Published online Nov 21, 2007. doi: 10.3748/wjg.v13.i43.5707
Revised: August 16, 2007
Accepted: October 12, 2007
Published online: November 21, 2007
AIM: To understand the interactions between iron and zinc during absorption in iron- and zinc-deficient rats, and their consequences on intestinal oxidant-antioxidant balance.
METHODS: Twenty-four weanling Wistar-Kyoto rats fed an iron- and zinc-deficient diet (< 6.5 mg Fe and 4.0 mg Zn/kg diet) for 4 wk were randomly divided into three groups (n = 8, each) and orally gavaged with 4 mg iron, 3.3 mg zinc, or 4 mg iron + 3.3 mg zinc for 2 wk. On the last day of repletion, 3 h before the animals were sacrificed, they received either 37 mBq of 55Fe or 65Zn, to study their localization in the intestine, using microautoradiography. Hemoglobin, iron and zinc content in plasma and liver were measured as indicators of iron and zinc status. Duodenal sections were used for immunochemical staining of ferritin and metallothionein. Duodenal homogenates (mitochondrial and cytosolic fractions), were used to assess aconitase activity, oxidative stress, functional integrity and the response of antioxidant enzymes.
RESULTS: Concurrent repletion of iron- and zinc-deficient rats showed reduced localization of these minerals compared to rats that were teated with iron or zinc alone; these data provide evidence for antagonistic interactions. This resulted in reduced formation of lipid and protein oxidation products and better functional integrity of the intestinal mucosa. Further, combined repletion lowered iron-associated aconitase activity and ferritin expression, but significantly elevated metallothionein and glutathione levels in the intestinal mucosa. The mechanism of interactions during combined supplementation and its subsequent effects appeared to be due to modulation of cytosolic aconitase, which in turn influenced the labile iron pool and metallothionein levels, and hence reduced intestinal oxidative damage.
CONCLUSION: Concurrent administration of iron and zinc corrects iron and zinc deficiency, and also reduces the intestinal oxidative damage associated with iron supplementation.
- Citation: Bodiga S, Krishnapillai MN. Concurrent repletion of iron and zinc reduces intestinal oxidative damage in iron- and zinc-deficient rats. World J Gastroenterol 2007; 13(43): 5707-5717
- URL: https://www.wjgnet.com/1007-9327/full/v13/i43/5707.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i43.5707
Iron-deficiency anemia is frequently the result of low intake of dietary iron, and high intake of phytate and tannins, which inhibit iron absorption. An iron-deficient population can generally have zinc deficiency, due to similar influences of various dietary factors on iron and zinc absorption[1]. There is strong evidence in humans that a blockage of zinc absorption is a consequence of daily intake of high amounts of iron[2]. Studies in pregnant women also provide evidence that the absorption of supplemental iron is lower from multimineral antenatal supplements, particularly in the presence of high calcium and magnesium than when it is administered alone[3,4]. It has therefore become evident during the past decade that deficiencies of iron and zinc co-exist, and those vulnerable groups may benefit from iron as well as zinc supplementation, rather than individual supplementation with these minerals. However, iron and zinc are known to interact, either at the site of absorption or post-absorptively, because of competition for similar transport pathways[5-8]. Moreover, these two minerals have opposing effects on oxidant-antioxidant balance in either iron- or zinc-deficient intestines[8,9]. As mineral supplementation programs are designed for populations at risk of both iron and zinc deficiencies, understanding of quantitative and qualitative aspects of the potential of the two metals is critical. Iron and zinc have been found to interact antagonistically at the site of absorption, and this interaction helps in reducing the iron-mediated generation of hydroxyl radicals and subsequent intestinal oxidative damage during repletion in a single mineral deficiency[8,9]. Other cascading effects of such interactions are the reduction of iron and zinc uptake, aconitase activity, and ferritin expression in the intestinal mucosa. Besides, zinc induction of metallothionein in the intestinal mucosa helps to restore its functional integrity[8]. However, absorption of these two minerals is regulated at the intestinal level[10,11], and hence the nature and extent of interactions may differ when the deficiencies co-exist, because of molecular adaptations that modulate the absorption of these minerals. Therefore, in the present study, we depleted iron and zinc in weanling rats and studied the nature of the interactions between two metals, and their consequences on intestinal oxidant-antioxidant balance; finally we postulated a probable mechanism.
This study employed a typical depletion-repletion design for understanding the potential interactions of iron and zinc during supplementation in iron- and zinc-deficient rats. The Institutional Animal Ethical and Bio-safety Committee of the National Institute of Nutrition approved the study.
Twenty-four weanling female Wistar-Kyoto rats, (National Center for Laboratory Animal Science at the National Institute of Nutrition, Hyderabad, India) weighing 35-45 g, were fed an egg-albumin-based, semi-synthetic, purified iron- and zinc-deficient diet (< 6.5 mg Fe and 4.0 mg Zn/kg diet) for 4 wk (Table 1). Rats were housed individually in polypropylene cages with stainless steel wire floors (45 cm × 16 cm, 7.5 mm mesh, 1 mm wire diameter) to prevent coprophagy, in a room maintained at 23°C and 60% humidity, with a 12-h light: dark cycle. Deionized distilled water, in plastic bottles with stainless steel sippers, was freely available to all rats. Body weight was recorded weekly and blood was collected by orbital sinus puncture for determination of iron and zinc status at the end of depletion and repletion.
At the end of depletion phase, rats were randomly divided into three groups and assigned to either Fe, Zn and Fe + Zn groups for repletion (n = 8, each). In order to achieve complete repletion of both the minerals, while minimizing the intestinal oxidative damage, we used a dose of 4 mg iron and 3.3 mg zinc for repletion. Force feeding was performed daily during the repletion period, either with a dose of 4.0 mg iron (Fe group), 3.3 mg zinc (Zn group) or a combination of 4.0 mg iron and 3.3 mg zinc (Fe + Zn group) in 1.0 mL 0.01 mol/L HCl, via an orogastric plastic tube, for 2 wk[12]. During this period, the rats were fed an iron- and zinc-deficient diet ad libitum. The doses were prepared by dissolving FeSO4 and ZnSO4 salts, either individually or in combination, in dilute HCl[8]. Administration of 0.01 mol/L HCl alone to the rats had no significant effect on the parameters studied[12]. Therefore, vehicle controls were not included in the present study. All the rats were fasted for 16 h before administering the last dose for uptake studies and for studying various other parameters.
Three rats, each from the Fe and Zn repleted groups, along with six rats from the Fe + Zn repleted group (three each for 55Fe and 65Zn) were randomly selected. Fe group rats received 4.0 mg Fe with 37 mBq of 55Fe (specific activity, 46.9 mCi/g), Zn group rats received 3.3 mg Zn with 37 mBq of 65Zn (specific activity, 1.14 Ci/g), and the Fe + Zn group rats received 4.0 mg Fe and 3.3 mg Zn with either 37 mBq of 55Fe (n = 3) or 37 mBq of 65Zn (n = 3) (BRIT, Mumbai, India). The rats were killed after collecting blood. Proximal 10-cm portion of the duodenum was excised for mucosa collection. Samples of liver were collected from the rats for iron and zinc determination by atomic absorption spectrophotometry.
The tissues were placed immediately on ice, trimmed of excess fat and mesentery, and weighed. The intestinal segments were flushed with ice-cold saline. Of the 10-cm portion, the first 2.0 cm was used for studying iron and zinc uptake, and localization of their responsive proteins, while the remaining portion was saved and processed for obtaining mucosal homogenates. The 2.0-cm portion was placed in 10% neutral-buffered formalin for 12 h, washed in PBS for 24 h, using a "Swiss roll" technique[13] to evaluate the entire longitudinal section on one slide and embedded in paraffin at 58°C. Multiple serial sections of 4-μm thickness were obtained from these blocks. These sections were dewaxed, defatted, mounted on chrome alum-gelatin coated slides, and dip-coated with Amersham LM1 photographic emulsion (Amersham, UK). These were then exposed for 30 d in a light-proof desiccator at 4°C, before developing and fixing in Kodak D-19 developer and Kodak Rapid fix (Kodak, Rochester NY), respectively. Sections were stained with Mayer's hematoxylin and eosin, and then examined using a Nikon Microfot-FX microscope (Nikon, Japan) at 100 × magnification, and photographed. In separate serial sections, localization of ferritin and metallothionein was probed using immunohistochemistry. Briefly, dewaxed and defatted sections were incubated overnight with a 1:500 dilution of rabbit anti-rat liver ferritin[12], or 1:50 dilution of metallothionein antibody (Clone E9; Dako Corp, Carpinteria, CA), or with IgG from non-immune rabbit serum. The specific binding was detected by biotinylated goat anti-rabbit IgG and avidin-biotin peroxidase complex (Sigma, St. Louis, MO) using hydrogen peroxide and diaminobenzidine. The endogenous peroxidase was quenched with 0.3% hydrogen peroxide in 100% methanol for 15 min. The sections were counterstained with hematoxylin and eosin and observed by light microscopy at 250 × magnification, and photographed.
We used the remaining 7-cm intestinal segment from the radioisotope-treated animals and the whole segment from non radioisotope-treated animals, for preparation of intestinal homogenates. All the animals were sufficiently repleted for 2 wk. However, radioisotope-treated animals received an additional dose of iron and/or zinc at the end of repletion. This single additional dose is unlikely to contribute much to the nutrient status compared to animals that have not received radioisotope.
The duodenal segments collected from each rat in ice-cold 1.15% KCl were scraped and a 10% homogenate of the mucosal suspension was prepared in 1.15% KCl containing 0.5 mmol/L butylated hydroxy tolune (to prevent ex vivo peroxidation) as described previously[12]. The homogenate was subjected to differential centrifugation (Sorvall RC-5B, Du Pont Instruments, CT) at 800 g for 30 min, 12000 g for 30 min, and 100000 g for 1 h at 4°C, and the respective supernatants were collected and stored at -20°C until further analysis. The protein content in these supernatants was determined using a modified Lowry method, with BSA as the standard.
Iron and zinc concentrations in the diet, liver, mineral solutions and plasma were determined by atomic absorption spectrophotometry using a SpectrAA-400 atomic absorption spectrometer (Varian, Sunnyvale, Melbourne, Australia). The mineral deficient diet fed to the rats was digested with concentrated nitric acid and 70% perchloric acid by method (II) A of the Analytical Methods Committee (1960)[14]. One g of liver tissue was ashed in a muffle furnace at 600°C for 6 h and was used to prepare the mineral solution.
Activity of alkaline phosphatase and lys-ala-dipeptidyl aminopeptidase was measured in the 12000 g supernatant to assess the functional integrity of the intestinal mucosa, because of the differential localization of these two enzymes within the mucosal cells: the maximum activity of the former towards the upper half of the villus, and that of the latter towards the lower half[8,12]. Alkaline phosphatase activity was determined using β-glycerophosphate and lys-ala-dipeptidyl aminopeptidase activity was measured using lys-ala-7-amido-4-methyl coumarin.
The amount of thiobarbituric acid-reactive substance (TBARS) in the 12000 g supernatant was measured using malondialdehyde as a standard, and was quantified, using a reverse-phase silica-based C18 column, according to Templar et al[15]. Elution was done for 10 min at a flow rate of 1.2 mL/min with 65% 50 mmol/L KH2PO4-KOH, pH 7.0, and 35% methanol buffer, while monitoring at 532 nm. Protein carbonyls were estimated by incubation of 1 mL of the 100000 g supernatant with 10 mmol/L dinitrophenyl hydrazine (DNPH) for 1 h in the dark, followed by protein precipitation with trichloroacetic acid according to the method of Reznick & Packer[16]. The final DNPH and lipid-free precipitate was dissolved in 6 mol/L guanidine hydrochloride and the absorbance was read at 375 nm. The amount of protein in the final pellet was quantified at 280 nm using BSA in guanidine hydrochloride as a standard.
Activity of total superoxide dismutase (SOD), glutathione peroxidase (100000 g supernatant), Mn-SOD and catalase (12000 g supernatant) was determined as previously described[17-19]. Glutathione (reduced GSH and oxidized GSSH) levels were determined using HPLC equipped with a fluorimetric detector (excitation at 350 nm and emission at 420 nm), according to Pastore et al[20]. Briefly, thiols were extracted using sulfosalicylic acid and injected onto a 150 mm × 4.6 mm Hypersil ODS column, equilibrated with 30 mmol/L ammonium nitrate and 40 mmol/L ammonium formate buffer, pH 3.6. For GSH determinations, NaBH4 was substituted with 2 mmol/L EDTA-DTT. The thiols were eluted from the column with a 6-min gradient of acetonitrile (0-4 min, 0%-30% acetonitrile; 4-5 min, 30%-100% acetonitrile; 5-6 min, 100% acetonitrile) at a flow rate of 1.5 mL/min.
The activity of aconitase in cytosolic (100000 g supernatant) and mitochondrial (12000 g pellet) was measured by a coupled isocitrate dehydrogenase reaction, monitored at 37°C (Cary 100 Bio UV-Vis spectrophotometer, Varian Inc., CA) at 340 nm for 1 h [21,22].
Statistical analysis was performed using SPSS/PC+, version 5.0 (SPSS, Chicago, IL, USA). Comparison among the three groups was tested by one-way ANOVA with post-hoc multiple comparison tests. For measurements of body weight and iron and zinc status, comparisons were made with littermates maintained in the animal colony. The values were considered significantly different at P < 0.05.
Rats fed an iron- and zinc-deficient diet for 4 wk weighed 57% less than their littermates in the animal colony (62.0 ± 8.0 g and 108.2 ± 7.5 g, respectively). However, the final bodyweight of Fe-, Zn- and Fe + Zn-repleted rats was similar, but weighed 50% of their littermates (95.6 ± 10.2 g and 190.0 ± 14.8 g).
Iron and zinc depletion were confirmed by lowered hemoglobin and plasma zinc concentrations compared to their littermates in the animal colony, which were fed an iron- and zinc-adequate diet (35.0 mg Fe and 30.0 mg Zn/kg diet). Concurrent depletion of iron and zinc resulted in reduction of hemoglobin to 58%, plasma iron to 86% and plasma zinc to 38% compared to littermates. A significant lowering in all the above measured variables indicated the co-existence of iron and zinc deficiency in the experimental rats (Table 2). On repletion, hemoglobin and plasma and liver iron concentrations were significantly higher in the Fe and Fe + Zn groups compared to the Zn group (Table 2). Among the iron-repleted groups, iron status was significantly higher in the Fe than in the Fe + Zn group. Similarly, plasma and liver zinc concentrations were significantly higher in the Zn and Fe + Zn groups than in the Fe group. However, the Fe + Zn group showed significantly lower zinc status compared to the Zn group (Table 2).
| Group | Hemoglobin (g/L) | Iron | Zinc | ||
| Plasma (μmol/L) | Liver (μg/g) | Plasma (μmol/L) | Liver (μg/g) | ||
| -Fe - Zn | 82.0a± 3.2 | 65.0a± 4.6 | - | 12.3a± 2.0 | - |
| Fe | 126.9b± 3.0 | 94.8b± 7.2 | 192.8a± 12.5 | 13.0a± 1.8 | 22.8a± 4.5 |
| Zn | 80.0a± 2.8 | 64.0a± 5.5 | 43.7b± 6.6 | 29.6b± 3.0 | 48.6b± 4.6 |
| Fe + Zn | 106.8c± 3.0 | 82.4c± 3.8 | 157.5c± 7.7 | 20.5c± 3.2 | 37.1c± 4.2 |
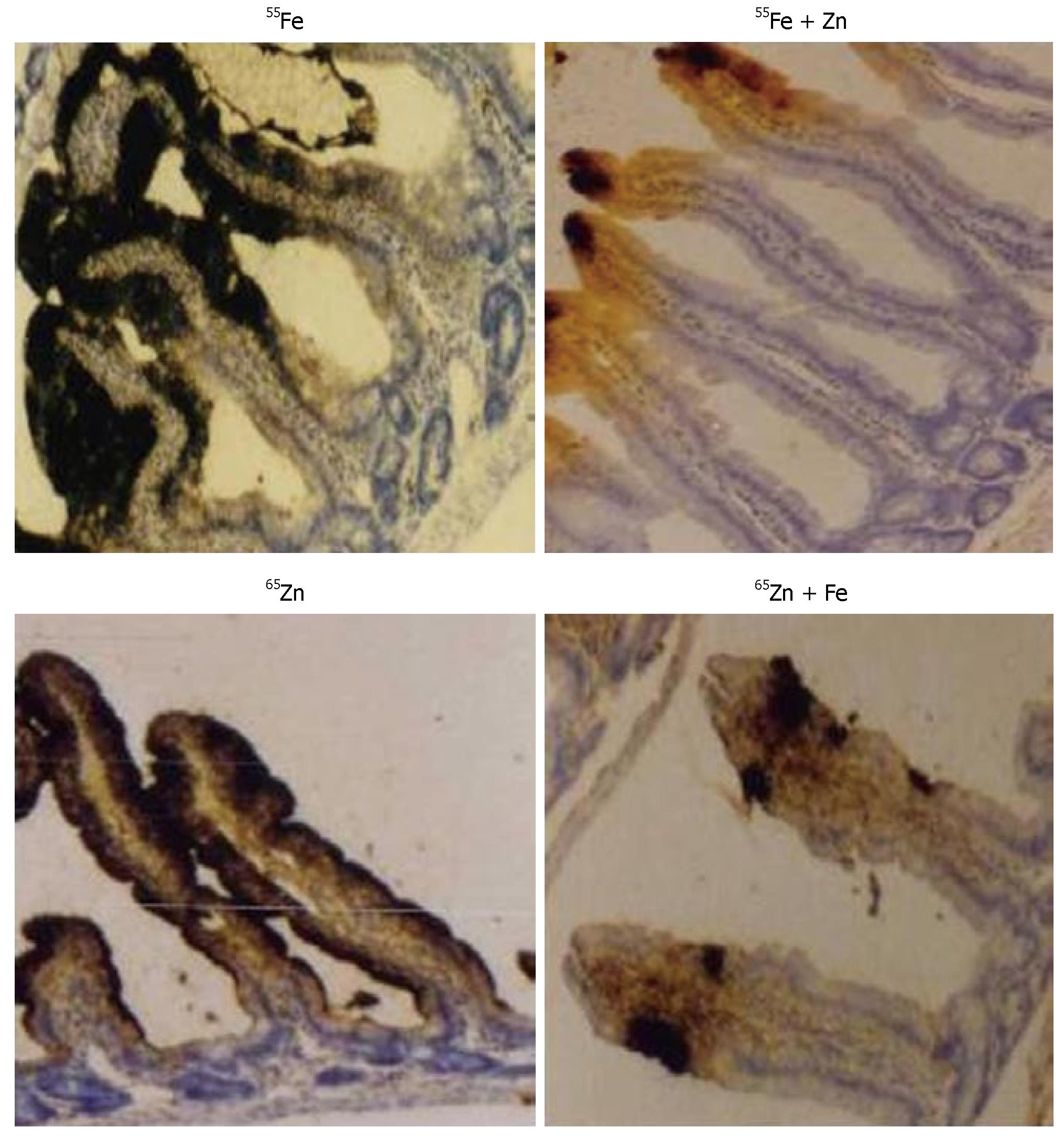
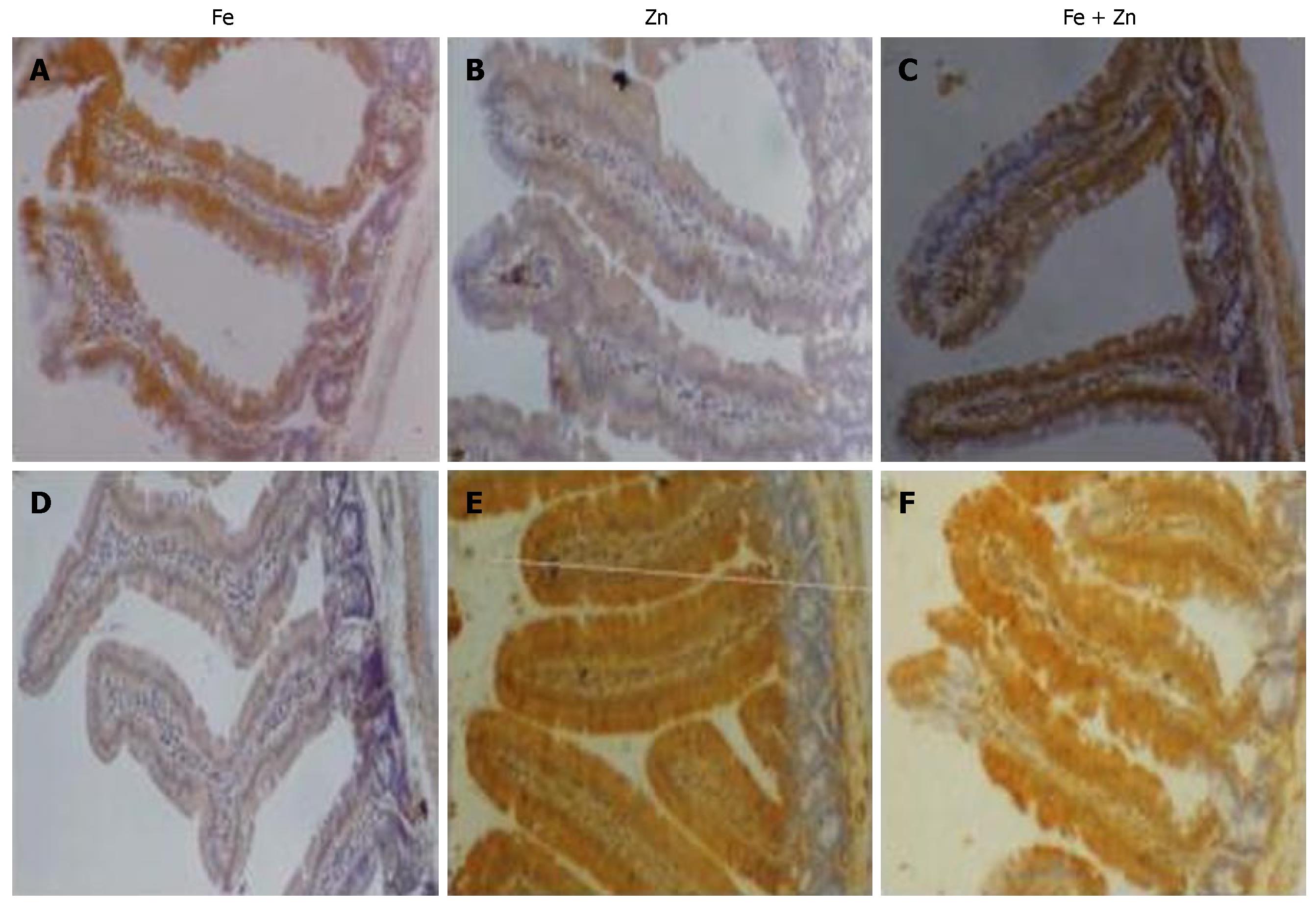
To understand the effect of interactions following concurrent repletion of iron and zinc to depleted rats, we looked for their presence in the site of absorption. Uptake of 55Fe and 65Zn at the end of repletion in the intestinal mucosa was reduced in Fe + Zn-supplemented rats compared with Fe- or Zn-supplemented rats, respectively (Figure 1). Intestinal ferritin concentration was higher in the Fe than in the Fe + Zn group (Figure 2, upper panel). Identically, metallothionein concentration in the intestinal mucosa was high and appeared similar in the Zn and Fe + Zn groups, but relatively weak in the Fe group (Figure 2, Lower panel).
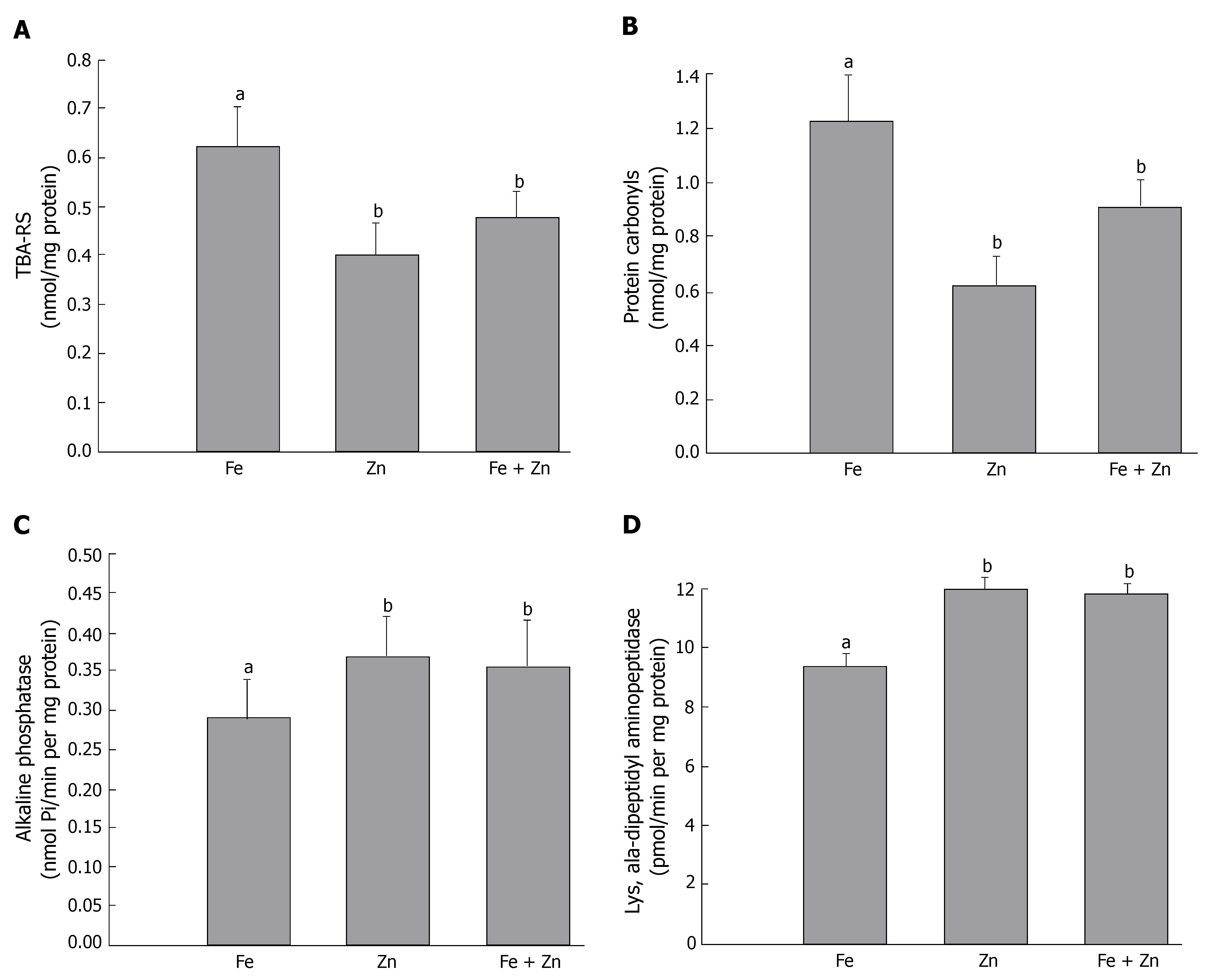
Interactions between iron and zinc and their functional relevance to intestinal oxidative stress were assessed by as TBARS and protein carbonyls. TBARS and protein carbonyl concentrations were 1.5-fold higher in the Fe than in the Fe + Zn group. These indices did not differ in the Zn and Fe + Zn groups (Figure 3A and B). Activity of the marker enzymes alkaline phosphatase and lys-ala-dipeptidyl aminopeptidase in the intestinal mucosa was about 20% lower in the Fe than in the Fe + Zn group. The activity did not differ between the Zn and Fe + Zn groups (Figure 3C and D).
Activity of intestinal SOD and glutathione peroxidase, except Mn-SOD, was significantly higher in the Fe than in the Zn and Fe + Zn groups. Catalase activity was significantly higher in the Fe and Fe + Zn groups compared to the Zn group. These enzyme activities did not differ between the Zn and Fe + Zn groups (Figure 4A-D), except for catalase activity. Glutathione concentration was significantly lower in the Fe than in the Zn and Fe + Zn groups. On the other hand, oxidized glutathione (GSSG) concentration was significantly higher in the Fe than in the Zn and Fe + Zn groups (Figure 5A and B).
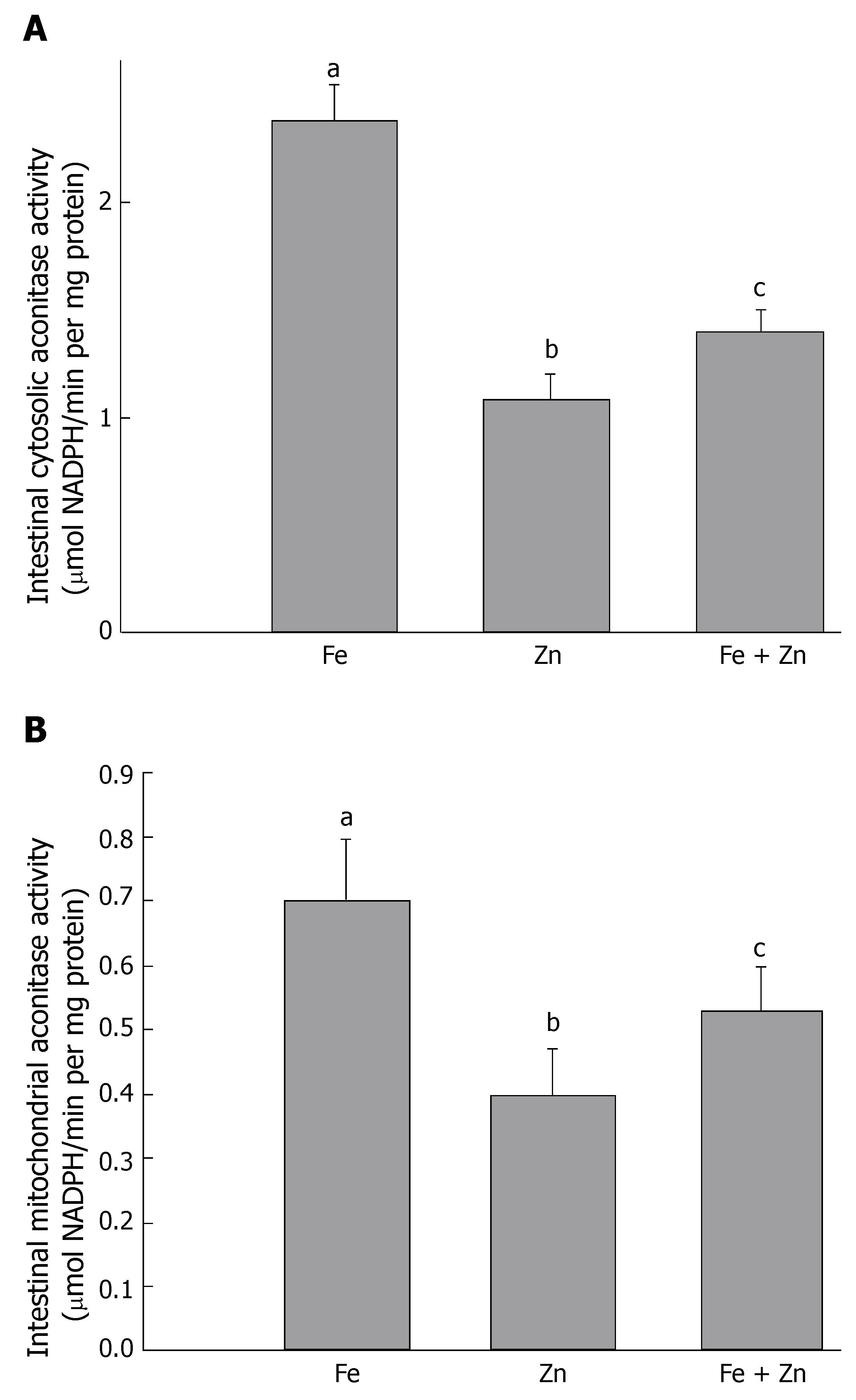
Activity of cytosolic and mitochondrial aconitase was measured as an indicator of the cellular labile iron pool. Activity was higher in the Fe group, but lowered significantly in the Zn and Fe + Zn groups. Activity in the Fe + Zn group was higher than that in the Zn group (Figure 6A and B).
In the light of high prevalence of anemia, emerging incidence of zinc deficiency, and interactions during supplementation, we studied the interactive effects of iron and zinc repletion on oxidant/antioxidant status in combined deficient rats. Metabolic studies and supplementation trials suggest an antagonistic relationship between iron and zinc, in which zinc reduces the positive effects of iron supplementation and vice versa. For example, inorganic iron was found to compete for absorption with zinc when given to adults in solution in ratios > 2:1[2]. Zinc absorption in fasting pregnant Peruvian women treated with Fe or Fe + Zn was significantly reduced compared with non-metals supplemented women[23]; which indicates a post-absorptive effect of iron on zinc absorption. In women treated only with Fe, plasma zinc concentrations was also lower, compared with controls. Conversely, there were smaller improvements in hemoglobin and serum ferritin concentrations in Indonesian children treated both with Fe and Zn than in children treated with Fe alone[24]. Two recent studies on iron and zinc supplementation of Indonesian infants show that iron and zinc interactions occur when they are given as supplements[25,26]. In the study by Dijkhuizen et al, infants were treated with iron alone (10 mg/d), zinc alone (10 mg/d), both elements together (10 + 10 mg/d) and placebo from 4 to 10 mo of age. Supplementation significantly reduces the prevalence of iron deficiency anemia and zinc deficiency. Iron supplementation does not negatively affect plasma zinc concentration, and zinc supplementation does not increase the prevalence of anemia. However, combined iron and zinc supplementation is less efficacious than iron supplementation alone in reducing the prevalence of anemia (20% vs 38% reduction) and in increasing hemoglobin and plasma ferritin concentrations. In the study by Lind et al, infants received the same treatments, but from 6 to 12 mo of age. After supplementation, the Fe group had higher hemoglobin and serum ferritin than the Fe + Zn group; this fact indicates an effect of zinc on iron absorption. The Zn group had higher serum zinc than the placebo group, whereas this was not the case for the Fe and Fe + Zn groups, this fact suggests an effect of iron on zinc absorption. Thus, supplementation with Fe + Zn is less efficacious than single supplements alone in improving iron and zinc status, with evidence of negative interactions between iron and zinc when a combined supplement is given. However, the crucial question is whether such interactions between iron and zinc have any functional consequences for intestinal oxidative stress and functional integrity.
Both these nutrients are essential for growth, and in populations in which deficiencies of these minerals co-exist, stunting of growth has been reported[27]. Depletion of both iron and zinc in the diet has a significant impact on the growth of rats, and causes a marked reduction in iron and zinc status. Thus, the model developed in the present study had characteristic clinical and sub-clinical manifestations of iron and zinc deficiency, respectively. Although several studies have examined the effect of iron supplementation on zinc absorption, few have considered the effect of zinc supplementation on iron absorption. The molar ratio of iron and zinc seems to be critical in determining the interactive effects. One study has shown that zinc inhibited iron absorption when the zinc-iron ratio was 1.14:1 (1:1 molar ratio), but not when it was 0.36:1 (0.4:1 molar ratio)[28]. Another study has shown that a zinc-iron ratio of 5:1 significantly reduced iron absorption from an aqueous solution, but did not affect heme iron absorption from a hamburger meal in humans[29]. In the present study, we used a 1:1 molar ratio for repletion of iron- and zinc-deficient rats.
Several possible sites of interaction during transfer from the apical membrane into the intestinal cytosolic compartment, and from the basolateral membrane into plasma have been suggested by Fairweather-Tait[30]. The possibility of iron and zinc inhibiting each other’s intestinal uptake through competition for a common pathway, has been studied by measuring ferritin and metallothionein concentrations, and aconitase activity in the site of absorption. Intestinal uptake of iron and zinc was significantly reduced in the presence of the other metal during repletion in rats with combined deficiencies of iron and zinc. This may have been due to increased competition between iron and zinc for the ligands/transporters at the site of absorption. Reduced uptake of 55Fe or 65Zn in the Fe + Zn group compared to the Fe or Zn groups provides clear evidence for their antagonistic interaction during repletion. Reduced plasma and liver iron and zinc concentrations in the Fe + Zn group compared to individual supplementation groups suggests that iron and zinc interactions affect not only the uptake, but also retention of these minerals. Thus, this study clearly demonstrates that a combined supplement is less efficacious than a single supplement in improving iron or zinc status.
Another intriguing possible site of interaction between iron and zinc is the duodenal transport protein divalent cation transporter 1 (DCT-1). DCT-1 appears to be a key transporter that is involved in iron absorption, but can also transport many other metals, including zinc[31]. It is possible that iron and zinc can inhibit each other’s absorption by competing for DCT-1, and their effects are expected to be most noticeable when one metal is in relative excess compared with the other, or when both deficiencies co-exist. Although we have not studied DCT-1 expression, the influence of iron and zinc on their responsive proteins clearly suggests that both interact at the site of absorption during concurrent repletion.
The intestine is vulnerable to oxidative damage during iron and/or zinc depletion and repletion. Incessant pulses of iron during repletion leave excess unabsorbed iron in the intestinal mucosa, which is a potential pro-oxidant. Moreover, electron paramagnetic resonance spectroscopy in rats has shown that oral iron therapy with ferrous sulfate results in iron-mediated oxidative stress, through hydroxyl radicals in the small intestine. This stress-stimuli results in a decrease in cell turnover, shortening of microvillus height, and partial or complete erosion of the microvilli in the duodenum[32-34]. On the other hand, zinc deficiency impairs intestinal antioxidant capacity by lowering the expression of metallothionein, an effective scavenger of hydroxyl radicals that can play a major role in the development of oxidative damage. In an earlier study, we have shown increased hydroxyl radical production, associated decrease in turnover of intestinal epithelial cells, and compromised functional integrity of the mucosa, when iron-deficient rats were repleted with 8.0 mg Fe[12]. This dose may be relatively high and could have produced structural and functional impairment at the site of absorption. To reduce these effects, we used 4.0 mg Fe for repletion and maintained a 1:1 molar ratio of iron and zinc in the present study. Iron repletion resulted in significantly higher levels of peroxidation products, i.e., TBARS and protein carbonyls in the intestinal mucosa, even with lower doses of iron. This also decreased the alkaline phosphatase and dipeptidase activity, which indicates compromised functional integrity. Although zinc negatively affected iron uptake, concurrent repletion of iron and zinc significantly reduced the oxidative damage and improved the functional integrity. The reason for the beneficial effects with co-administration of iron and zinc may be due to the changes in antioxidant balance. We have observed increased localization of metallothionein in the intestinal mucosa during zinc repletion. These findings support the view that zinc per se can act at various levels and exert its antioxidant effect. Incorporation of zinc along with iron in supplements seems to be efficacious in improving the antioxidant capacity.
To determine the role of antioxidant defense enzymes in reducing iron-induced oxidative damage, activity of SOD, catalase and glutathione peroxidase were determined. Cu, Zn-SOD was more active in the Fe group compared to that in the Zn and Fe + Zn groups. On the other hand, Mn-SOD activity was similar in all three groups. This indicates that the formation of superoxide anion and its conversion to hydrogen peroxide is greater in the cytosolic compartment than in the mitochondria. The other antioxidant enzyme that showed a difference in the Fe and Zn + Fe groups was glutathione peroxidase (Gpx), which implies that reduction of peroxides to water was more prevalent in the Fe than in the Fe + Zn group. No increase was observed in the catalase activity of iron-treated animals, which suggests that hydrogen peroxide conversion to water was similar among all groups, but was compensated by an increase in Gpx in the Fe group. This increase in Gpx is indicative of excess formation of organic peroxides in the Fe group, which may have enhanced oxidative stress. In a recent study, we have shown that zinc per se can reduce iron-mediated production of hydroxyl radicals and thereby protect against oxidative stress[8]. Hence, concurrent administration of iron and zinc or zinc alone substantially enhances the intestinal non-enzymatic antioxidant capacity. In addition, we have observed in zinc-supplemented groups, increased intestinal metallothionein concentrations, which has been reported to be more effective than GSH on a molar basis for preventing oxidative damage of various cellular components[35]. Quenching of hydroxyl radicals by sulfhydryl groups in metallothionein, releasing zinc and its subsequent uptake by the membranes can protect both cellular components and membranes against oxidative damage[36,37]. Thus, zinc-induced metallothionein not only reduces the oxidative stress, but also improves the functional integrity of the mucosa.
Control of iron uptake and storage through regulation of transferrin receptor and ferritin proteins represents an important avenue through which cellular iron homeostasis is modulated and maintained[38,39]. The expression of ferritin and transferrin receptor is linked to iron status through the action of two iron-regulated RNA-binding proteins, iron regulatory proteins, IRP1 and IRP2. Iron regulates the RNA-binding function of IRP1 and IRP2 through fundamentally different mechanisms[40,41]. For IRP1, which is a bifunctional protein, iron inhibits RNA-binding activity by promoting assembly of an iron-sulfur cluster in the binding protein, thereby converting it to cytosolic aconitase[42]. Walter et al[43] has suggested that iron deficiency can induce the IRP-mediated cellular iron signaling pathway, which leads to enhanced intracellular iron levels. Therefore, we assessed the activity of cytosolic and mitochondrial aconitase. Cytosolic aconitase activity was found to be suppressed in the presence of zinc in the Fe + Zn group compared to that in the Fe group. A decrease in cytosolic aconitase activity implies a decrease in cellular iron availability in the presence of zinc, or inhibition of aconitase per se by zinc. It has been shown that zinc competitively inhibits duodenal cytosolic aconitase activity[22]. Reduced ferritin protein in the intestinal mucosa of the Fe + Zn group when compared to that of the Fe group supports the decrease in cytosolic aconitase activity in the Fe + Zn group. We also observed a decrease in mitochondrial aconitase activity in the Fe + Zn compared to the Fe group. This may have been due to a decrease in citrate availability or post-transcriptional regulation by IRP1, as reported by Chen et al[44]. Nevertheless, zinc seems to act as a buffer against free-radical damage invoked by the presence of free iron. The presence of zinc during supplementation had a significant effect on the levels of various antioxidants, including GSH and metallothionein.
Thus, this study clearly demonstrates that the interactions between iron and zinc during absorption in iron- and zinc-deficient rats are mutually antagonistic. Competition of iron and zinc for common transporters (DMT1) at the site of absorption results in reduced uptake of these minerals during concurrent administration. The presence of zinc during iron administration also influences aconitase activity, ferritin expression and thereby the labile iron pool. Furthermore, zinc induction of metallothionein and scavenging of hydroxyl radicals helps to control iron-mediated oxidative stress.
Iron deficiency is the single most common nutritional disorder worldwide and the main cause of anemia in infancy, childhood and pregnancy. It is prevalent in most of the developing world, where it coexists with other micronutrient deficiencies such as zinc, vitamin A and folate. The exact prevalence of zinc deficiency is not known, but it is estimated that the magnitude might not be too different from that for iron deficiency. This is probably because the diet of populations in the developing world is based mainly on foodstuffs that have low iron and zinc concentrations, and with a poor bioavailability of these minerals. Combined supplementation with iron and zinc in target populations may be effective in preventing deficiencies of these micronutrients, but knowledge of their potential interactions when given together is inadequate.
Combined supplementation with both micronutrients is one strategy that can be used to improve the iron and zinc status of a population. However, there is concern about the negative interactions between these two minerals. Studies performed in humans have shown an inhibitory effect of zinc on iron absorption, but it is not well established whether this interaction depends on the absolute amount of iron and zinc in the supplement and/or on the molar ratio between these two minerals, or on nutritional status. This information could help design rational guidelines for iron and zinc supplementation programs. A review of the randomized trials that have assessed the effects of iron and zinc supplementation on iron and zinc status shows that zinc supplementation alone does not appear to have a clinically important negative effect on iron status. However, when zinc is given with iron, iron indicators do not improve as greatly as when iron is given alone. In most of the studies, iron supplementation did not affect the biochemical status of zinc, but the data are not clear regarding morbidity outcomes. Although some trials have shown that joint iron and zinc supplementation has less effect on biochemical or functional outcomes than supplementation with either mineral alone, there is no strong evidence to discourage joint supplementation. Supplementation programs that provide iron and zinc together are an efficient way to provide both micronutrients, provided the benefits of individual supplementation are not lost. Further research is needed before health policies on joint supplementation programs can be established.
Oral iron supplementation is a widely used practice to correct iron-deficiency anemia. Exposure of iron-deficient intestine to large doses of iron is known to induce oxidative damage, which leads to loss of functional integrity and reduced mucosal cell turnover. Intestinal conditioning with anti-oxidants during iron administration has been shown to suppress iron-induced oxidative damage. Zinc is known to protect cells from peroxidative damage by inducing metallothionein and maintaining sulfhydryl group stability. Nevertheless, co-administration of iron and zinc may antagonize each other with respect to absorption. In the present study, we showed that although combined supplementation of iron and zinc marginally inhibits iron uptake, it significantly attenuates oxidative stress by induction of metallothionein and elevation of GSH level. Furthermore, the presence of zinc in situ reduced the iron-induced hydroxyl radical production in the intestinal mucosa, as assessed by electron paramagnetic resonance spectroscopy. These results strongly suggest a protective role for zinc on iron-induced oxidative stress, which might have implications in anemia control programs.
Supplementation with multiple micronutrients is an appealing strategy for the prevention and treatment of anemia and common morbidities that affect women and young children. However, drawing definitive conclusions regarding the potential benefit or harm of joint supplementation, based on a variety of study designs, target populations and outcome measures, has proven challenging.
Nutrient-nutrient interactions: Although the term interaction denotes a bidirectional effect, many interactions are unidirectional, i.e., one nutrient affects the biological disposition of another, which remains more or less passive. Bidirectional interactions are most common among nutrients with similar physicochemical properties and that share a common mechanism of absorption or metabolism. Some uni- or bidirectional interactions are affected by the presence of a third dietary constituent. Nutrient interactions are not usually additive. From the physiological standpoint, nutrient interactions can occur at several different levels: (1) In the diet. The mode of preparation of diets may be as important as their composition in determining nutrient interactions. For example, cooking in an alkaline medium may decrease the interaction between ascorbic acid and iron by destroying the vitamin; (2) In the intestinal lumen. Interactions at this level have received the most attention, because they determine the true availability of a nutrient for translocation through the enterocytes. Most luminal interactions consist of direct nutrient-nutrient interactions, but certain nutrients can indirectly affect the absorption of others by modifying gastrointestinal physiological activities. For example, certain dietary fibers can stimulate gastrointestinal hormone secretion or inhibit micellar formation, thus indirectly affecting nutrient absorption (Table 2); (3) In the post-absorptive phase. Many interactions take place after the process of absorption has been completed. These interactions may be in the form of physiological synergism, such as the effect of vitamin A and zinc on the visual process, or between vitamin A and iron mobilization. Conversely, negative interactions may affect circulating or storage levels of nutrients.
In this study, the authors investigated the nature of interactions between iron and zinc, and their consequences on intestinal oxidant-antioxidant balance. They demonstrated that the interactions between iron and zinc during absorption in iron- and zinc-deficient rats were antagonistic. They demonstrated that the antioxidant status was significantly lower in the Fe than in the Zn and Fe + Zn groups, whereas the aconitase activity was higher in the Fe than in the Zn and Fe + Zn groups. In conclusion, they reported that the competition of iron and zinc for common transporters such as DCT-1, at the site of absorption, resulted in reduced uptake of Fe and Zn during concurrent administration. Moreover, they suggested that zinc induction of metallothionein and scavenging of hydroxyl radicals assisted in controlling iron-mediated oxidative stress.
S- Editor Liu Y L- Editor Kerr C E- Editor Li HY
| 2. | Solomons NW. Competitive interaction of iron and zinc in the diet: consequences for human nutrition. J Nutr. 1986;116:927-935. [PubMed] |
| 3. | Seligman PA, Caskey JH, Frazier JL, Zucker RM, Podell ER, Allen RH. Measurements of iron absorption from prenatal multivitamin--mineral supplements. Obstet Gynecol. 1983;61:356-362. [PubMed] |
| 4. | Babior BM, Peters WA, Briden PM, Cetrulo CL. Pregnant women's absorption of iron from prenatal supplements. J Reprod Med. 1985;30:355-357. [PubMed] |
| 5. | Solomons NW, Ruz M. Zinc and iron interaction: Concepts and perspectives in the developing world. Nutr Res. 1997;17:177-189. [RCA] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 44] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Isfaoun A, Bureau F, Mouly-Boudey M, Drosdowsky M, Arhan P, Bouglé D. Relationships between iron and zinc metabolism: predictive value of digestive absorption on tissue storage. J Trace Elem Med Biol. 1997;11:23-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Bouglé D, Isfaoun A, Bureau F, Neuville D, Jauzac P, Arhan P. Long-term effects of iron: zinc interactions on growth in rats. Biol Trace Elem Res. 1999;67:37-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Sreedhar B, Subramaniyan R, Nair KM. A protective role for zinc on intestinal peroxidative damage during oral iron repletion. Biochem Biophys Res Commun. 2004;318:992-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Sreedhar B, Nair KM. Modulation of aconitase, metallothionein, and oxidative stress in zinc-deficient rat intestine during zinc and iron repletion. Free Radic Biol Med. 2005;39:999-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Lönnerdal B. Trace element nutrition in infants. Annu Rev Nutr. 1989;9:109-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Lombard M, Chua E, O'Toole P. Regulation of intestinal non-haem iron absorption. Gut. 1997;40:435-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 60] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Srigiridhar K, Nair KM. Iron-deficient intestine is more susceptible to peroxidative damage during iron supplementation in rats. Free Radic Biol Med. 1998;25:660-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Moolenbeek C, Ruitenberg EJ. The "Swiss roll": a simple technique for histological studies of the rodent intestine. Lab Anim. 1981;15:57-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 316] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 14. | Analytical Methods Committee Methods of destruction of organic matter. Analyst. 1960;85:643-656. [RCA] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 102] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Templar J, Kon SP, Milligan TP, Newman DJ, Raftery MJ. Increased plasma malondialdehyde levels in glomerular disease as determined by a fully validated HPLC method. Nephrol Dial Transplant. 1999;14:946-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 112] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 16. | Reznick AZ, Packer L. Oxidative damage to proteins: spectrophotometric method for carbonyl assay. Methods Enzymol. 1994;233:357-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1645] [Cited by in RCA: 1739] [Article Influence: 56.1] [Reference Citation Analysis (0)] |
| 17. | Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6497] [Cited by in RCA: 6574] [Article Influence: 128.9] [Reference Citation Analysis (1)] |
| 18. | Aebi HE. Catalase. Methods in Enzymatic analysis [Vol.III]. Weinheim, Germany: Verlag Chemiel 1983; 273-282 [Book chapter]. |
| 19. | Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158-169. [PubMed] |
| 20. | Pastore A, Massoud R, Motti C, Lo Russo A, Fucci G, Cortese C, Federici G. Fully automated assay for total homocysteine, cysteine, cysteinylglycine, glutathione, cysteamine, and 2-mercaptopropionylglycine in plasma and urine. Clin Chem. 1998;44:825-832. [PubMed] |
| 21. | Rose IA, O'Connell EL. Mechanism of aconitase action. I. The hydrogen transfer reaction. J Biol Chem. 1967;242:1870-1879. [PubMed] |
| 22. | Sreedhar B, Nair KM. Iron dependence and zinc inhibition of duodenal cytosolic aconitase of rat. Indian J Biochem Biophys. 2004;41:250-253. [PubMed] |
| 23. | O'Brien KO, Zavaleta N, Caulfield LE, Wen J, Abrams SA. Prenatal iron supplements impair zinc absorption in pregnant Peruvian women. J Nutr. 2000;130:2251-2255. [PubMed] |
| 24. | Schultink W, Merzenich M, Gross R, Shrimpton R, Dillon D. Effects of iron zinc supplementation on the iron, zinc, and vitamin A status of anaemic preschool children in Indonesia. Food Nutr Bull. 1997;18:311-317. |
| 25. | Dijkhuizen MA, Wieringa FT, West CE, Martuti S. Effects of iron and zinc supplementation in Indonesian infants on micronutrient status and growth. J Nutr. 2001;131:2860-2865. [PubMed] |
| 26. | Lind T, Lönnerdal B, Stenlund H, Gamayanti IL, Ismail D, Seswandhana R, Persson LA. A community-based randomized controlled trial of iron and zinc supplementation in Indonesian infants: effects on growth and development. Am J Clin Nutr. 2004;80:729-736. [PubMed] |
| 27. | Rosado JL. Separate and joint effects of micronutrient deficiencies on linear growth. J Nutr. 1999;129:531S-533S. [PubMed] |
| 28. | Crofton RW, Gvozdanovic D, Gvozdanovic S, Khin CC, Brunt PW, Mowat NA, Aggett PJ. Inorganic zinc and the intestinal absorption of ferrous iron. Am J Clin Nutr. 1989;50:141-144. [PubMed] |
| 29. | Rossander-Hultén L, Brune M, Sandström B, Lönnerdal B, Hallberg L. Competitive inhibition of iron absorption by manganese and zinc in humans. Am J Clin Nutr. 1991;54:152-156. [PubMed] |
| 30. | Fairweather-Tait SJ. Iron-zinc and calcium-Fe interactions in relation to Zn and Fe absorption. Proc Nutr Soc. 1995;54:465-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 31. | McMahon RJ, Cousins RJ. Regulation of the zinc transporter ZnT-1 by dietary zinc. Proc Natl Acad Sci USA. 1998;95:4841-4846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 231] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 32. | Srigiridhar K, Nair KM. Protective effects of antioxidant enzymes and GSH in vivo on iron mediated lipid peroxidation in gastrointestinal tract of rat. Indian J Biochem Biophys. 1997;34:402-405. [PubMed] |
| 33. | Srigiridhar K, Nair KM. Supplementation with alpha-tocopherol or a combination of alpha-tocopherol and ascorbic acid protects the gastrointestinal tract of iron-deficient rats against iron-induced oxidative damage during iron repletion. Br J Nutr. 2000;84:165-173. [PubMed] |
| 34. | Srigiridhar K, Nair KM, Subramanian R, Singotamu L. Oral repletion of iron induces free radical mediated alterations in the gastrointestinal tract of rat. Mol Cell Biochem. 2001;219:91-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 35. | Abel J, de Ruiter N. Inhibition of hydroxyl-radical-generated DNA degradation by metallothionein. Toxicol Lett. 1989;47:191-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 160] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 36. | Thornalley PJ, Vasák M. Possible role for metallothionein in protection against radiation-induced oxidative stress. Kinetics and mechanism of its reaction with superoxide and hydroxyl radicals. Biochim Biophys Acta. 1985;827:36-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 737] [Cited by in RCA: 716] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 37. | Thomas JP, Bachowski GJ, Girotti AW. Inhibition of cell membrane lipid peroxidation by cadmium- and zinc-metallothioneins. Biochim Biophys Acta. 1986;884:448-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 158] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 38. | Theil EC. Iron regulatory elements (IREs): a family of mRNA non-coding sequences. Biochem J. 1994;304:1-11. [PubMed] |
| 39. | Eisenstein RS, Kennedy MC, Beinert H. In: Silver S, Walden W, editors. Metal Ions in Gene Regulation. New York: Chapman and Hall, Inc 1997; 157-216. |
| 40. | Guo B, Phillips JD, Yu Y, Leibold EA. Iron regulates the intracellular degradation of iron regulatory protein 2 by the proteasome. J Biol Chem. 1995;270:21645-21651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 230] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 41. | Henderson BR, Kühn LC. Differential modulation of the RNA-binding proteins IRP-1 and IRP-2 in response to iron. IRP-2 inactivation requires translation of another protein. J Biol Chem. 1995;270:20509-20515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 78] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 42. | Kennedy MC, Mende-Mueller L, Blondin GA, Beinert H. Purification and characterization of cytosolic aconitase from beef liver and its relationship to the iron-responsive element binding protein. Proc Natl Acad Sci USA. 1992;89:11730-11734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 250] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 43. | Walter PB, Knutson MD, Paler-Martinez A, Lee S, Xu Y, Viteri FE, Ames BN. Iron deficiency and iron excess damage mitochondria and mitochondrial DNA in rats. Proc Natl Acad Sci USA. 2002;99:2264-2269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 256] [Article Influence: 11.1] [Reference Citation Analysis (0)] |