Published online Nov 21, 2007. doi: 10.3748/wjg.v13.i43.5736
Revised: August 25, 2007
Accepted: October 10, 2007
Published online: November 21, 2007
AIM: To study the predictive value of the vegetative-depressive symptoms of the Zung Depression Rating Scale for the occurrence of depression during treatment with peg-interferon α-2b of chronic hepatitis C (CHC) patients.
METHODS: The predictive value of vegetative-depressive symptoms at 4 wk of treatment for the occurrence of a subsequent diagnosis of major depressive disorder (MDD) was studied in CHC patients infected after substance use in a prospective, multi-center treatment trial in Belgium. The presence of vegetative-depressive symptoms was assessed using the Zung Scale before and 4 wk after the start of antiviral treatment.
RESULTS: Out of 49 eligible patients, 19 (39%) developed MDD. The area under the ROC curve of the vegetative Zung subscale was 0.73, P = 0.004. The sensitivity at a cut-point of > 15/35 was 95% (95% CI: 74-100). The positive predictive value equalled 44% (95% CI: 29-60).
CONCLUSION: In this group of Belgian CHC patients infected after substance use, antiviral treatment caused a considerable risk of depression. Seven vegetative-depressive symptoms of the Zung scale at wk 4 of treatment predicted 95% of all emerging depressions, at a price of 56% false positive test results.
- Citation: Robaeys G, De Bie J, Wichers MC, Bruckers L, Nevens F, Michielsen P, Van Ranst M, Buntinx F. Early prediction of major depression in chronic hepatitis C patients during peg-interferon α-2b treatment by assessment of vegetative-depressive symptoms after four weeks. World J Gastroenterol 2007; 13(43): 5736-5740
- URL: https://www.wjgnet.com/1007-9327/full/v13/i43/5736.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i43.5736
Following the recent expert consensus, patients with chronic hepatitis C (CHC) should now receive interferon (IFN) and ribavirin regardless of psychiatric status, except in uncontrolled psychiatric disease[1-3]. As 23%-44% of patients treated with IFN develop depressive symptoms during treatment[4,5] and since, more patients are treated, interferon-induced psychiatric illness is likely to become an increasingly important clinical problem[4,6-14].
IFN-induced depression appears to be a depressive disorder that is unusually responsive to antidepressant treatment. Approximately 80% of patients are responsive within 4 wk[15]. This high response rate may be related to the usually short duration of this illness when treatment is initiated, to the mild to moderate severity of illness in most cases[1], but also to the mainly interferon-induced influences on the central nervous system[16].
Based on the results of a small scale study (n = 16), it has been hypothesised that vegetative symptoms are early predictors for the emergence of full depression[9]. We wanted to confirm or refute this finding in a larger patient group of CHC positive substance users, classically considered as predisposed for depression[17].
Therefore, the predictive value of an increase in the score of the seven vegetative-depressive symptoms from the 20-item Zung Depression Rating Scale[18] was studied in a new study in substance users, using the emergence of a DSM-IV based major depression during treatment as the outcome measure.
Previously untreated patients, 18 years or older, with compensated chronic HCV infection acquired after substance use, with a serum alanine aminotransferase level above the upper limit of normal, and positive serum HCV RNA were eligible for the study.
HCV patients infected after substance use were defined as patients who were HCV-positive and who had used cocaine, heroin or LSD at least once. All of them were currently or previously cared for in multidisciplinary and specialised programs, according to criteria defined in the Belgian guidelines for the treatment of CHC patients infected after substance use (Table 1)[1]. Patients, who had co-infections such as hepatitis B virus or human immunodeficiency virus, or patients with a diagnosis of uncontrolled neurological, cardiovascular, endocrine, haematological, hepatic or renal disease or patients with insufficient knowledge of the Dutch or French language were excluded.
| Characteristics at baseline | n | Thrice in wk 3 MIU interferon | Weekly pegylated interferon | Total | Wilcoxon rank sum test or fishers exact test |
| Age at baseline (yr) | 49 | 37 | 37 | 37 | 0.99 |
| At exposure (yr) | 49 | 27 | 28 | 27 | 0.55 |
| At infection (yr) | 49 | 33 | 36 | 35 | 0.58 |
| Body mass index | 33 | 25 | 25 | 25 | 0.93 |
| Genotype (%) | |||||
| -non 2-3 | 25 | 73 | 36 | 53 | 0.02a |
| -5 | 22 | 27 | 64 | 47 | |
| Gender (%) | |||||
| F | 11 | 26 | 19 | 22 | 0.73 |
| M | 38 | 74 | 81 | 78 | |
| Methadone user (%) | 49 | 39 | 35 | 37 | 0.77 |
| Drug user active (%) | 48 | 59 | 54 | 56 | 0.77 |
| IVDU (%) | 49 | 91 | 92 | 92 | 1.00 |
| Activity1 | |||||
| A0 + 1 | 40 | 81 | 96 | 89 | 0.17 |
| A2 + 3 | 5 | 19 | 4 | 11 | |
| Fibrosis1 | |||||
| F0 + 1 | 20 | 38 | 50 | 44 | 0.19 |
| F2 + 3 | 22 | 48 | 50 | 49 | |
| -F4 | 3 | 14 | 0 | 7 | |
| Steatosis1 | |||||
| S0 | 20 | 40 | 52 | 47 | 0.73 |
| S1 | 12 | 30 | 26 | 28 | |
| S2 + 3 | 11 | 30 | 22 | 26 | |
| ASAT at baseline (IU/L) | 49 | 59 | 77 | 68 | 0.2098 |
| ALAT at baseline (IU/L) | 49 | 87 | 127 | 108 | 0.0635 |
The study was approved by the ethical review board of the Catholic University of Leuven Medical School and by the Medical Ethical Committees of each of the different centres, and carried out in accordance with the Declaration of Helsinki. Written informed consent was obtained from each subject prior to participation.
Patients were treated in a prospective, randomised, multi-center study in Belgium between November 2001 and February 2004 with pegylated interferon α-2b, 1.5 μg/kg weekly by subcutaneous injection in combination with ribavirin (administered orally, 1000-1200 mg/d, depending on body weight) or interferon α-2b PEN 3 MIU subcutaneously 3 times/wk in combination with ribavirin (administered orally, 1000-1200 mg/d, depending on body weight). The total duration of therapy for the two study medication arms was 48 wk if the patient was infected with genotype non-2-3 and 24 wk if the patient was infected with genotype 2-3. The patients were required to use effective birth control. A subgroup out of the study centers that collaborated to the study, also recorded the emergence of depression. In this group we systematically investigated the occurrence of depression during therapy in patients who were not already depressed at baseline.
A complete medical history, physical examination, laboratory blood test and liver biopsy were performed before study entry. A liver biopsy was performed before randomisation.
In total, 12 hepatologists in collaborating treatment centres (in whom 49 patients were treated) agreed to apply a 20-item Zung self-rating depression scale (SDS) at wk 0, 4 wk after start of treatment and after the treatment regimen was completed. Zung scale results could score between 25 (normal) and 100 points. A high score indicates a depressive attitude. A cut-off of equal to and higher than 60/100 was considered as indicative for diagnosable depression[18].
Cognitive-depressive and vegetative-depressive symptom dimensions were constructed as described earlier[19]. The vegetative-depressive symptoms (Diurnal variation, lack of sleep, loss of appetite, loss of sex drive, weight loss, constipation, fatigue) were scored from 1 to 4 (in the analysis multiplied with 5/4).
Clinical diagnosis of depression was confirmed by a qualified psychiatrist on the basis of DSM-IV criteria for major depression[20].
Quantitative measures are expressed as mean or as median. Qualitative variables are presented as counts and percentages.
The χ2 test statistic and logistic regression methodology were used to compare two patient subgroups with respect to categorical outcome measures. T-tests for independent samples and linear regression methodology were applied to compare subgroups for differences in means of continuous variables. Non-parametric test statistics such as the Wilcoxon Rank Sum test were used if normality was absent.
In identifying an optimal cut-point for the Zung-vegetative symptoms score, we searched for a maximal sensitivity with a positive predictive value of at least around 50%. We report the full ROC curve, the AUC and its confidence interval for the four-week vegetative subscale of the Zung and the AUC and the P-values for possible alternative measures.
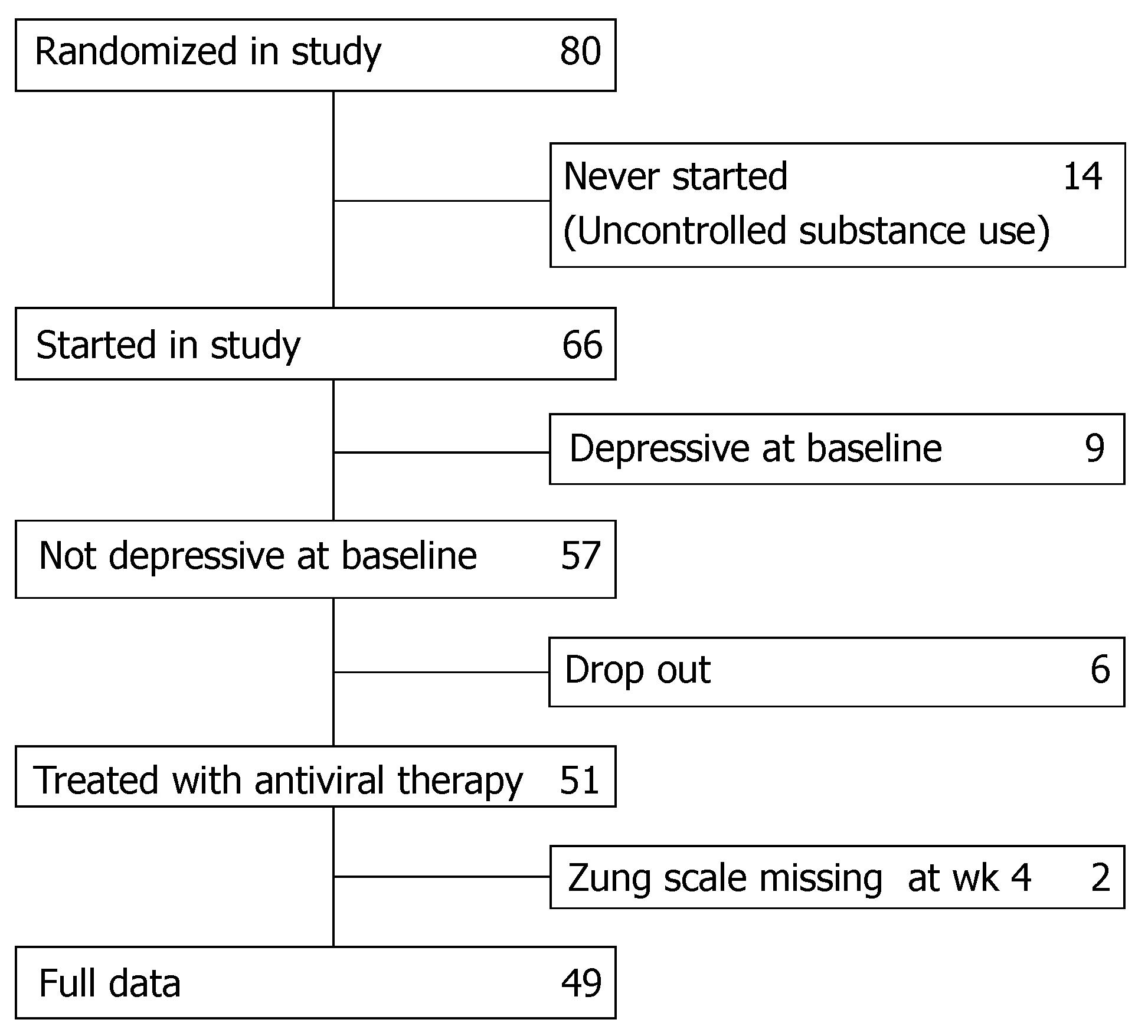
Eighty patients in whom chronic hepatitis C was diagnosed as acquired after substance use were randomised to treatment with either interferon α-2b or pegylated interferon α-2b combined with ribavirin and were followed by a hepatologist who accepted to apply the Zung rating scales. Fourteen patients never started the study because they were still consuming substances in an uncontrolled way. Nine had major depressive disorder (MDD) at baseline and were therefore excluded. For 8 patients data collection was incomplete. In total 49 eligible patients were included in this analysis (Figure 1).
Demographic characteristics and clinical data for all eligible patients not having depression at baseline (n = 49) are summarized in Table 1. There were no baseline differences between the two treatment groups, with the exception of HCV genotype distribution (P = 0.02) and alanine aminotransaminase (ALT) level (P = 0.06).
Nineteen patients became depressed during antiviral treatment. Median time to depression ranged from 4 to 26 wk and was distributed over this whole period with a mean of 10 wk.
At baseline there was no difference in total, vegetative or cognitive Zung score between patients who subsequently became depressed or not.
At wk 4 after treatment start the mean vegetative Zung scale was higher among the patients developing depression later on compared to other patients (P = 0.008). It decreased slightly from baseline to wk 4 (difference: -0.72; P = 0.49) for patients not getting depressed. Patients getting depressed increased on average 4.13 points on the vegetative Zung scale during the first four weeks of therapy (P = 0.03).
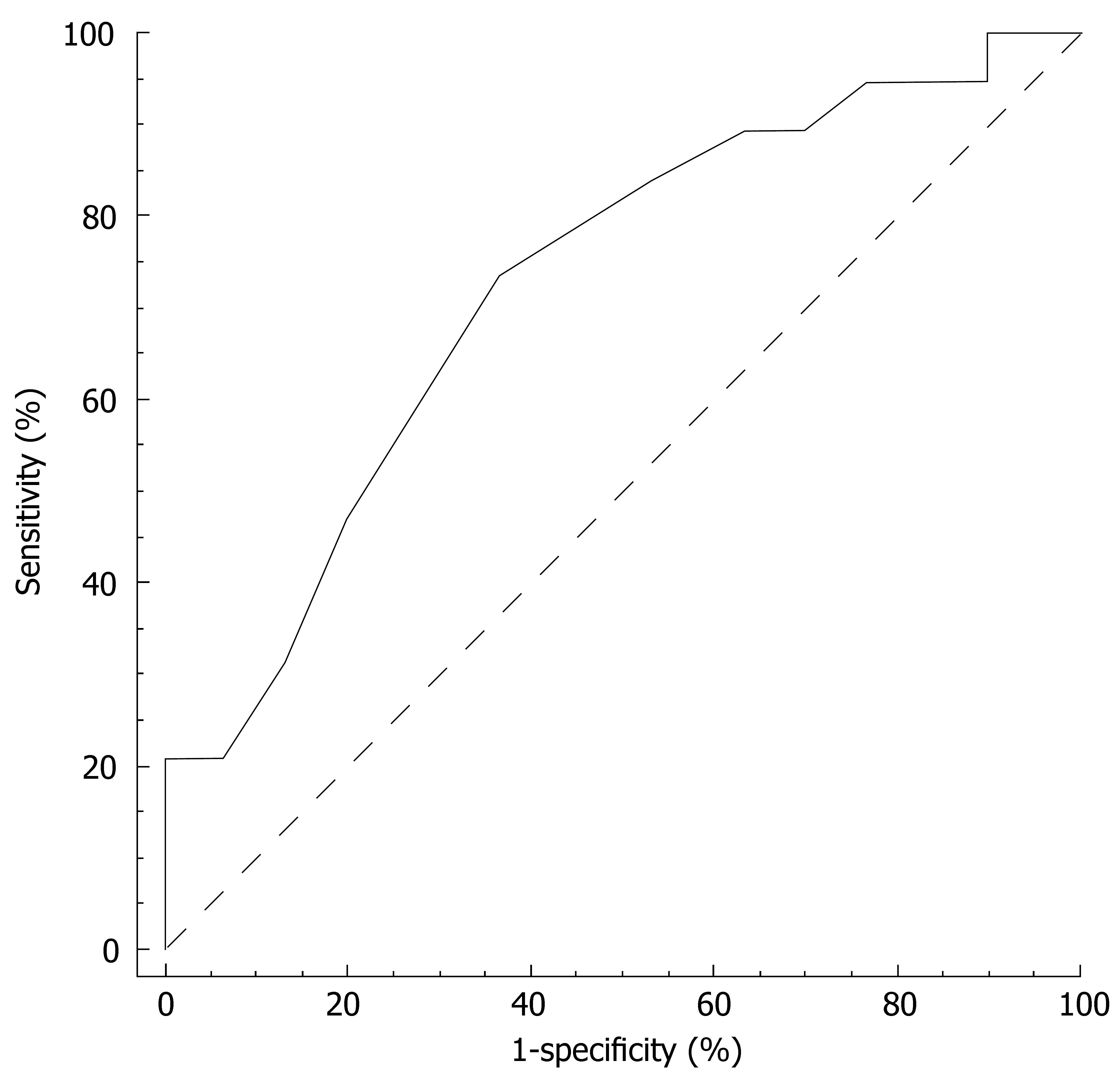
The ROC curve for the prediction of depression emerging during treatment by the vegetative subscale at wk 4 is reported in Figure 2. The area under the curve (AUC) was 0.73 (95% CI 0.58-0.84; P = 0.004). At a cut-off point of > 17/35, the sensitivity and specificity of the vegetative subscale at wk 4 were 90% and 30%, respectively. The positive and negative predictive value equalled 45% and 82%. For a sensitivity of 95% (95% CI 74-100) the vegetative subscale criterion was > 15 and the corresponding specificity 23% (95% CI 10-42), the positive predictive value 44% (95% CI 39-60) and negative predictive value 88% (95% CI 47-100).
The increased vegetative Zung scale was not merely a measure of depression at that moment, but predicted the occurrence of depression scattered over the weeks and months afterwards. Results were not different according to gender, age and genotype.
The change in vegetative symptoms between wk 0 and 4 was marginally predictive (AUC = 0.68; P = 0.06).
The cognitive subscale at wk 4 was not able to predict a clinical diagnosis of depression (AUC = 0.62; P = 0.15). The total Zung at wk 4 did only have marginal predictive power (AUC = 0.67; P = 0.05).
The early presence of vegetative symptoms seems to be predictive for the occurrence of depression later on. In a small scale prospective study (n = 16)[9] it was found that the increase in the vegetative subscale score of the Montgomery Asberg Depression Rating Scale (MADRS) or the Hamilton Depression Rating Scale scales after one week of antiviral treatment following the start of antiviral treatment in CHC patients was predictive for MDD. We could confirm these findings in this prospective study during treatment with regular interferon or pegylated interferon in combination with ribavirin. Our study was larger and we had a more vulnerable population resulting in a higher number of patients becoming depressed during treatment. In this study, we found that a cut-off point of > 15/35 at the vegetative subscales at wk 4 resulted in a sensitivity of 95% at an acceptable positive predictive value of 44%.
This high sensitivity was found using a questionnaire of 7 items, part of the 20-item Zung scale. A similar sensitivity was found using the Minnesota Multiphasic Personality Inventory[21]. However, this rating scale includes 566 items and is more elaborate to perform in a routine outpatient clinic.
There was no relation between a higher score or a significant increase in the cognitive subscale at wk 4 and a higher risk of subsequent clinical depression. This is in agreement with the hypothesis formulated in reference[9].
Also, the Zung depression rating scale as a whole was not predictive for the development of depression in substance use patients, at least after exclusion of the patients that were already depressed at baseline. In other studies the baseline value was found to be predictive for patients with the Minnesota Multiphasic Personality Inventory[22], the Hamilton Depression Rating Scale score (HDRSS)[23] and the Centre for Epidemiological Studies-Depression Scale, CES-D[24] higher than a certain cut-off level[21,25,26]. However, in these studies people that were depressed at baseline were not always excluded[25,26].
In our study, an increased vegetative subscale score at 4 wk of treatment predicted diagnosis of major depression scattered over the weeks and months afterwards. This scatter is comparable to results found in other recent studies[4,7-10,25].
Although depressive symptomatology occurring in patients treated with interferon has usually been called ‘depression’ or ‘major depressive episode’, the more appropriate term according to new DSM-IV-TR should be ‘substance induced mood disorder’. There are psychopathological (e.g., more irritability)[27], epidemiological (the gender difference in major depressive episode is different from the gender difference in interferon induced mood disorder)[28], genetic[29] and treatment (more patients than in major depressive episode respond well to SSRIs)[30] arguments to support this really to be a different disorder.
This study has some limitations. It was restricted to substance users with CHC infection and antiviral treatment. Therefore, it is not possible to extrapolate to other patient groups. However, from small studies there seems no difference in the development or the mechanism of depression in other patient groups[9,30]. Secondly, although larger than previous studies[9] our sample size is still relatively low resulting in large confidence intervals.
In conclusion, the development of depression can reliably be predicted at wk 4 after start of interferon treatment by an increase in vegetative symptoms of depression (subscale score) to at least 15/35. This results in a prediction with a sensitivity of 95%, at the price of 56% false-positive test results, which for a screening test is considered to be acceptable. Treatment with antidepressants may cure or prevent depression and so increase the treatment adherence. This may improve the SVR in patients evolving to depression and make the SVR comparable to SVR in non-depressed patients. To formally prove this, however, it should be the subject of another study.
Interferon derived treatment will remain the cornerstone for antiviral therapy in chronic hepatitis C patients in the near future. Interferon induced depression is a frequent side effect that may influence the outcome of the treatment. Since interferon-induced depression is unusually responsive to antidepressant treatment, it is important to detect the patients who are at risk for depression and who can benefit from antidepressive treatment.
At this moment very few tools have been extensively studied to detect the patients at risk of depression during treatment with interferon. The Minnesota Multiphasic Personality Inventory is thoroughly studied but questioning 566 items makes it very elaborate to perform in a routine outpatient clinic.
Using only seven questions at week four of treatment from the vegetative-depressive symptoms of the Zung scale, it is possible to predict 95% of all emerging depressions, at a price of 56% false positive test results.
Treatment with antidepressants may cure or prevent depression and so increase the treatment adherence. This may improve the SVR in patients evolving to depression and make the SVR comparable to SVR in non-depressed patients. To formally prove the hypothesis that antidepressants may improve SVR in patients at risk for depression, should be the subject of further studies.
Dr. Robaeys and co-workers-including the BASL Steering Committee and the Belgian Study Group: M Adler in Erasme University Hospital, Brussels; B Bastens in St Joseph Hospital, Liège; N Bourgeois, S Bourgeois in Ziekenhuis Netwerk Antwerpen (Stuivenberg), Antwerpen; R Brenard in St Joseph Hospital, Gilly; C Brixko in Hôpital de la Citadelle, Liège; Ph Caenepeel in Ziekenhuis Oost-Limburg, Genk; B Caucheteur in University Hospital St Pierre, Brussels; I Colle in University Hospital, Gent; C de Galocsy in Bracops Hospital, Brussels; J Delwaide in CHU Univertity of Liege, Liege; S Francque University Hospital Antwerpen, Antwerpen; J Henrion in Hôpital de Jolimont, Haine-Saint-Paul; J Holvoet in Ziekenhuis Netwerk Antwerpen (Middelheim), Antwerpen; Y Horsmans in Cliniques universitaires St-Luc UCL, Brussels; Ph Langlet in Clinique Edith Cavell, Brussels; P Laukens in St Jansziekenhuis, Brugge; V Lefebvre in CHR de Namur, Namur; H Louis1, P Michielsen10, JP Mulkay7, F Nevens in UZ Gasthuisberg KUL, Leuven; C Preux in Centre Hospitalier de Tivoli, La Louvière; G Robaeys6, H Van Vlierberghe8, Ph Warzée in Reine Fabiola Hospital, Montignies sur Sambre - have presented a clinical study in which they aimed to show the predictive value of vegetative-depressive symptoms according to the Zung depression rating for subsequent clinical depression of HCV infected patients during antiviral treatment with interferon and Ribavirin after 4 wk of treatment. The manuscript is well written and presents a very interesting clinical study showing convincing data.
S- Editor Zhu LH L- Editor Rippe RA E- Editor Lu W
| 1. | Robaeys G, Buntinx F, Bottieau E, Bourgeois S, Brenard R, Colle I, De Bie J, Matheï C, Mulkay JP, Van Damme P. Guidelines for the management of chronic hepatitis C in patients infected after substance use. Acta Gastroenterol Belg. 2005;68:38-45. [PubMed] |
| 2. | Sylvestre D. Hepatitis C treatment in drug users: perception versus evidence. Eur J Gastroenterol Hepatol. 2006;18:129-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Robaeys G, Van Vlierberghe H, Matheï C, Van Ranst M, Bruckers L, Buntinx F. Similar compliance and effect of treatment in chronic hepatitis C resulting from intravenous drug use in comparison with other infection causes. Eur J Gastroenterol Hepatol. 2006;18:159-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Horikawa N, Yamazaki T, Izumi N, Uchihara M. Incidence and clinical course of major depression in patients with chronic hepatitis type C undergoing interferon-alpha therapy: a prospective study. Gen Hosp Psychiatry. 2003;25:34-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 95] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 5. | Miyaoka H, Otsubo T, Kamijima K, Ishii M, Onuki M, Mitamura K. Depression from interferon therapy in patients with hepatitis C. Am J Psychiatry. 1999;156:1120. [PubMed] |
| 6. | Edlin BR, Seal KH, Lorvick J, Kral AH, Ciccarone DH, Moore LD, Lo B. Is it justifiable to withhold treatment for hepatitis C from illicit-drug users? N Engl J Med. 2001;345:211-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 149] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 7. | Dieperink E, Ho SB, Thuras P, Willenbring ML. A prospective study of neuropsychiatric symptoms associated with interferon-alpha-2b and ribavirin therapy for patients with chronic hepatitis C. Psychosomatics. 2003;44:104-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 176] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 8. | Hauser P, Khosla J, Aurora H, Laurin J, Kling MA, Hill J, Gulati M, Thornton AJ, Schultz RL, Valentine AD. A prospective study of the incidence and open-label treatment of interferon-induced major depressive disorder in patients with hepatitis C. Mol Psychiatry. 2002;7:942-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 227] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 9. | Wichers MC, Koek GH, Robaeys G, Praamstra AJ, Maes M. Early increase in vegetative symptoms predicts IFN-alpha-induced cognitive-depressive changes. Psychol Med. 2005;35:433-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 66] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Capuron L, Gumnick JF, Musselman DL, Lawson DH, Reemsnyder A, Nemeroff CB, Miller AH. Neurobehavioral effects of interferon-alpha in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology. 2002;26:643-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 551] [Cited by in RCA: 556] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 11. | Dalakas MC, Mock V, Hawkins MJ. Fatigue: definitions, mechanisms, and paradigms for study. Semin Oncol. 1998;25:48-53. [PubMed] |
| 12. | Dusheiko G. Side effects of alpha interferon in chronic hepatitis C. Hepatology. 1997;26:112S-121S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 280] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 13. | Licinio J, Kling MA, Hauser P. Cytokines and brain function: relevance to interferon-alpha-induced mood and cognitive changes. Semin Oncol. 1998;25:30-38. [PubMed] |
| 14. | Schaefer M, Schmidt F, Folwaczny C, Lorenz R, Martin G, Schindlbeck N, Heldwein W, Soyka M, Grunze H, Koenig A. Adherence and mental side effects during hepatitis C treatment with interferon alfa and ribavirin in psychiatric risk groups. Hepatology. 2003;37:443-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 234] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 15. | Kraus MR, Schäfer A, Faller H, Csef H, Scheurlen M. Paroxetine for the treatment of interferon-alpha-induced depression in chronic hepatitis C. Aliment Pharmacol Ther. 2002;16:1091-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 119] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 16. | Asnis GM, De La Garza R. Interferon-induced depression in chronic hepatitis C: a review of its prevalence, risk factors, biology, and treatment approaches. J Clin Gastroenterol. 2006;40:322-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 124] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 17. | De Bie J, Robaeys G, Buntinx F. Hepatitis C, interferon alpha and psychiatric co-morbidity in intravenous drug users (IVDU): guidelines for clinical practice. Acta Gastroenterol Belg. 2005;68:68-80. [PubMed] |
| 18. | Zung WW. A self-rating depression scale. Arch Gen Psychiatry. 1965;12:63-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5900] [Cited by in RCA: 6052] [Article Influence: 208.7] [Reference Citation Analysis (0)] |
| 19. | Miura H, Kitagami T, Ohta T. Application of the Zung self-rating depression scale to patients before and after introduction to haemodialysis. Psychiatry Clin Neurosci. 1999;53:381-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington DC, USA: Swets & Zeitlinger BV 1994; 210-216. |
| 21. | Scalori A, Pozzi M, Bellia V, Apale P, Santamaria G, Bordoni T, Redaelli A, Avolio A, Parravicini P, Pioltelli P. Interferon-induced depression: prevalence and management. Dig Liver Dis. 2005;37:102-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Mosticoni R, Chiari G. Una descrizione obiettiva della personalit`a. Il Minnesota Multphasic Personality Inventory (MMPI). Firenze, Italy: Organizzazioni Speciali. Rot Off Set 1993; 15–28. |
| 23. | Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6:278-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5785] [Cited by in RCA: 5844] [Article Influence: 100.8] [Reference Citation Analysis (0)] |
| 24. | Bono C, Ried LD, Kimberlin C, Vogel B. Missing data on the Center for Epidemiologic Studies Depression Scale: a comparison of 4 imputation techniques. Res Social Adm Pharm. 2007;3:1-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 99] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 25. | Beratis S, Katrivanou A, Georgiou S, Monastirli A, Pasmatzi E, Gourzis P, Tsambaos D. Major depression and risk of depressive symptomatology associated with short-term and low-dose interferon-alpha treatment. J Psychosom Res. 2005;58:15-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Guadagnino V, Trotta MP, Carioti J, Caroleo B, Antinori A. Does depression symptomatology affect medication compliance during the first weeks of anti-HCV therapy in intravenous drug users? Dig Liver Dis. 2006;38:119-124. [PubMed] |
| 27. | Constant A, Castera L, Dantzer R, Couzigou P, de Ledinghen V, Demotes-Mainard J, Henry C. Mood alterations during interferon-alfa therapy in patients with chronic hepatitis C: evidence for an overlap between manic/hypomanic and depressive symptoms. J Clin Psychiatry. 2005;66:1050-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 115] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 28. | Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB. Sex and depression in the National Comorbidity Survey. I: Lifetime prevalence, chronicity and recurrence. J Affect Disord. 1993;29:85-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1396] [Cited by in RCA: 1359] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 29. | Rifai MA. Implementing genomic research in Psychosomatic Medicine, studying vulnerability to depression in patients with hepatitis C receiving interferon-alpha and ribavirin. Psychosomatics. 2007;48:107-110. |
| 30. | Kraus MR, Schäfer A, Csef H, Scheurlen M. Psychiatric side effects of pegylated interferon alfa-2b as compared to conventional interferon alfa-2b in patients with chronic hepatitis C. World J Gastroenterol. 2005;11:1769-1774. [PubMed] |