Published online Sep 14, 2007. doi: 10.3748/wjg.v13.i34.4641
Revised: June 2, 2007
Accepted: June 9, 2007
Published online: September 14, 2007
AIM: To investigate if the nucleoside analogue lamivudine (LAM), a potent inhibitor of HBV replication, could restore the function of dendritic cells derived from patients with chronic hepatitis B (CHB) in an Asian population.
METHODS: Dendritic cells (DCs) derived from mononuclearcytes of patients with chronic HBV infection were cultured in the presence of IL-4, granulocyte-macrophage colony-stimulating factors (GM-CSF) and gradient concentrations of LAM (0-2 mmol/L). Cell morphology was observed under light microscopy. Cell surface molecules, including HLA-DR, CD80, CD83, and CD1α, were analyzed with flow cytometry. The concentrations of IL-6 and IL-12 in the supernatant were assayed by ELISA. T cell proliferation was assayed by methyl thiazolyl tetrazolium (MTT).
RESULTS: The expression of CD1α on DC treated with 0.5 mmol/L LAM (LAM-DC 0.5 mmol/L) was significantly higher than that of DC untreated with LAM (54.1 ± 4.21 vs 33.57 ± 3.14, P < 0.05), and so was the expression of CD83 (20.24 ± 2.51 vs 12.83 ± 2.12, P < 0.05) as well as the expression of HLA-DR (74.5 ± 5.16 vs 52.8 ± 2.51, P < 0.05). Compared with control group, LAM-DC group (0.5 mmol/L) secreted significantly more IL-12 (910 ± 91.5 vs 268 ± 34.3 pg/mL, P < 0.05), had lower levels of IL-6 in the culture supernatant (28 ± 2.6 vs 55 ± 7.36 pg/mL, P < 0.05), markedly enhanced the stimulatory capacity in the allogeneic mixed leukocyte reaction (MLR) (1.87 ± 0.6 vs 1.24 ± 0.51, P < 0.05).
CONCLUSION: The lower expression of phenotypic molecules and impaired allogeneic mixed lymphocyte reaction function of dendritic cells derived from patients with HBV infection could be restored in vitro by incubation with LAM.
- Citation: Zheng PY, Zhang DY, Lu GF, Yang PC, Qi YM, Wang BS. Effects of lamivudine on the function of dendritic cells derived from patients with chronic hepatitis B virus infection. World J Gastroenterol 2007; 13(34): 4641-4645
- URL: https://www.wjgnet.com/1007-9327/full/v13/i34/4641.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i34.4641
More than 300 million people worldwide are chronically infected with hepatitis B virus (HBV), and the majority of them are in Asia, especially in China. One of the important mechanisms resulting in the immune tolerance by chronic hepatitis B (CHB) is the impaired function of dendritic cells (DCs) derived from patients with CHB[1]. DCs are antigen-presenting cells (APC) responsible for initiating immunity and play an important role in the induction of antiviral immune responses[2]. Recently there has been considerable interest in using DCs as adjuvants to enhance host immunity against vial infection[3].
Lamivudine (LAM), a nucleoside analogue, is a potent and selective inhibitor of HBV replication. LAM specifically inhibits hepadnaviral DNA polymerase by competing with the corresponding dNTPs for incorporation into nascent DNA acting as a chain terminator after incorporation[4]. In addition to anti-HBV replication, the potential role of LAM in the regulation of immune response in HBV patients has been noticed recently. LAM treatment can restore T cell responsiveness in chronic hepatitis B[5]. Furthermore, it has been reported that LAM could up-regulate major histocompatibility complex class II (MHC-II) expression and restore impaired allostimulatory function of mononuclear cell-derived DC in the Caucasian patients with HBV infection[6]. In China, the influence of LAM on function of DC has not been investigated. We, therefore, HBsAg-pulsed DCs derived from the mononuclear cells of patients with chronic HBV infection with different LAM concentrations (0-2 mmol/L) in vitro and observed whether LAM could enhance DCs function. We attempted to explore a new approach to combine LAM and dendritic cell-based immunotherapy for CHB infection.
rhGM-CSF, rhIL-4, mouse anti-human HLA-DR-PE, CD80-FITC, CD1α-FITC, CD83-PE were purchased from BioLegend. RPMI-1640 was purchased from GIBCO (USA), and fetal calf serum (FCS) from Hangzhou Sijiqing Biological Engineering, China. Ficoll-Hypaque density gradient separate solution was purchased from Tianjin Jinmai Gene Biotechnology Co., China. rhIL-6, IL-12 ELISA Kit (Peprotech) were purchased from Shanghai Shenxiong Technology Company, China. LAM was purchased from GlaxoSmithKline Company.
Fifteen outpatients with HBeAg-positive CHB infection participated in this study. Written informed consent was obtained from all the patients and the study protocol was approved by the Ethics Committee of Zhengzhou University.
Peripheral blood mononuclear cells (PBMCs) were prepared from CHB patients. Briefly, PBMCs were washed and resuspended at 2 × 106 cells/mL. The cells were cultured in RPMI 1640 media containing 100 mL/L autologous serum, 100 U/mL penicillin, 100 μg/mL streptomycin. After incubation for 2 h at 37°C, non-adherent cells were removed; the adherent cells were cultured with RPMI 1640 media with recombinant GM-CSF and IL-4 in a 24-well culture plate at 37°C in a humidified atmosphere containing 50 mL/L CO2. Mononuclear cells were assigned to two groups on d 3: LAM-treated group (experimental group), and non-LAM-treated group (control group). The experimental group was further divided into subgroups according to the different concentrations (0.125, 0.25, 0.5, 1, 2 mmol/L) of LAM. Half of the medium was replaced with fresh medium every other day. DCs were harvested on the 8th d.
DCs were observed on an inverted microscope dynamically, and the phenotypes of DCs, such as CD1a, CD80, CD83 and HLA-DR, were analyzed by FACS on the 8th day. FITC-labelled mouse anti-human CD1α, CD80 monoclonal antibody (mAb) and phycoerythrin-conjugated mouse anti-human CD83, HLA-DR mAb were used. Staining was performed as described previously[7].
To clarify whether the antigen-presenting capacity of LAM-treated DCs (LAM-DC) was different from that of non-LAM-treated DCs (non-LAM-DC), mononuclear cells were isolated from the peripheral blood of healthy subjects. After incubation for 2 h, the non-adherent cells were collected as lymphocyte cells. DCs were collected as stimulator cells at a concentration of 1 × 105/mL and added into a 96-well culture plate. DCs were incubated with mitomycin C (50 μg/mL) for 30 min, and then lymphocytes were added as responder cells at a concentration of 1 × 106/mL; each group was set to triple wells. After being cultured for 3 d, absorbance at 570 nm (A570) was assayed by MTT and the stimulator index (SI) was calculated using a formula: SI = Aexperiment/(Aresponder cells + Astimulator cells).
Levels of IL-12 and IL-6 in the supernatant of DC were determined using an enzyme-linked immunosorbent assay (ELISA) kit following the manufacturer’s instructions. Samples of each group were set to triple wells.
Data were analyzed with SPSS10.0 statistical software. The significant difference between groups was determined by one-way ANOVA test. Two-sided P < 0.05 was considered statistically significant.
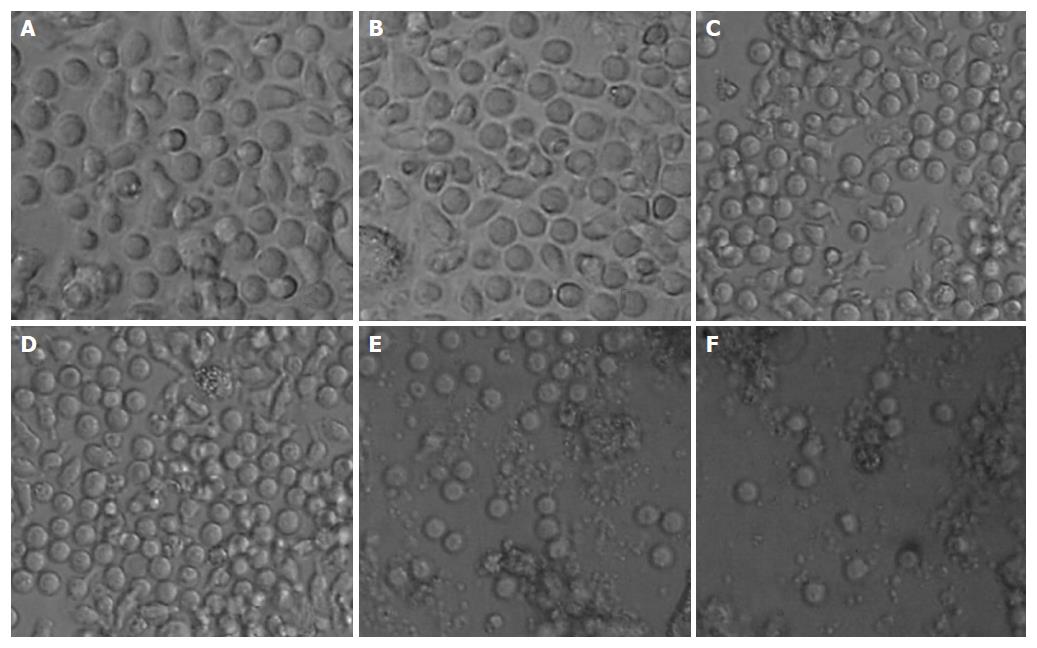
After being cultured for 24 h, DCs began to grow as fully differentiated swarm cells as observed under a microscope; 3 d later there was an increase in size and in numbers of DCs; 6 d later much salience on the surface of DCs was observed; many nebulous substance floating among culture media were also found on the 8th day. However, the LAM-DC at low doses had no distinct difference in morphology from control group (Figure 1A and B). There was an increase in number of grainy substance in culture media of the LAM-DC at a high dose on the 2nd day (Figure 1F), which might be cell debris resulted from the medication toxicity.
The phenotypes of DCs were determined by FACS on the 8th day. The expressions of phenotype molecules in DCs diversified among groups treated with different concentrations of LAM. The expressions of CD1α on LAM-DC (0.5 mmol/L) was significantly higher than those in naïve controls and other groups (P < 0.05), and so was the expression of CD83 (P < 0.05) and the expression of HLA-DR (P < 0 .05); there was no difference in CD80 expression between LAM-DC (0.5 mmol/L) group and control group. The results demonstrated that HBsAg-pulsed DCs with LAM at certain concentration can enhance the capacities of antigen-presentation (Table 1).
| CD83 | CD80 | CD1α | HLA-DR | |
| DC | 12.83 ± 2.12 | 35.82 ± 2.41 | 33.57 ± 3.14 | 52.80 ± 2.51 |
| DC + LAM 0.125 mmol/L | 13.40 ± 2.16 | 37.73 ± 3.24 | 40.26 ± 2.09 | 53.70 ± 3.27 |
| DC + LAM 0.25 mmol/L | 18.30 ± 3.15 | 41.13 ± 3.61 | 53.10 ± 2.79 | 67.83 ± 4.15 |
| DC + LAM 0.5 mmol/L | 20.24 ± 2.51a | 41.73 ± 4.18 | 54.10 ± 4.21a | 74.50 ± 5.16a |
| DC + LAM 1 mmol/L | 12.81 ± 2.31 | 25.66 ± 3.05 | 16.55 ± 1.95 | 42.11 ± 3.91 |
| DC + LAM 2 mmol/L | 11.92 ± 1.28 | 24.67 ± 2.62 | 16.54 ± 1.76 | 41.01 ± 3.52 |
In the allogeneic mixed leukocyte reaction (MLR), the lymphocyte proliferation reflected the stimulatory function of DCs. The stimulatory capacity of the LAM-DC (0.5 mmol/L) in the allogeneic mixed leukocyte reaction (MLR) was markedly enhanced as compared with control group (P < 0.05). On the contrary, there was no significant difference between LAM-DC (0.125 mmol/L) and control group (Table 2).
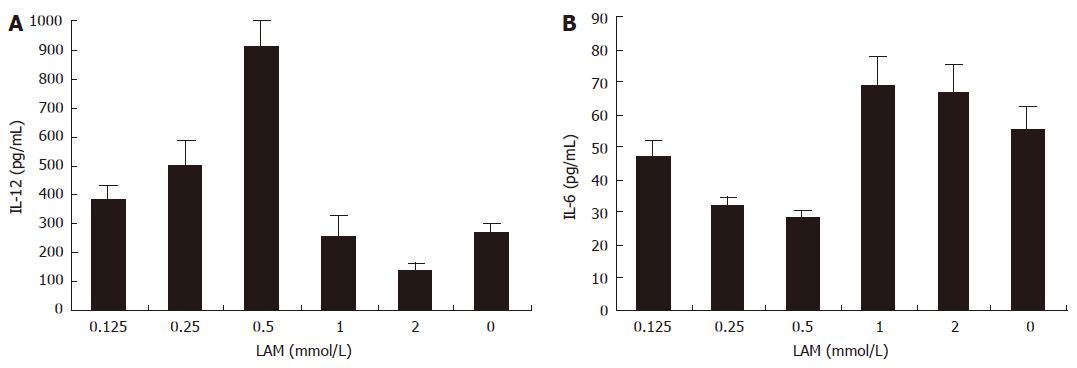
The concentrations of IL-12 in the supernatant of LAM-DC (0, 0.125, 0.25, 0.5, 1, 2 mmol/L) were 268 ± 34.3, 380 ± 51.2, 500 ± 89.4, 910 ± 91.5, 255 ± 73.1, 138 ± 27.9 pg/mL, respectively. The concentrations of IL-6 in the supernatant of LAM-DC (0, 0.125, 0.25, 0.5, 1, 2 mmol/L) were 55 ± 7.36, 47 ± 5.2, 32 ± 2.7, 28 ± 2.6, 69 ± 8.7, 67 ± 8.4 pg/mL, respectively. The secretion of IL-12 significantly increased in the LAM-DC (0.5 mmol/L) group in comparison with control group (P < 0.05). However, LAM-DC (0.5 mmol/L) group produced a lower level of IL-6 as compared with control group (P < 0. 05) (Figure 2).
Experimental data from HBV-transgenic mice demonstrate that the immune system plays a key role in HBV clearance[8]. After HBV infection, the body starts a series of non-specific immunology responses, including activation of natural-killing cells and secretion of interferon. However, the complete clearance of HBV relies on the activation of HBV-specific T lymphocytes[9]. The body can produce HBV antigen-specific CTL response to kill target cells related to HBV. DCs are professional antigen-presenting cells that link innate and adaptive immunity, and are essentially involved in the initiation of primary immune responses and in the establishment of peripheral tolerance[10,11]. The infection of CHB relates to the impairment of immunity by the mechanism resulting from the deficiency of DC function[12]. Selective modification of DC function and promotion of HBV-specific immune responses during CHB treatment have shown therapeutic significance[13].
DCs could be assigned to two groups by their immunocompetence: mature DCs and immature DCs. Mature DCs have already encountered antigens, whereas immature DCs are able to internalize antigens, process them in the extranuclear compartment, and convert them on the cell surface in the compound of MHC-antigen peptides and present antigenic peptides to T cells. It activates the antigen-specific T cells and initiates immune response with the help of costimulatory molecules binding the corresponding T cell receptors[14]. In the present study, we detected the expression of CD1α, CD80, CD83 and HLA-DR on DCs. CD1α is the specific sign of DC’s function; the more CD1α expresses on DCs, the strong function DCs have. CD83 is a mature sign of DCs; HLA-DR, one of the MHC II molecules, mostly takes part in the process of antigen presentation; CD80, one of the costimulatory molecules, promotes T cell activation via combining the corresponding T cell receptors[15,16].
Beckebaum et al[6] proposed that LAM could facilitate the maturation of DCs isolated from Caucasian patients with HBV infection by increasing the expression of HLA-DR and further promote DCs function. In the present study, the expression of CD1α, CD83 and HLA-DR on DCs derived from Chinese patients with CHB infection incubated with LAM (0.5 mmol/L) in vitro could be enhanced; however, the increase of CD80 was not statistically different as compared with that of control group. Here we found that the lower expression of phenotypic molecules and impaired allogeneic mixed lymphocyte reaction function of DCs from patients with HBV infection could be restored in vitro by incubation with LAM. The present study demonstrated that LAM did promote DCs maturation and enhance DCs function.
DCs regulate Th0 cell proliferation towards Th1 cells and Th2 cells through the secretion of IL-6, IL-12 and IFN-γ[17]. IL-12 secreted by mature DCs regulates Th0 cells proliferation towards Th1 cells, promotes the secretion of IL-2, IFN-γ and participates in the cellular immune response. IL-6 from immature DCs drives Th0 cells to proliferate towards Th2 cells, restrains the cellular immune and induces the immune tolerance[17]. In the current experiment, we found that the secretion levels of IL-12, IL-6 by DCs could be affected by LAM in vitro. The secretion of IL-12 increased in the LAM-DC. LAM-DC produced lower levels of IL-6 as compared with non-LAM-DC. These results indicate that DCs treated with LAM at certain concentrations can promote T cell proliferation and enhance the cellular immunity.
The present data confirmed that the defect of DC function could be partially restored by LAM in vitro as marked by the enhanced expression of CD1α, CD83 and HLA-DR on DCs and increased secretion of IL-12, reduced secretion of IL-6 in DCs, and enhanced mixed lymphocyte reaction ability. This indicates that, in addition to its potent anti-HBV replication role, LAM is able to modify the biological activities of DCs derived from patients with CHB infection. Therefore, LAM can be a potential candidate as an immunoregulatory therapeutic remedy used in the treatment of patients with CHB infection. Since treatment with LAM can overcome T cell hyporeactivity[5] and restore DCs function, combination of LAM with DCs may be an effective therapeutic strategy to obtain eradication of chronic HBV infection.
Hepatitis B virus (HBV) infection is a global public health problem. The immune response of the host plays an important role in the pathogenesis of chronic HBV infection.
One of the important reasons responsive for the immune tolerance in chronic hepatitis B (CHB) is impaired function of dendritic cells (DCs) which cannot efficiently present HBV antigens to host immune system.
DC-based therapeutic vaccine has recently been a potential approach to treat CHB. Lamivudine (LAM), a nucleoside analogue, specifically inhibits the hepadnaviral DNA polymerase.
The present study provides a new support for the application of LAM and DC-based immunotherapy in clinical practice of CHB therapy.
This is a good paper indicating that combining LAM with DCs may be an effective therapeutic approach to obtain eradication of chronic HBV infection.
S- Editor Zhu LH L- Editor Kumar M E- Editor Lu W
| 1. | Hilleman MR. Critical overview and outlook: pathogenesis, prevention, and treatment of hepatitis and hepatocarcinoma caused by hepatitis B virus. Vaccine. 2003;21:4626-4649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 65] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 2. | Akbar SM, Inaba K, Onji M. Upregulation of MHC class II antigen on dendritic cells from hepatitis B virus transgenic mice by interferon-gamma: abrogation of immune response defect to a T-cell-dependent antigen. Immunology. 1996;87:519-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | Moll H. Dendritic cells as a tool to combat infectious diseases. Immunol Lett. 2003;85:153-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Wolters LM, Niesters HG, de Man RA. Nucleoside analogues for chronic hepatitis B. Eur J Gastroenterol Hepatol. 2001;13:1499-1506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Boni C, Bertoletti A, Penna A, Cavalli A, Pilli M, Urbani S, Scognamiglio P, Boehme R, Panebianco R, Fiaccadori F. Lamivudine treatment can restore T cell responsiveness in chronic hepatitis B. J Clin Invest. 1998;102:968-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 369] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 6. | Beckebaum S, Cicinnati VR, Zhang X, Ferencik S, Frilling A, Grosse-Wilde H, Broelsch CE, Gerken G. Hepatitis B virus-induced defect of monocyte-derived dendritic cells leads to impaired T helper type 1 response in vitro: mechanisms for viral immune escape. Immunology. 2003;109:487-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 112] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 7. | Beckebaum S, Cicinnati VR, Dworacki G, Müller-Berghaus J, Stolz D, Harnaha J, Whiteside TL, Thomson AW, Lu L, Fung JJ. Reduction in the circulating pDC1/pDC2 ratio and impaired function of ex vivo-generated DC1 in chronic hepatitis B infection. Clin Immunol. 2002;104:138-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 88] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 8. | Kurose K, Akbar SM, Yamamoto K, Onji M. Production of antibody to hepatitis B surface antigen (anti-HBs) by murine hepatitis B virus carriers: neonatal tolerance versus antigen presentation by dendritic cells. Immunology. 1997;92:494-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Hasebe A, Akbar SM, Furukawa S, Horiike N, Onji M. Impaired functional capacities of liver dendritic cells from murine hepatitis B virus (HBV) carriers: relevance to low HBV-specific immune responses. Clin Exp Immunol. 2005;139:35-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Foti M, Granucci F, Ricciardi-Castagnoli P. Dendritic cell interactions and cytokine production. Ernst Schering Res Found Workshop. 2006;61-80. [PubMed] |
| 11. | Kunitani H, Shimizu Y, Murata H, Higuchi K, Watanabe A. Phenotypic analysis of circulating and intrahepatic dendritic cell subsets in patients with chronic liver diseases. J Hepatol. 2002;36:734-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 68] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Szabolcs P, Moore MA, Young JW. Expansion of immunostimulatory dendritic cells among the myeloid progeny of human CD34+ bone marrow precursors cultured with c-kit ligand, granulocyte-macrophage colony-stimulating factor, and TNF-alpha. J Immunol. 1995;154:5851-5861. [PubMed] |
| 13. | Rodríguez-Fernández JL, Corbí AL. Adhesion molecules in human dendritic cells. Curr Opin Investig Drugs. 2005;6:1103-1111. [PubMed] |
| 14. | Rouard H, Léon A, Klonjkowski B, Marquet J, Tennezé L, Plonquet A, Agrawal SG, Abastado JP, Eloit M, Farcet JP, Delfau-Larue MH. Adenoviral transduction of human 'clinical grade' immature dendritic cells enhances costimulatory molecule expression and T-cell stimulatory capacity. J Immunol Methods. 2000;241:69-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Seager Danciger J, Lutz M, Hama S, Cruz D, Castrillo A, Lazaro J, Phillips R, Premack B, Berliner J. Method for large scale isolation, culture and cryopreservation of human monocytes suitable for chemotaxis, cellular adhesion assays, macrophage and dendritic cell differentiation. J Immunol Methods. 2004;288:123-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 93] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 16. | Kolb-Mäurer A, Bröcker EB. The role of dendritic cells during infection. J Dtsch Dermatol Ges. 2003;1:438-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Talmor M, Mirza A, Turley S, Mellman I, Hoffman LA, Steinman RM. Generation or large numbers of immature and mature dendritic cells from rat bone marrow cultures. Eur J Immunol. 1998;28:811-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |