Published online Sep 14, 2007. doi: 10.3748/wjg.v13.i34.4636
Revised: April 23, 2007
Accepted: April 26, 2007
Published online: September 14, 2007
AIM: To study the postoperative complications in patients with preoperative portal vein thrombosis (PVT) undergoing liver transplantation (LT) and to evaluate the complications with Doppler ultrasonography.
METHODS: Retrospective studies were performed on 284 patients undergoing LT (286 LT) with respect to pre- and postoperative clinical data and Doppler ultrasonography. According to the presence and grade of preoperative PVT, 286 LTs were divided into three groups: complete PVT (c-PVT), partial PVT (p-PVT) and non-PVT, with 22, 30 and 234 LTs, respectively. Analyses were carried out to compare the incidence of early postoperative complications.
RESULTS: PVT, inferior vena cava (IVC) thrombosis, hepatic artery thrombosis (HAT) and biliary complications were found postoperatively. All complications were detected by routine Doppler ultrasonography and diagnoses made by ultrasound were confirmed by clinical data or/and other imaging studies. Nine out of 286 LTs had postoperative PVT. The incidence of the c-PVT group was 22.7%, which was higher than that of the p-PVT group (3.3%, P < 0.05) and non-PVT group (1.3%, P < 0.005). No difference was found between the p-PVT and non-PVT groups (P > 0.25). Of the 9 cases with postoperative PVT, recanalizations were achieved in 7 cases after anticoagulation under the guidance of ultrasound, 1 case received portal vein thrombectomy and 1 case died of acute injection. Ten LTs had postoperative IVC thrombosis. The c-PVT group had a higher incidence of IVC thrombosis than the non-PVT group (9.1% vs 2.6%, P < 0.05); no significant difference was found between either the c-PVT and p-PVT groups (9.1% vs 6.7%, P > 0.5) or between the p-PVT and non-PVT groups (P > 0.25). Nine cases with IVC thrombosis were cured by anticoagulation under the guidance of ultrasound, and 1 case gained natural cure without any medical treatment after 2 mo. HAT was found in 2 non-PVT cases, giving a rate of 0.7% among 286 LTs. Biliary complications were seen in 12 LTs. The incidence of biliary complications in the c-PVT, p-PVT and non-PVT groups was 9.1%, 3.3% and 4.3%, respectively (P > 0.25 for all), among which 2 stenosis led retransplantations and others were controlled by relative therapy.
CONCLUSION: C-PVT patients tend to have a higher incidence of PVT and IVC thrombosis than non-PVT patients after LT. The incidence of postoperative complications in p-PVT patients does not differ from that of non-PVT patients. A relatively low incidence of HAT was seen in our study. Doppler ultrasonography is a convenient and efficient method for detecting posttransplant complications and plays an important role in guiding treatment.
- Citation: Jia YP, Lu Q, Gong S, Ma BY, Wen XR, Peng YL, Lin L, Chen HY, Qiu L, Luo Y. Postoperative complications in patients with portal vein thrombosis after liver transplantation: Evaluation with Doppler ultrasonography. World J Gastroenterol 2007; 13(34): 4636-4640
- URL: https://www.wjgnet.com/1007-9327/full/v13/i34/4636.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i34.4636
Portal vein thrombosis (PVT) is a common complication of chronic liver disease and malignant liver tumors. The incidence of PVT in patients undergoing liver transplantation (LT) ranges from 4.9% to 10.6%[1-4]. In the past, technical difficulties and the potential risk of vessel reconstruction, as well as the higher rate of postoperative complications made PVT a contraindication of LT[1,5]. However, improvement of operative management and advancement of postoperative care have meant that PVT is no longer a contraindication of LT[6]. Only a few studies have looked in detail at the actual impact of preoperative PVT on postoperative complications. The understanding of pretransplant PVT still remains a problem. Since 1999, our center has begun to perform LT, and a portion of LT recipients have suffered from preoperative PVT. The aim of this study was to analyze the probable impact of preoperative PVT on postoperative complications, and to discuss the value of Doppler ultrasonography on monitoring the complications and guiding treatment by comparing results of LT in patients with and without PVT.
Between February 1999 and April 2006, 302 patients received cadaveric liver transplantations. The inclusion standard was as follows: records of clinical data and ultrasonographic exams were complete. Exclusion standard was as follows: postoperative routine Doppler ultrasonography studies were not performed. Of the entire population, 284 patients (286 LT) were included in our study, with 284 primary grafts and 2 regrafts due to biliary stenosis after the primary graft. Patient group characters were as follows. There were 237 males and 47 females with an age range of 4-65 years (mean age, 43.7). The number of LTs was used as the frame of reference in our study.
Primary disease and previous surgery were as follows. There were 131 malignant liver tumors (2 regrafts), among which 8 had hepatic segment excision, 6 had splenectomy and 1 had transjugular intrahepatic portosystemic shunt (TIPS). One hundred and forty-eight cases had chronic liver disease, among which 8 had splenectomy and 3 had splenic artery embolization. Four patients had hepatic hydatidosis, 1 patient had congenital Caroli’s disease, 1 patient had acute hepatic failure after liver contusion, and 1 patient had fulminant hepatitis. Orthotopic liver transplantations were performed on all patients including 284 full-size and 2 reduced-size grafts.
PVT patients: Of 286 LTs, 52 (18.2%) had preoperative PVT, with 48 males and 4 females ranging in age from 28 to 65 years (mean age, 44.3). Primary disease and previous surgery were as follows. There were 46 malignant liver tumors, with 1 liver segment excision and 3 splenectomies; 6 patients had cirrhosis, with 1 splenectomy and 2 splenic artery embolizations. PVT was confirmed by Doppler ultrasonography, contrast-enhanced CT and/or surgery as follows; there were 44 cases with tumor thrombosis and 8 with thrombus. All 52 PVT patients received full-size grafts. Portal vein (PV) reconstruction was as follows. Forty-seven patients accepted end-to-end PV anastomosis, 3 accepted porto-caval hemitransposition, 1 accepted PV to mesenteric vein anastomosis and 1 accepted PV to renal vein anastomosis.
According to the presence and grade of PVT, 286 LTs were divided into three groups. (1) Complete PVT (c-PVT) group: 22 cases, all with complete main portal vein (PV) thrombosis and 15 of them had PV branch thrombosis, 3 with superior mesenteric vein (SMV) thrombosis, 2 with splenic vein (SPV) thrombosis, and 2 with both SMV and SPV thrombosis. (2) Partial PVT (p-PVT) group: 30 cases, including 2 with partial main PV thrombosis and 28 with PV branch thrombosis. (3) Non-PVT group: 234 cases, which were the control group.
An Agilent Sonos 4500 (HP, USA), Logiq 500 (GE, USA) and HDI 5000 (ATL, USA) were utilized in our study. The probe frequency was 2-5 MHz. Preoperative ultrasound scan included etiology of the liver, presence of PVT, and grade and extent of PVT. Postoperative ultrasound scan included size, configuration and echo of graft, the diameter of the hepatic vein, the PV and hepatic artery, anastomotic stoma, presence of thrombosis, blood flow and spectrum, presence of biliary obstruction, presence of perihepatic space effusion, and hydrothorax and ascites. Examination intervals were on the operation day and then daily or every 2 d during the first week. If any complications occurred, a scan was performed daily or according to the clinical situation.
Our study focused on early postoperative complications, which was defined as having been diagnosed within the initial hospital stay[7].
Statistical analysis was performed with SPSS 10.0 (Chicago, IL, USA). A probability value less than 0.05 was considered significant. Differences in incidence between c-PVT, p-PVT and non-PVT groups were determined by Chi-square analysis.
Postoperative complications included PVT, inferior vena cava (IVC) thrombosis, hepatic artery thrombosis (HAT) and biliary complications (BC). Postoperative complications of the three groups are listed in Table 1.
| Postoperative complications | c-PVTn = 22 (%) | p-PVTn = 30 (%) | non-PVTn = 234 (%) |
| PVT | 5 (22.7) | 1 (3.3) | 3 (1.3) |
| IVC thrombosis | 2 (9.1) | 2 (6.7) | 6 (2.6) |
| BC | 2 (9.1) | 1 (3.3) | 10 (4.3) |
| HAT | 0 | 0 | |
| 0 | 0 | 2 (0.9) |
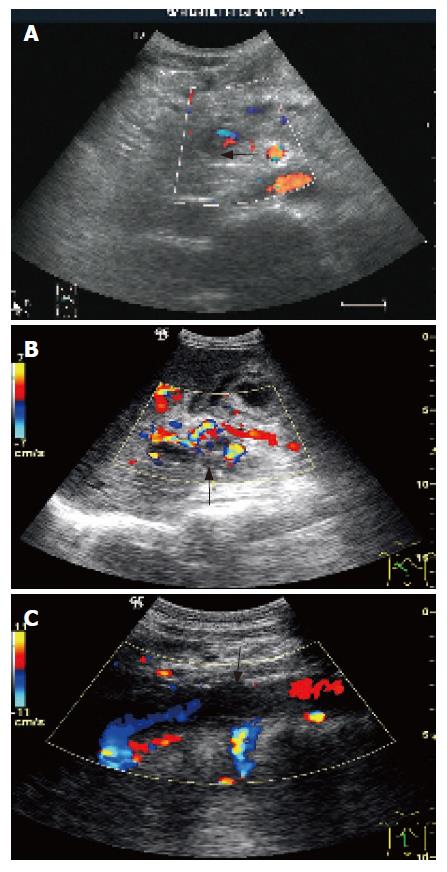
Postoperative PVT was observed in 9 cases (3.1%), among which 6 were rethrombosis (11.5%). Five PVTs were found in the c-PVT group (22.7%), 1 was found in the p-PVT group (3.3%) and 3 were found in the non-PVT group (1.3%). The grades of PVT were 3 complete main PVT (Figure 1A and B), 1 partial main PVT and 5 PV branch thromboses. Nine PVTs were confirmed clinically as thrombus, appearing on 2-11 d, among which recanalizations were achieved in 7 after anticoagulation, 1 received portal vein thrombectomy on the 5th d and 1 died of acute injection on the 12th d postoperatively.
IVC thrombosis was seen in 10 cases on 5-13 d postoperatively. All were subhepatic IVC thrombosis (Figure 1C), with 4 complete and 6 partial thromboses. Recanalizations were obtained in 9 cases after anticoagulation. One case with partial thrombosis got natural cure after 2 mo.
HAT was seen in 2 non-PVT patients on d 3 and 5. Thrombectomy was successfully performed on 2 patients.BC.
Thirteen cases suffered from BC postoperatively. Four of these were stenosis, leading to cholangioplasty in 2 cases and retransplantation in the other 2 cases. Two cases had cholangitis and were cured with drainage and anti-infective treatment. Four cases had evanescent dilation of the intrahepatic duct. One case had biliary fistula and a repair operation was necessary. One case had secondary biliary obstruction.
The incidence of postoperative PVT in the c-PVT group was higher than in the p-PVT (P < 0.05) and non-PVT groups (P < 0.005), and there were no significant differences found between the p-PVT and non-PVT groups (P > 0.25). The c-PVT group had more IVC thromboses than the non-PVT group (P < 0.05) and did not differ from the p-PVT group (P > 0.5). There was no difference for IVC thromboses between the p-PVT and non-PVT groups (P > 0.1). Among the three groups, the incidence of BC between every two groups was not different (P > 0.25 for all groups).
PVT is a common preoperative complication in patients undergoing LT. In China, posthepatitic cirrhosis and advanced malignant liver cancer form the majority of primary diseases in the LT population[8], which results in an even higher incidence of PVT in LT patients. In our series, the incidence of PVT was 18.2%, which is higher than reports elsewhere[1-4,6,16]. Gayowski et al[5] also found an extremely high incidence of PVT (26%) in a predominantly male group of patients mostly with postnecrotic cirrhosis. Our study is consistent with the finding in that most of our cases were male and primary diseases were posthepatitic cirrhosis and liver cancer accompanied with cirrhosis.
In past years, PVT has been taken a contraindication for LT. With improvement of surgical techniques and enhancement of posttransplant care, the outcomes of PVT patients are very close to those of non-PVT patients. The one-year survival rate of PVT patients is reported to be 81%[6], and 1-, 2- and 4-year survival rates are not significantly different in patients with or without PVT[5]. However, several reports confirmed that PVT had a substantial impact on increased blood loss, a higher mortality rate, a higher graft loss and more posttransplant complications[1,5,9,10]. The mortality rate of patients with PVT and extensive splanchnic venous thrombosis undergoing LT is as high as 33%[10]. As shown by Zhou et al[11], PV tumor thrombosis is one of two major prognostic factors of HCC after LT. Therefore, strict preoperative screening and efficient postoperative examinations such as laboratory examinations, Doppler ultrasound, CT, MRI, and angiography when needed should be executed on PVT recipients[4,12]. In addition, such surgeries should be handled by an experienced transplantation center.
PVT is a common postoperative complication of LT. Especially in children, PVT is the top transplant vascular complication[13]. The clinical presentations include intractable ascites, variceal bleeding, shrunken livers, splenomegaly, and hepatic failure[1-3]. It’s widely accepted that risk factors of posttransplant PVT include severe pretransplant portal hypertension, experience of treatment for portal hypertension (e.g., TIPS, portocaval shunt, splenectomy, and splenic vein embolization) and preoperative PVT[4,14-16]. The incidence of postoperative PVT in our study was 3.1%, which is similar to 1%-3% of other reports[2,4,17,18]. The incidence of rethrombosis in LT recipients with native PVT has been reported to range from 5% to 21%[2,4,10,16]. A significantly increased incidence compared to the whole group of rethrombosis (11.5%) was also seen in our study, which is much higher than 1.3% of non-PVT recipients (P < 0.005). Although Settmacher et al[16] associated splenectomy during LT with an increased incidence (10.5%) of PV complications, none of our patients endured splenectomy during LT.
In the study by Yerdel et al[4], PVT was classified into four grades as follows: grade 1; < 50% PVT ± minimal obstruction of SMV, grade 2; grade 1 but > 50% PVT, grade 3; complete PVT and proximal SMV thrombosis, and grade 4; complete PVT and entire SMV thrombosis. By Yerdel’s research, postoperative complications of grade 1 did not differ from those of non-PVT, while grades 2, 3 and 4 exceeded non-PVT. We only divided the PVT patients into the p-PVT and c-PVT groups instead of classifying the recipients as in the study by Yerdel et al because there were too few grade 3 and 4 patients to perform statistical analysis. Our results also revealed the impact of grade and extent of PVT on postoperative complications. Rethrombosis of c-PVT recipients was 22.7%, which was higher than that of p-PVT and non-PVT groups.
With regard to the probable cause of posttransplant PV complications, Settmacher et al[16] took pathological changes of the vessel wall for an important reason, because all cirrhosis patients may have experienced partial or complete PVT and pathological changes of the vessel wall may increase the incidence of PV complications to 17.9%. In our study, PV branch thrombosis was predominant in the p-PVT group, which had a minute influence on PV reconstruction, while blood vessel pathological changes caused by partial thrombosis of main PV were relatively milder than c-PVT. We attribute these probable reasons to the lower incidence of postoperative PVT in p-PVT than c-PVT and similar incidences of postoperative complications between p-PVT and non-PVT groups. With regard to LT in children, postoperative PVT is much more common with the incidence ranging from 8% to 33%, which is attributed to donor/recipient portal vein diameter mismatch and application of graft veins[7,13,19]. One of the patients with postoperative PVT in our study was a 4-year child without preoperative PVT. However, it is difficult to reach a conclusion that mismatch of donor/receptor vein diameter may lead to posttransplant PVT due to too few child cases.
IVC thrombosis occurred in 3.5% of our series (10/286), with more in the c-PVT group than in the non-PVT group and adjacent occurrences were seen between p-PVT and non-PVT groups. Donor/recipient vessel mismatch and recurrence of the underlying disease (e.g. Budd-Chiari syndrome) account for the majority of complications of the vena cava, while unknown causes account for the minority[16]. At this time we can not explain the higher incidence of IVC thrombosis in c-PVT.
HAT is a common posttransplant complication, which is associated with operation techniques[20]. In our center, vascular anastomoses are accomplished by a vascular surgeon with microsurgical techniques, which remarkably improves the quality of vascular reconstruction, especially on small vessels like the hepatic artery. Thus, posttransplant HAT in our center has been decreased to an ideal extent, which is much less than in other centers[18,21].
Biliary stenosis, biliary fistula and cholangitis account for most of the postransplant BC, for which technical failure or local ischemia are major causes[22]. BC occurred after 13 LTs (4.5%), affecting 11 recipients in our series. No difference was found between groups with or without PVT. Biliary cirrhosis caused by biliary obstructions in two recipients led to retransplantations in the 7th and 12th mo, which suggests that BC may be the main reason for late retransplantation.
Vascular complications after LT may be more prone for placing the patient and allograft in jeopardy than BC, but they rarely necessitate retransplantations with early management[18,23]. In our study, although 2 patients suffering from postoperative HAT endured re-exploration and thrombectomy, and 1 patient with postoperative PVT died of acute rejection, no retransplantations were caused by PVT or IVC thrombosis if early diagnosis and treatment were administered. Settmacher et al[16] studied 1000 LTs and demonstrated that more than half of the patients suffering from PV complications did not require any treatment at all and treatment became necessary only when additional complications such as arterial occlusion or bile duct injuries occurred.
In China, cirrhosis and unresectable liver cancer forms the majority of primary disease in LT recipients, among which PVT has a high incidence. Preoperative PVT should have the requirement for pretransplant screening and postoperative monitoring of a high quality. Since Doppler ultrasonography is rapidly available, inexpensive and provides reasonable accuracy, it is predominant in pre-, intra- and posttransplant evaluations[4,21,24]. Accurate diagnosis of vascular abnormalities helps to determine the operation agenda[4]. Early detection and management of postoperative complications, especially clinically unsuspected vascular complications, can satisfactorily reduce the mortality rate and avoid retransplantation[2,21]. Conventional Doppler ultrasound provides an ideal specificity on diagnosing PVT ranging from 97% to 100%[4,24,25]. In our series, Doppler ultrasonography detected all of the complications and got confirmation of clinical presentations and other image examinations. In our center, if anticoagulation is essential when the posttransplant patient suffers from PVT or IVC thrombosis, a daily ultrasound examination will be performed to observe dissolution of thrombosis. Termination of treatment is thrombosis dissolution and vessel recanalization determined by ultrasound examination.
In a previous study by Hom et al[25], diagnostic validity of contrast-enhanced ultrasound (CEUS) was comparable to that of angiography, and CEUS shortened study time markedly. In addition, we are currently working on CEUS on posttransplant patients and will have results when more cases are studied.
In conclusion, the incidence of PVT after LT in our study was higher in the c-PVT group than in the p-PVT and non-PVT groups. Compared to non-PVT patients, c-PVT patients are more likely to suffer from IVC thrombosis postoperatively. The incidence of BC did not differ between c-PVT, p-PVT and non-PVT groups. In our center, postoperative HAT is relatively uncommon. Doppler ultrasonography provides an accurate diagnosis of complications after LT, plays a very important role in posttransplant monitoring and is essential in guiding treatment.
S- Editor Zhu LH L- Editor Knapp E E- Editor Wang HF
| 1. | Egawa H, Tanaka K, Kasahara M, Takada Y, Oike F, Ogawa K, Sakamoto S, Kozaki K, Taira K, Ito T. Single center experience of 39 patients with preoperative portal vein thrombosis among 404 adult living donor liver transplantations. Liver Transpl. 2006;12:1512-1518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 71] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 2. | Davidson BR, Gibson M, Dick R, Burroughs A, Rolles K. Incidence, risk factors, management, and outcome of portal vein abnormalities at orthotopic liver transplantation. Transplantation. 1994;57:1174-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 94] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Seu P, Shackleton CR, Shaked A, Imagawa DK, Olthoff KM, Rudich SR, Kinkhabwala M, Busuttil RW. Improved results of liver transplantation in patients with portal vein thrombosis. Arch Surg. 1996;131:840-844; discussion 840-844;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 73] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Yerdel MA, Gunson B, Mirza D, Karayalçin K, Olliff S, Buckels J, Mayer D, McMaster P, Pirenne J. Portal vein thrombosis in adults undergoing liver transplantation: risk factors, screening, management, and outcome. Transplantation. 2000;69:1873-1881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 527] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 5. | Gayowski TJ, Marino IR, Doyle HR, Echeverri L, Mieles L, Todo S, Wagener M, Singh N, Yu VL, Fung JJ. A high incidence of native portal vein thrombosis in veterans undergoing liver transplantation. J Surg Res. 1996;60:333-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 85] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Langnas AN, Marujo WC, Stratta RJ, Wood RP, Ranjan D, Ozaki C, Shaw BW. A selective approach to preexisting portal vein thrombosis in patients undergoing liver transplantation. Am J Surg. 1992;163:132-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Millis JM, Seaman DS, Piper JB, Alonso EM, Kelly S, Hackworth CA, Newell KA, Bruce DS, Woodle ES, Thistlethwaite JR. Portal vein thrombosis and stenosis in pediatric liver transplantation. Transplantation. 1996;62:748-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 122] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 8. | Wang DC, Zhang TL, Song SB, Yuan J, Xiu DR, Yang XX. A report of 28 cases of 3-year follow-up after liver transplantation for advanced hepatocellular carcinoma. World J Gastroenterol. 2004;10:2134-2135. [PubMed] |
| 9. | Marini M, Gómez-Gutierrez M, Cao I, Sellés C, Aguirrezabalaga J, Otero A, Soler R. Endovascular treatment of splenomesenteric-portal vein thromboses during orthotopic liver transplantation. J Vasc Interv Radiol. 2005;16:1135-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Manzanet G, Sanjuán F, Orbis P, López R, Moya A, Juan M, Vila J, Asensi J, Sendra P, Ruíz J. Liver transplantation in patients with portal vein thrombosis. Liver Transpl. 2001;7:125-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 138] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 11. | Zhou LX, Yan LN. Prognostic factors of hepatocellular carcinoma after liver transplantation. Ai Zheng. 2006;25:736-739. [PubMed] |
| 12. | Bhattacharjya T, Olliff SP, Bhattacharjya S, Mirza DF, McMaster P. Percutaneous portal vein thrombolysis and endovascular stent for management of posttransplant portal venous conduit thrombosis. Transplantation. 2000;69:2195-2198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Yamanaka J, Lynch SV, Ong TH, Fawcett J, Robinson HE, Beale K, Balderson GA, Strong RW. Surgical complications and long-term outcome in pediatric liver transplantation. Hepatogastroenterology. 2000;47:1371-1374. [PubMed] |
| 14. | Luo Y, Li B, Cai DM, Ma BY, Lin L. Color Doppler ultrasound in diagnosis of portal vein complication of orthotopic liver transplantation. Zhongguo Yixue Yingxiang Jishu. 2004;20:558-560. |
| 15. | Lu MQ, Chen GH, Yang Y, Cai CJ, Wang GD, Zhu XF, He XS. Analysis of vascular complications after liver transplantation. Zhongguo Xiandai Yixue Zazhi. 2003;13:57-59. |
| 16. | Settmacher U, Nüssler NC, Glanemann M, Haase R, Heise M, Bechstein WO, Neuhaus P. Venous complications after orthotopic liver transplantation. Clin Transplant. 2000;14:235-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 132] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 17. | Lu MQ, Chen GH, Yang Y, Cai CJ, Wang GD, Zhu XF, He XS. Etiology and Management of Vascular Complications After Liver Transplantation. Zhongshan Daxue Xuebao (Yixue Kexue Ban). 2003;24:485-487. |
| 18. | Langnas AN, Marujo W, Stratta RJ, Wood RP, Shaw BW. Vascular complications after orthotopic liver transplantation. Am J Surg. 1991;161:76-82; discussion 82-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 325] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 19. | Aw MM, Phua KB, Ooi BC, Da Costa M, Loh DL, Mak K, Tan KC, Isaac J, Prabhakaran K, Quak SH. Outcome of liver transplantation for children with liver disease. Singapore Med J. 2006;47:595-598. [PubMed] |
| 20. | Tiao GM, Alonso M, Bezerra J, Yazigi N, Heubi J, Balistreri W, Bucuvalas J, Ryckman F. Liver transplantation in children younger than 1 year--the Cincinnati experience. J Pediatr Surg. 2005;40:268-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Nĕmec P, Ondrásek J, Studeník P, Hökl J, Cerný J. Biliary complications in liver transplantation. Ann Transplant. 2001;6:24-28. [PubMed] |
| 22. | Gustafsson BI, Backman L, Friman S, Herlenius G, Lindnér P, Mjornstedt L, Olausson M. Retransplantation of the liver. Transplant Proc. 2006;38:1438-1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Kok T, Slooff MJ, Thijn CJ, Peeters PM, Verwer R, Bijleveld CM, van den Berg AP, Haagsma EB, Klompmaker IJ. Routine Doppler ultrasound for the detection of clinically unsuspected vascular complications in the early postoperative phase after orthotopic liver transplantation. Transpl Int. 1998;11:272-276. [RCA] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 66] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Tamsel S, Demirpolat G, Killi R, Aydin U, Kilic M, Zeytunlu M, Parildar M, Oran I, Ucar H. Vascular complications after liver transplantation: evaluation with Doppler US. Abdom Imaging. 2007;32:339-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 61] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 25. | Hom BK, Shrestha R, Palmer SL, Katz MD, Selby RR, Asatryan Z, Wells JK, Grant EG. Prospective evaluation of vascular complications after liver transplantation: comparison of conventional and microbubble contrast-enhanced US. Radiology. 2006;241:267-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 68] [Article Influence: 3.6] [Reference Citation Analysis (0)] |