Published online Nov 21, 2007. doi: 10.3748/wjg.v13.i43.5765
Revised: August 23, 2007
Accepted: October 18, 2007
Published online: November 21, 2007
AIM: To construct a new target-oriented conjugate of humanized carcinoembryonic antigen (CEA) specific single chain variable fragment (scFv) and mitomycin (MMC) against colorectal cancer, and to investigate its influence on the growth and apoptosis of colorectal cancer cells.
METHODS: The primer was designed according to the gene sequence described in reference 16, which respectively contains restriction enzyme cleavage sites BamHI and EcoRI in its upstream and downstream. PCR was performed with the plasmid as template containing genes of humanized anti-CEA scFv. The product was digested by BamHI and EcoRI, and connected to an expression vector which also has the restriction enzyme cleavage sites BamHI and EcoR. Expression of the reaction was induced by isopropy-β -D-thiogalactoside (IPTG). Then the expression product was covalently coupled with MMC by dextran T-40. The immunoreactivity of the conjugate against colorectal cancer cells as well as CEA was measured by enzyme linked immunosorbent assay (ELISA). The inhibiting ratio of conjugate on the growth of colorectal cancer cells was also measured by ELISA. The effect of conjugate on the apoptosis of colorectal cancer cells was determined by flow cytometry (FCM).
RESULTS: Restriction endonuclease cleavage and gene sequencing confirmed that the expression vector was successfully constructed. Sodium dodecyl sulfate polyacrylamide gel electropheresis (SDS-PAGE) confirmed that this vector correctly expressed the fusion protein. ELISA confirmed that the conjugate had quite a strong immunoreactivity against colorectal cancer cells and CEA. The conjugate had inhibitory effects on colorectal cancer cells in a concentration-dependent manner and could induce apoptosis of colorectal cancer cells in a concentration-dependent manner.
CONCLUSION: The CEA-scFv-MMC conjugate can be successfully constructed and is able to inhibit the growth and induce apoptosis of colorectal cancer cells.
- Citation: Chen DJ, Tan Z, Chen F, Du T. Construction of humanized carcinoembryonic antigen specific single chain variable fragment and mitomycin conjugate. World J Gastroenterol 2007; 13(43): 5765-5770
- URL: https://www.wjgnet.com/1007-9327/full/v13/i43/5765.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i43.5765
Significant improvement has been achieved in the treatment of advanced colorectal cancer in the past decade[1]. New cytotoxic agents and new monoclonal antibodies (mAb) have been shown to substantially improve patient outcomes in randomized trials[2]. Nevertheless, the prognosis of patients with advanced colorectal cancer remains relatively poor with a median survival of approximately 20 mo in optimally treated patients[3]. Therefore, additional treatment strategies are needed in order to further improve the outcomes.
With advances in oncomolecular biology, the mechanism of tumor genesis and development is better understood[4], which provides a new medication pattern against tumors. Since a conception of target-oriented medication against tumors has been recently brought forward[5], we can design and develop medications aiming at target spots of specific molecules and genes to selectively kill tumor cells according to the known abnormal molecules and genes related to the genesis of tumors. Target-oriented medication against tumors provides a new effective way of treating malignant tumors such as mammary cancer, intestinal cancer and lung cancer[6-8], and can directly deliver chemotherapeutics for the tumor and form a high local drug concentration so as to decrease the total dosage and further reduce the toxic and side effects[9].
An ideal target spot of tumor treatment has the following features. (1) It is a kind of macromolecules which are rather critical for malignancy phenotype; (2) It has no obvious expression in important organs and tissues; (3) It has biological correlativity; (4) It can be repeatedly tested on clinical specimens; (5) It has apparent correlativity with the clinical outcome[10]. Carcinoembryonic antigen (CEA) is a 180 ku cell surface-expressed glycoprotein antigen present in a number of adenocarcinomas, especially in colorectal cancer[11]. The gene sequence and three-dimensional structure of CEA have been reported elsewhere[12]. CEA is a member of the immunoglobulin superfamily and has cell adhesion properties as well as other less clearly defined roles[13]. Since it corresponds to all features of the target spot of tumor treatment, it is the ideal target spot of treating colorectal cancer.
At present, research on target-oriented medication against advanced colorectal cancer involved 3 different target-oriented drugs that have achieved significant curative effect: epidermal growth factor receptor (EGFR) inhibitors, vascular endothelial growth factor receptor (VEGFR) inhibitors, and cyclooxygenase-2 (COX-2) inhibitors[14]. By coupling radio immunity drugs with anti-CEA monoclonal antibody, Vogel[15] has achieved favorable therapeutic effectiveness on nude mice model of liver metastasis of colorectal cancer, thus opening up a new idea for target-oriented medication against advanced colorectal cancer. Based on the above-mentioned theory, we constructed a humanized anti-CEA single-chain antibody (scFv) and coupled it with mitomycin (MMC), a chemotherapeutic agent against colorectal cancer, and investigated the influence of this conjugate on the growth and apoptosis of colorectal cancer cells.
Plasmid pUC18 containing humanized anti-CEA-scFv was kindly provided by Professor Mark Sherman (the Berkman Research Institute, USA). E.coli DH5α and expression plasmid pGEX-4T-1 were stored in the Virus Research Institute, Medical College of Wuhan University. T4 DNA ligase and restriction endonuclease were purchased from New England Biolabs (USA). High fidelity DNA polymerase and DNA gel extraction kits were purchased from the Promega Company (USA). Plasmid extracting and purifying kits were the products of Vegen Company (Hangzhou, China). Glutathione acyltransferase purification kits were purchased from the Clonetech Company (USA). MMC was purchased from the Roche Company (USA). Bovine serum albumin (BSA), Tween 20, PI dyebath and Hanks solution were bought from Fuzhou Maixin Biology Company (China). Glucosan T-40, cysteine, N-2 N-ethylmaleimide (NEMI), horseradish peroxidase-labeled goat anti-mouse IgG, dimethyl sulphoxide (DMSO) were bought from the Sigma Company (USA). RPMI 1640 culture medium was bought from the Gibico Company (USA). CEA was produced by the Zymed Company (USA). Colorectal cancer cell lines SW480, SW620 and LoVo were provided by China Center for Typical Culture Collection (CCTCC) (Wuhan, China). Primer synthesis and gene sequencing were performed by the Sangon Company (Shanghai, China).
Primers were designed using the program Oligo4.1 and synthesized by Sangon Company (Shanghai, China) as previously described[16], a restriction enzyme cleavage site BamHI was added to the end 5’, and a restriction enzyme cleavage site EcoRI was added to the end 3’. Upstream primer: 5’CGGGATCCATGGACAGAGTCACA3’, downstream primer: 5’CCGAATTCTCCACGTGCACTCGAGACGGTGAC3’. The underlined parts are the restriction enzyme cleavage sites.
Plasmid pUC18 containing humanized anti-CEA scFv T84.66 was taken as the template to perform PCR with its upstream and downstream primers in 50 μL reaction system containing 5 μL template, 5 μL reaction buffer, 2 μL upstream and downstream primers, 0.5 μL high fidelity DNA polymerase, 4 μL dNTP and 33.5 μL deionized water. Samples were heated at 94°C for 5 min, followed by 35 cycles of heating at 94°C for 30 s, at 55°C for 30 s and at 72°C for 30 s. The temperature was held constant at 72°C for 7 min to ensure complete extension. The completed PCR reaction mix was electrophoresed on 1% agarose gel and the desired product was extracted from 200 mg gel slice. The purified product was digested with BamHI and EcoR. cDNA of anti-CEA scFv T84.66 containing correspondence restriction enzyme sites to pGEX-4T-1 was produced. Prokaryon expression vector pGEX-4T-1 was also digested with BamHI and EcoR. After identified by agarose gel electrophoresis, the plasmid with a cohesive end was connected to the cDNA of anti-CEA scFv T84.66 acquired previously. Connection reaction was performed in 10 μL system. The reaction product was transformed into competent cell line DH5α and the positive clone of recombined CEA-scFv-pGEX-4T-1 was acquired. Restriction endonuclease cleavage and gene sequencing confirmed that the scFv fragment was correctly interpolated into pGEX4-T-1. Positive plasmid was extracted and purified with the kits from Vegen Company (Hangzhou, China).
Expression of E. coli DH5α containing the positive plasmid was induced by isopropyl-β-D thiogalactoside (IPTG) followed by sodium dodecyl sulfate polyacrylamide gel electropheresis (SDS-PAGE). The level of this protein in total bacterial protein was detected with a thin layer chromatogram (TLC) scanner. The correctly expressed DH5α containing the recombinant plasmid was cultured in 500 mL triangular flask. After IPTG was added to induce expression of the protein, the product was purified with glutathione acyltransferase purification kit according to its manufacturer’s instructions. The purified protein was collected with a step-by-step collector containing 500 μL per tube, and the absorbance value was measured at 280 nm to quantify the protein.
A pertinent amount of dextran T-40 was admixed with sodium periodate as previously described[17], and the mixture reacted for 3 h at ordinary temperature away from light, then sufficiently dialyzed with deionized water. After cryodesiccation, poly aldehyde dextran (PAD) was produced. Fifty mg of PAD was admixed with 100 mg of MMC, and the mixture was incubated at 4°C for 12 h away from light. Then 80 mg of CEA-scFv purified protein was added, stirred reaction was carried out for 12 h and terminated with sodium borohydride. The reaction product was purified with Sephadex G-75 for CEA-scFv-MMC conjugate. The molecular constitution of the conjugate was measured by spectrophotometry. The absorbance value of the conjugate was measured respectively at 280 nm and 488 nm, and at 595 nm after stained with Coomassie brilliant blue. The mole ratio of each composition was recorded.
Colorectal tumor cells SW480, SW620 and LoVo were cultured in vitro in 96-well culture plates (104 cells per well). Twenty-four hours later, 2.5 g/L glutaraldehyde precooled at 4°C was added (50 μL per well). The cells were fixed at 4°C for 5min, washed 3 times with PBS and stored at -20°C. When it was used, 10 g/L BSA was added (200 μL per well), sealed overnight at 4°C, then washed with PBS-Tween 20 (PBS-T), and CEA-scFv-MMC diluted with multiple proportion was added (50 μL per well). The mixture was left to react at 37°C for 1 h, then washed 3 times with PBS-T. Horseradish peroxidase-labeled goat anti-mouse IgG antibody was added (50 μL per well). The mixture was incubated at 37°C for 1 h, washed 5 times with PBS-T. Enzyme reaction substrate was added (200 μL per well), the mixture was incubated to react for 15 min at room temperature away from light and terminated by adding 2 mol/L sulfuric acid. The absorbance value was measured at 490 nm with enzyme labelling instruments to determine the immunoreactivity of CEA-scFv-MMC against colorectal cancer cells. CEA (pH 7.5) at the concentration of 1.35 g/L was put into culture plates (100 μL per well), the mixture was incubated over night at 4°C. The supernatant was discarded. The mixture was washed 3 times with PBS and stored at -20°C. When it was used, 10 g/L BSA was added (200 μL per well), sealed overnight, and washed with PBS-T buffer solution. Other steps were the same as above, and the immunoreactivity of CEA-scFv-MMC against CEA was detected.
The colorectal cancer cells LoVo were cultured in RPMI1640 culture medium, routine serial subculture was carried out in an incubator containing 50 mL/L CO2 at 37°C. The culture medium was replaced with drug-containing culture medium 24 h after the cells adherently grew to 70%-80% monolayer, then the experiment was carried out. The cells were digested with trypsin and blown to single cell suspension. The cell concentration was counted, adjusted to 1 × 108 cells/L, which were inoculated into 96-well culture plates (0.2 mL per well). The original fluid was discarded after 24 h culture. CEA-scFv-MMC conjugate with different concentrations was added into the wells (0 mg/L in control group; 25 mg/L, 50 mg/L, 100 mg/L, 150 mg/L, 200 mg/L, 300 mg/L and 400 mg/L, respectively, in treatment group) and cultured for 48 h. Twenty μL of MTT solution (2.5 g/L) was added into each well, and cultured for 4 h. After the supernatant was carefully blotted, 150 μL of 100 g/L DMSO was added into each well. The absorbance value (A630nm) of each well was measured by enzyme labelling instruments after gently agitated on the oscillator. The growth inhibition ratio at each concentration in treatment group was calculated according to the following formula: growth inhibition ratio IR (%) = (1-average A630nm of treatment group/A630nm of control group) × 100%.
Colorectal cancer cells treated with different concentrations of CEA-scFv-MMC conjugate were cultured for 3 d and 106 cells were collected, fixed with 700 mL/L ethanol. The dying cell suspension was filtrated through nylon net (400 meshes) and washed 3 times with PBS followed by addition of 10 μL of Annexin V-fluorescein isothiocyanate and PI solution. The mixture was blended in ice bath for 10 min away from light. A flow cytometer (FCM) was used to detect the influence of CEA-scFv-MMC conjugate on the apoptosis of colorectal cancer cells.
Experiments approved by the local ethical committee were performed after the patients gave their informed consent. All the experimental data were expressed as mean ± SD. Comparison between treatment and control groups was made by t test and their ratios by χ2 test. The correlation between the two groups was analyzed with collinearity. P < 0.05 was considered statistically significant.
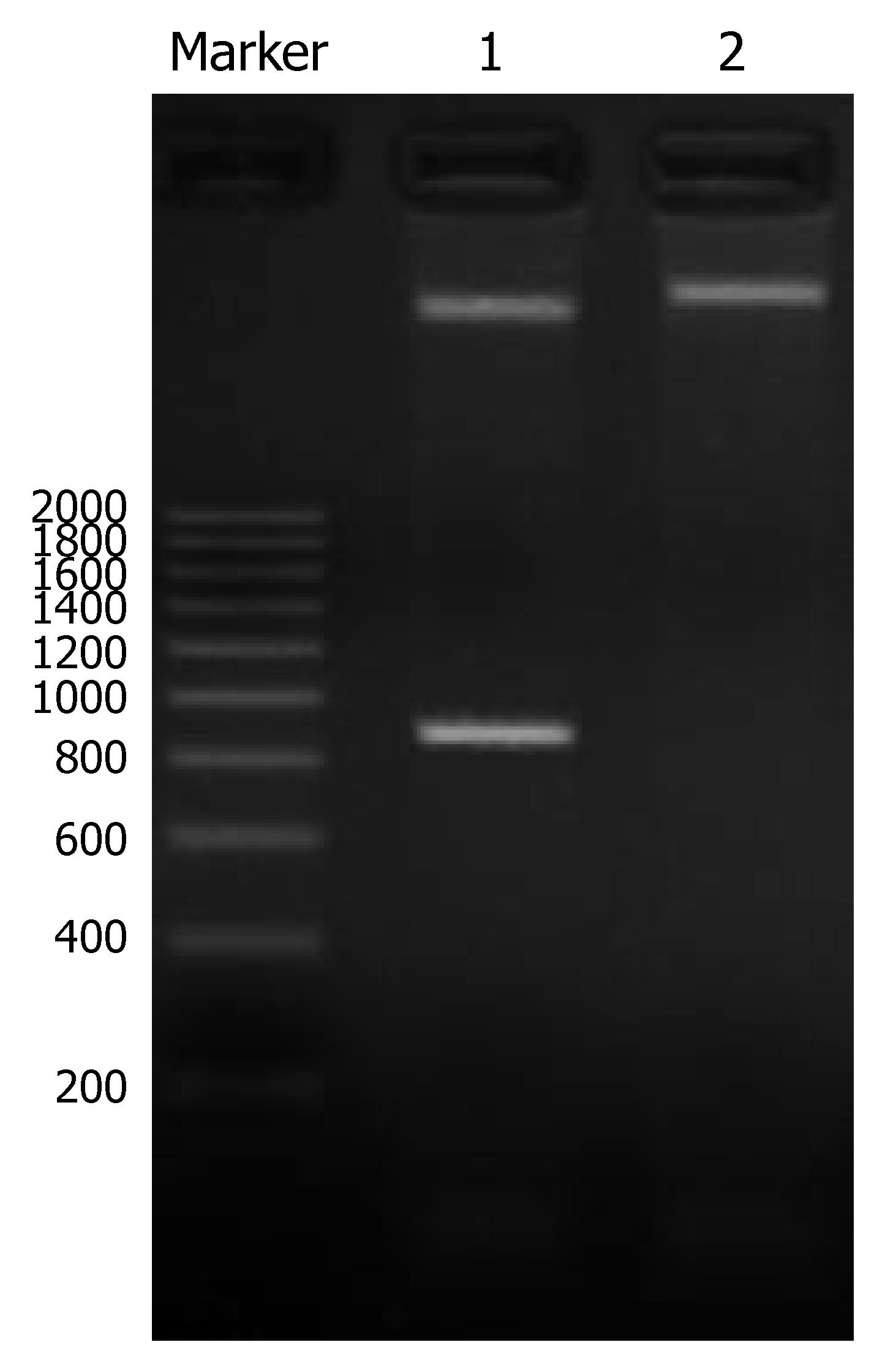
A 810 bp specific band was obtained by PCR amplification with pUC18 as template. cDNA of PCR amplification was completely in accordance with the gene sequence of humanized anti-CEA specific scFv T84.66. The positive clone of recombinant plasmid CEA-scFv-pGEX-4T-1 was identified by double restriction endonuclease cleavage with BamHI and EcoRI. Two specific bands were obtained in line 1, one was 4.7 kb and the other was 810 bp, and a 5.5 kb band was obtained in line 2. The 4.7 kb band represented pGEX-4T-1, 810 bp band anti-CEA-scFv, and the 5.5 kb band the recombinant plasmid CEA-scFv-pGEX-4T-1 (Figure 1). The result was completely consistent with our hypothesis. Meanwhile, the scFv gene was correctly inserted to the expression vector.
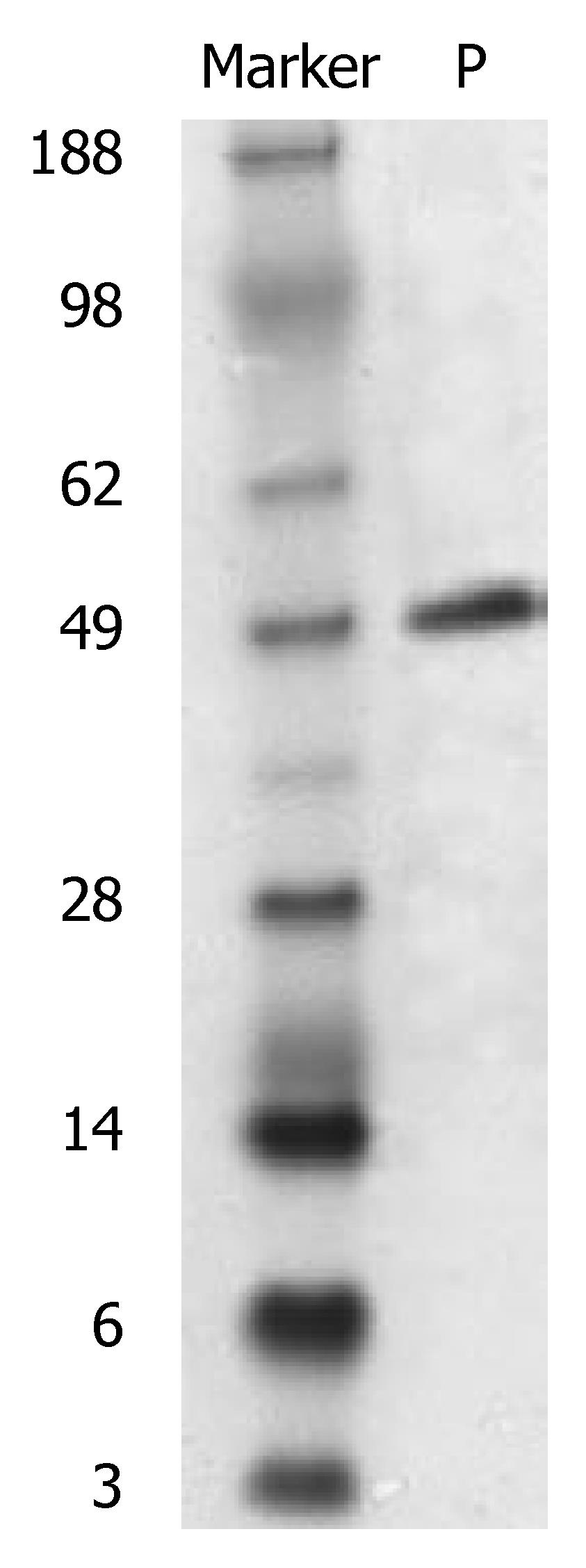
After induction of IPTG, expression of the scFv gene inserted to the pGEX-4T-1 was stable. A new protein band was obtained at Mr 49000 after SDS-PAGE, and its size was in accordance with GST (Mr 26000) and scFv-CEA (Mr 23000) fusion protein (Figure 2). This new protein band was preliminarily identified as the expressed CEA-scFv/GST fusion protein. TLC scan showed that the expressed fusion protein amounted to 26% of the total bacterial protein.
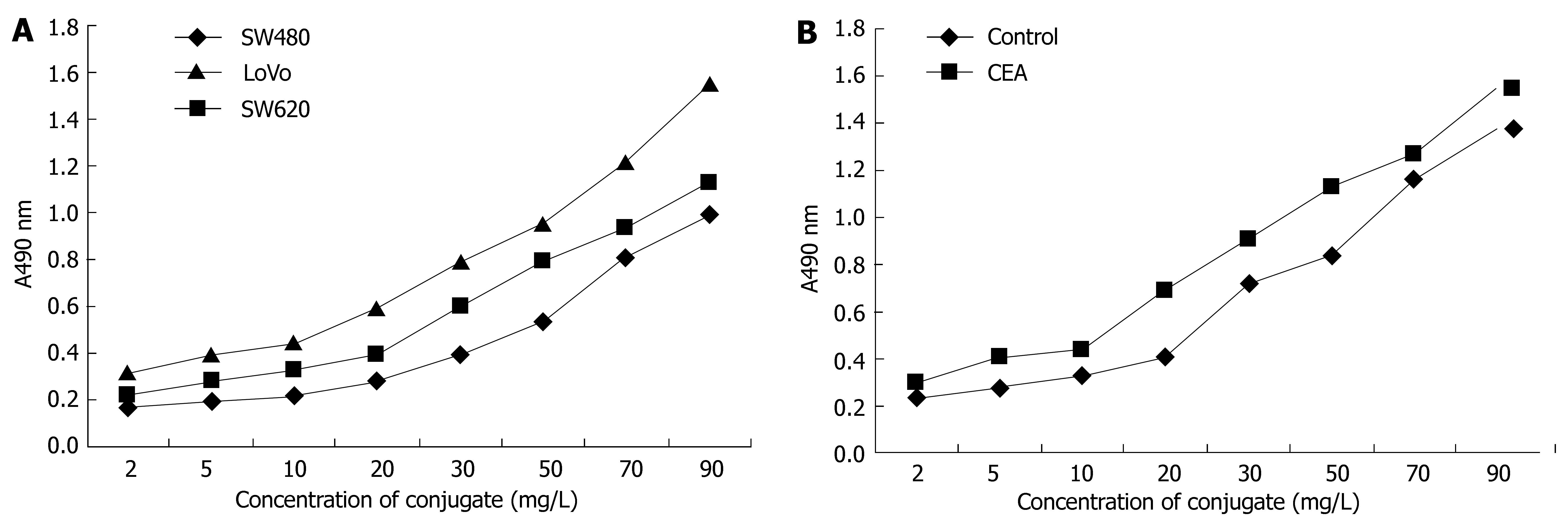
The components of the conjugate were calculated. The molecule ratio of CEA-scFv: Dextran T-40: MMC in the conjugate was 1:1.2:38. The immunoreactivity of the conjugate against colorectal cancer cells SW480, SW620 and LoVo was strong, and that against LoVo cells was the strongest (Figure 3A). The immunoreactivity of the conjugate against CEA was also strong, especially when the concentration of the conjugate was above 20 mg/L (Figure 3B).
After 48 h treatment, LoVo cells were treated with various concentrations of CEA-scFv-MMC, more or less restraining effect of the conjugate on the growth of LoVo cells was shown, significantly depending on the concentration. When the concentration was above 100 mg/L, it had an obvious restraining effect on the growth of the cells (aP < 0.05), and when the concentration was above 200 mg/L, it had a further restraining effect on the cells (bP < 0.01, Table 1).
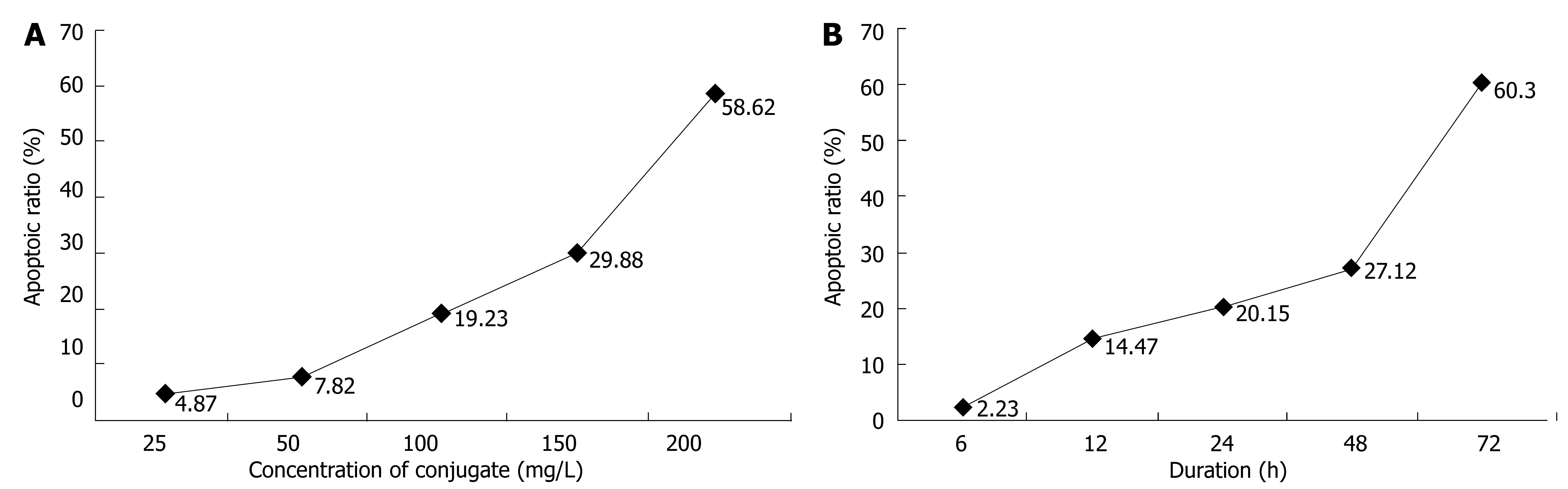
Different doses of CEA-scFv-MMC had different effects on apoptosis of LoVo cells. Apoptosis of LoVo cells began at the concentration of 25 mg/L CEA-scFv-MMC. The apoptosis rate increased with the increasing concentration of CEA-scFv-MMC (Figure 4A). Apoptosis of LoVo cells began 12 h after treatment of LoVo cells with CEA-scFv-MMC and the apoptosis rate reached its peak at 72 h (Figure 4B). The results showed that apoptosis of LoVo cells induced by CEA-scFv-MMC was highly dependent on its concentration and its duration of action.
Adjuvant chemotherapy has become more and more important in the treatment of colorectal cancer[18]. In recent years, though a variety of anti-cancer drugs are available, most of them could not distinguish cancer cells from normal cells[19]. Therefore, their clinical application is limited due to their toxic and side effects[20]. Target-oriented treatment directly delivers chemotherapeutic drugs to the tumor, resulting in a high drug concentration in the tumor[21]. Through decreasing the total dose, the toxic and side effects of drugs are decreased[22]. Single-chain antibody is an ideal vehicle for delivering chemotherapeutic drugs, because it is easy to reach the tumor due to its small molecular weight and strong penetrating force[23]. The scFv gene applied in this study is derived from monoclonal antibody T84.66, which has been humanized[16] and does not cause (HAMA) reaction in human body[24]. MMC, a broad spectrum anti-tumor drug, is nonspecific for cell cycle. However, it may depolymerize the DNA of cells and inhibit its replication, thus restraining the division of tumor cells[25]. The aim of this study was to construct a drug for target-oriented treatment of colorectal cancer, with MMC as the “warhead” and anti CEA svFv as the vehicle.
Taking plasmid pUC18 as template, we successfully amplified the CEA scFv gene, which is completely consistent with the reported gene sequence[16]. In order to make the fusion gene express effectively and prokaryotic cells express eukaryotic protein, we successfully constructed the GST fusion expressing vector (pGEX-4T-1/CEA-ScFv) and removed the repression effect of lac by IPTG, and made CEA scFv express highly effective in E.coli DH5α. The expressed fusion protein amounted to 26% of the total bacterial protein.
In this study, we successfully coupled anti-CEA scFv and MMC with dextran T-40 as a medium. The molecular ratio of scFv: dextran T-40: MMC in the conjugate was 1:1.2:38. Since the molecular weight of the antibody is only 49 ku, the conjugate could meet the requirements of antibody[26]. Measurement of immunoreactivity of the conjugate showed that it had a strong immunological activity against three kinds of colorectal cancer cells, among which immunoreactivity of the conjugate against LoVo cells was the strongest, which may be due to the high CEA expression in LoVo cells[27].
In the study, different concentrations of CEA-scFv-MMC had a different restraining effect on LoVo cells depending on the concentration. When the concentration was above 100 mg/L, it had an obvious restraining effect on the growth of LoVo cells. Apoptosis of LoVo cells began at the concentration of 25 mg/L, the apoptosis ratio increased with the increasing concentration of CEA-scFv-MMC. The optimal dose of CEA-scFv-MMC for inducing apoptosis was 200 mg/L. When time was studied as a variable, the apoptosis began 12 h after treatment of LoVo cells with CEA-scFv-MMC and reached its peak 72 h after CEA-scFv-MMC treatment, suggesting that apoptosis of LoVo cells induced by CEA-scFv-MMC is highly dependent on the concentration of CEA-scFv-MMC and its duration of action. However, a large dose or a long duration of CEA-scFv-MMC can result in cell necrosis, which may be due to the in vitro accumulation of cells undergoing apoptosis without pinocytosis of macrophages[28].
Taking the capacity of the protein yield into consideration, we did not remove GST from the GST/CEA-scFv fusion protein, but directly coupled the fusion protein with MMC. In in vitro experiment, GST had no effect on the immune activity of the conjugate and colorectal cancer cells. Further experiments are needed to demonstrate whether the conjugate can be applied in vivo. On the other hand, the reaction of the body and immunogenecity do not necessarily parallel the process of humanization[29]. Thus, the in vivo immunogenecity cannot be predicted .The next experiment will focus on more suitable expression vectors to make the expression of CEA-scFv more effectively. Animal experiments will be carried out to explore the target-oriented effect of CEA-scFv-MMC, immunogenecity of the conjugate and its therapeutic effect on colorectal cancer in vivo. Since only some kinds of colorectal cancer express CEA[30], this therapy would only be applied to such cancers.
In conclusion, CEA-scFv-MMC conjugate can be successfully constructed and restrains the growth of colorectal cancer cells and induces apoptosis of cancer cells in vitro.
The authors thank the teachers of the Virus Research Institute, Medical College, Wuhan University for their assistance with the experiments and Wuhan University Medical College for offering the scientific research fund.
Carcinoembryonic antigen (CEA) is present in a number of adenocarcinomas, especially in colorectal cancer. If a humanized anti-CEA single-chain antibody (scFv) can be coupled with a chemotherapeutics, the conjugate would be an ideal target-oriented medication for colorectal cancer.
An expression vector has been constructed to express the humanized anti-CEA scFv, with the protein coupled with mitomycin (MMC). The conjugate can restrain the growth of colorectal cancer cells and induce apoptosis of colorectal cancer cells in vitro.
Through restriction endonuclease cleavage and gene sequencing, the expression vector was successfully constructed. Applying SDS-PAGE and ELISA, we have confirmed that this vector can correctly express the fusion protein and the conjugate has quite a strong immunoreactivity against colorectal cancer cells and CEA. The conjugate has an inhibitory effect on colorectal cancer cells in a concentration-dependent manner, and induces apoptosis of colorectal cancer cells in a concentration-and time-dependent manner.
The conjugate may be a potential target-oriented medication for colorectal cancer expressing CEA.
CEA is a 180 ku cell-surface expressed glycoprotein antigen present in a number of adenocarcinomas, especially in colorectal cancer. It is a member of the immunoglobulin superfamily and has cell adhesion properties as well as other less clearly defined roles. scFv is an ideal vehicle for delivering chemotherapeutics, as it is easy for single-chain antibody to reach the tumor due to its small molecular weight and strong penetrating force. MMC is a broad spectrum anti-tumor medicine and nonspecific for cell cycle. However, it may depolymerize DNA of cells and inhibit its replication, thus restraining the division of tumor cells.
This paper reports the construction and in vitro effect of a humanized carcinoembryonic antigen specific single chain fragment mitomycin conjugate. The authors have demonstrated that CEA-scFv-MMC conjugate is able to inhibit the growth and induce the apoptosis of colorectal cancer cells.
S- Editor Liu Y L- Editor Wang XL E- Editor Li JL
| 1. | van Laarhoven HW, Punt CJ. Systemic treatment of advanced colorectal carcinoma. Eur J Gastroenterol Hepatol. 2004;16:283-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Krol M, Koopman M, Uyl-de Groot C, Punt CJ. A systematic review of economic analyses of pharmaceutical therapies for advanced colorectal cancer. Expert Opin Pharmacother. 2007;8:1313-1328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Punt CJ. New options and old dilemmas in the treatment of patients with advanced colorectal cancer. Ann Oncol. 2004;15:1453-1459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 87] [Article Influence: 4.4] [Reference Citation Analysis (1)] |
| 4. | Mulders P, Bleumer I, Oosterwijk E. Tumor antigens and markers in renal cell carcinoma. Urol Clin North Am. 2003;30:455-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Elfiky AA, Saif MW. The developing trend of monoclonal antibodies in the treatment of colorectal cancer. Expert Opin Biol Ther. 2007;7:871-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Herbst RS, Bajorin DF, Bleiberg H, Blum D, Hao D, Johnson BE, Ozols RF, Demetri GD, Ganz PA, Kris MG. Clinical Cancer Advances 2005: major research advances in cancer treatment, prevention, and screening--a report from the American Society of Clinical Oncology. J Clin Oncol. 2006;24:190-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 88] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 7. | Pfeiffer P, Qvortrup C, Eriksen JG. Current role of antibody therapy in patients with metastatic colorectal cancer. Oncogene. 2007;26:3661-3678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Takano T, Ohe Y, Sakamoto H, Tsuta K, Matsuno Y, Tateishi U, Yamamoto S, Nokihara H, Yamamoto N, Sekine I. Epidermal growth factor receptor gene mutations and increased copy numbers predict gefitinib sensitivity in patients with recurrent non-small-cell lung cancer. J Clin Oncol. 2005;23:6829-6837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 571] [Cited by in RCA: 590] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 9. | Guillem EB, Sampsel JW. Antitumor-associated antigens IgGs: dual positive and negative potential effects for cancer therapy. Adv Exp Med Biol. 2006;587:341-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Reichert JM, Valge-Archer VE. Development trends for monoclonal antibody cancer therapeutics. Nat Rev Drug Discov. 2007;6:349-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 272] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 11. | Gold P, Freedman SO. Demonstration of tumor-specific antigens in human colonic carcinomata by immunological tolerance and absorption techniques. J Exp Med. 1965;121:439-462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1572] [Cited by in RCA: 1544] [Article Influence: 53.2] [Reference Citation Analysis (0)] |
| 12. | Zimmermann W, Ortlieb B, Friedrich R, von Kleist S. Isolation and characterization of cDNA clones encoding the human carcinoembryonic antigen reveal a highly conserved repeating structure. Proc Natl Acad Sci USA. 1987;84:2960-2964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 77] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Shen LZ, Wu WX, Xu DH, Zheng ZC, Liu XY, Ding Q, Hua YB, Yao K. Specific CEA-producing colorectal carcinoma cell killing with recombinant adenoviral vector containing cytosine deaminase gene. World J Gastroenterol. 2002;8:270-275. [PubMed] |
| 14. | O'Dwyer PJ. The present and future of angiogenesis-directed treatments of colorectal cancer. Oncologist. 2006;11:992-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Vogel CA, Galmiche MC, Buchegger F. Radioimmunotherapy and fractionated radiotherapy of human colon cancer liver metastases in nude mice. Cancer Res. 1997;57:447-453. [PubMed] |
| 16. | Rodenburg CM, Mernaugh R, Bilbao G, Khazaeli MB. Production of a single chain anti-CEA antibody from the hybridoma cell line T84.66 using a modified colony-lift selection procedure to detect antigen-positive ScFv bacterial clones. Hybridoma. 1998;17:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Gladysheva IP, Polekhina OV, Karmakova TA, Nemtsova ER, Yakubovskaya RI, Shen WC, Kennedy AR, Larionova NI. Potential of block copolymer- and immuno-conjugates for tumor-targeted delivery of Bowman-Birk soybean proteinase inhibitor. J Control Release. 2001;74:303-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 18. | Balch GC, De Meo A, Guillem JG. Modern management of rectal cancer: a 2006 update. World J Gastroenterol. 2006;12:3186-3195. [PubMed] |
| 19. | Chung KY, Saltz LB. Antibody-based therapies for colorectal cancer. Oncologist. 2005;10:701-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Puleo S, Mauro L, Gagliano G, Lombardo R, Li Destri G, Petrillo G, Di Carlo I. Liver damage after transarterial chemoembolization without embolizing agent in unresectable hepatocellular carcinoma. Tumori. 2003;89:285-287. [PubMed] |
| 21. | Sharon J, Liebman MA, Williams BR. Recombinant polyclonal antibodies for cancer therapy. J Cell Biochem. 2005;96:305-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Ng EW, Adamis AP. Targeting angiogenesis, the underlying disorder in neovascular age-related macular degeneration. Can J Ophthalmol. 2005;40:352-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 193] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 23. | Batra SK, Jain M, Wittel UA, Chauhan SC, Colcher D. Pharmacokinetics and biodistribution of genetically engineered antibodies. Curr Opin Biotechnol. 2002;13:603-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 134] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 24. | Yazaki PJ, Sherman MA, Shively JE, Ikle D, Williams LE, Wong JY, Colcher D, Wu AM, Raubitschek AA. Humanization of the anti-CEA T84.66 antibody based on crystal structure data. Protein Eng Des Sel. 2004;17:481-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 53] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | Marinelli A, Vahrmeijer AL, van de Velde CJ. Phase I/II studies of isolated hepatic perfusion with mitomycin C or melphalan in patients with colorectal cancer hepatic metastases. Recent Results Cancer Res. 1998;147:83-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Carter P. Improving the efficacy of antibody-based cancer therapies. Nat Rev Cancer. 2001;1:118-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 614] [Cited by in RCA: 736] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 27. | Li Y, Chen Y, Dilley J, Arroyo T, Ko D, Working P, Yu DC. Carcinoembryonic antigen-producing cell-specific oncolytic adenovirus, OV798, for colorectal cancer therapy. Mol Cancer Ther. 2003;2:1003-1009. [PubMed] |
| 28. | Morand EF. New therapeutic target in inflammatory disease: macrophage migration inhibitory factor. Intern Med J. 2005;35:419-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 83] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 29. | Park TG, Jeong JH, Kim SW. Current status of polymeric gene delivery systems. Adv Drug Deliv Rev. 2006;58:467-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 782] [Cited by in RCA: 753] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 30. | Vallejo J, Torres-Avisbal M, Contreras P, Rodríguez-Liñán M, Rebollo A, González F, Benítez A, Infante J, Mateo A. CEA, CA 19.9 and CA 195 in patients with colorectal carcinoma. ROC analysis. Rev Esp Med Nucl. 1999;18:281-286. [PubMed] |