti-leukemic cytotoxic T lymphocytes by fusion of patient-derived dendritic cells with autologous myeloblasts. Leuk Res. 2004;28:1303-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Ye Z, Chen Z, Sami A, El-Gayed A, Xiang J. Human dendritic cells engineered to express alpha tumor necrosis factor maintain cellular maturation and T-cell stimulation capacity. Cancer Biother Radiopharm. 2006;21:613-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Kehrl JH. Chemoattractant receptor signaling and the control of lymphocyte migration. Immunol Res. 2006;34:211-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Stein JV, Nombela-Arrieta C. Chemokine control of lymphocyte trafficking: a general overview. Immunology. 2005;116:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 189] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 25. | Blaschke S, Middel P, Dorner BG, Blaschke V, Hummel KM, Kroczek RA, Reich K, Benoehr P, Koziolek M, Müller GA. Expression of activation-induced, T cell-derived, and chemokine-related cytokine/lymphotactin and its functional role in rheumatoid arthritis. Arthritis Rheum. 2003;48:1858-1872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | Kim BO, Liu Y, Zhou BY, He JJ. Induction of C chemokine XCL1 (lymphotactin/single C motif-1 alpha/activation-induced, T cell-derived and chemokine-related cytokine) expression by HIV-1 Tat protein. J Immunol. 2004;172:1888-1895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Huang H, Li F, Gordon JR, Xiang J. Synergistic enhancement of antitumor immunity with adoptively transferred tumor-specific CD4+ and CD8+ T cells and intratumoral lymphotactin transgene expression. Cancer Res. 2002;62:2043-2051. [PubMed] |
| 28. | Kurt RA, Bauck M, Harma S, McCulloch K, Baher A, Urba WJ. Role of C chemokine lymphotactin in mediating recruitment of antigen-specific CD62L(lo) cells in vitro and in vivo. Cell Immunol. 2001;209:83-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Pejawar-Gaddy S, Alexander-Miller MA. Ligation of CD80 is critical for high-level CD25 expression on CD8+ T lymphocytes. J Immunol. 2006;177:4495-4502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 30. | Storset AK, Berntsen G, Larsen HJ. Kinetics of IL-2 receptor expression on lymphocyte subsets from goats infected with Mycobacterium avium subsp. paratuberculosis after specific in vitro stimulation. Vet Immunol Immunopathol. 2000;77:43-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |