Published online Sep 14, 2007. doi: 10.3748/wjg.v13.i34.4626
Revised: June 20, 2007
Accepted: June 23, 2007
Published online: September 14, 2007
AIM: To explore the role of the matrix metalloproteinase-9 (MMP-9) polymorphism in colorectal cancer (CRC) in a northeast Chinese population.
METHODS: Genotyping of MMP-9-1562C>T and 279R>Q polymorphisms was carried out on blood samples from 137 colorectal cancer patients and 199 controls using polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP). Multivariate logistic regression models were used to calculate adjusted odds ratios (OR) and 95% confidence intervals (95% CI).
RESULTS: The distribution of MMP-9 -1562C>T and 279 R>Q genotype was not significantly associated with the risk of CRC. However, the risk of llymph node metastasis of CRC was increased in patients with the -1562T allele (OR = 2.601; 95% CI = 1.160-5.835; P = 0.022). The frequency of MMP-9 279RR + RQ genotype was higher than the QQ genotype among CRC patients younger than sixty years old (OR = 0.102; 95% CI = 0.013-0.812; P = 0.012).
CONCLUSION: Our results indicated that the MMP-9-1562C>T polymorphism affects lymph node metastasis of CRC. In addition, the MMP-9 279R allele may lead to a younger age of onset of colorectal cancer.
- Citation: Xing LL, Wang ZN, Jiang L, Zhang Y, Xu YY, Li J, Luo Y, Zhang X. Matrix metalloproteinase-9-1562C>T polymorphism may increase the risk of lymphatic metastasis of colorectal cancer. World J Gastroenterol 2007; 13(34): 4626-4629
- URL: https://www.wjgnet.com/1007-9327/full/v13/i34/4626.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i34.4626
Matrix metalloproteinases (MMPs) are a family of zinc-binding proteases that process growth factors, growth factor binding proteins, and cell surface proteins[1]. They also degrade extracellular matrix (ECM) components and thereby play a central role in tissue remodeling associated with various pathological processes, such as cancer invasion and metastasis[2].
MMP-9 is a member of the MMP family that is also known as gelatinase B or type IV collagenase. (92 kDa). MMP-9 possesses proteolytic activity against type IV collagen, a major component of the basement membrane, and has been shown to facilitate vascular smooth muscle cell migration[3]. The expression of MMP-9 is up-regulated in various types of human cancer, such as esophageal carcinogenesis[4], breast cancer[5], and gastric carcinoma[6]. MMP-9 expression is also significantly increased in CRC tissues[7]. Further, over-expression of MMP-9 represents an early event in colorectal carcinogenesis and may possibly have prognostic value[8].
The regulation of MMP-9 expression may be at the transcription level. Growing evidence indicates that genetic variants in the promoters of the MMP-9 gene may result in differential expression in different individuals[9]. A promoter variant, -1562C>T, a polymorphism due to a C to T substitution results in the loss of the binding site of a nuclear protein to this region of the MMP-9 gene promoter[10]. Polymorphisms in coding regions may also have altered function; a coding region polymorphism, 279R>Q, which is located in the catalytic domain, leads to substitution of arginine by glutamine.
We hypothesized with respect to the role of the two polymorphisms, speculating that they may contribute to CRC risk and metastasis. We thus conducted a case-control study to examine the relationship between MMP-9 polymorphisms and CRC.
The subjects for this case-control study of risk factors for CRC were unrelated and from Shenyang in northern China. The trial recruited 137 CRC patients and 199 healthy control subjects. The patients were comprised of 71 men and 66 women, median age 61.29 years, with a histologically confirmed new diagnosis of CRC, made at the First Affiliated Hospital of China Medical University and Shenyang Anal Hospital, between 2005 and 2006.The CRC patients were grouped according to TNM-classification (UICC) on the basis of postoperative histopathological evaluation.
The controls, 104 men and 95 women, with a median age of 60.65 years, were randomly selected among people admitted to the same hospital during the same period. These control subjects had no history of any cancer.
All subjects gave informed consent for the study, and allowed their blood samples to be analyzed. Detailed information on risk factors including tobacco (smokers were defined as the population who intake more than one cigarette per day; all others were non-smokers), alcohol intake (drinkers were defined as the population who intake more than 50 g alcohol per day), and BMI were obtained with a baseline questionnaire. This study used the suggested WHO BMI cutoff points for Asians to assess several variables; respondents whose BMI was < 23 kg/m2 were categorized as normal weight and respondents whose BMI was ≥ 23 kg/m2 were categorized as overweight and obese.
Five milliliters of venous blood was extracted from each subject. Genomic DNA was extracted using proteinase K digestion, followed by a salting out procedure.
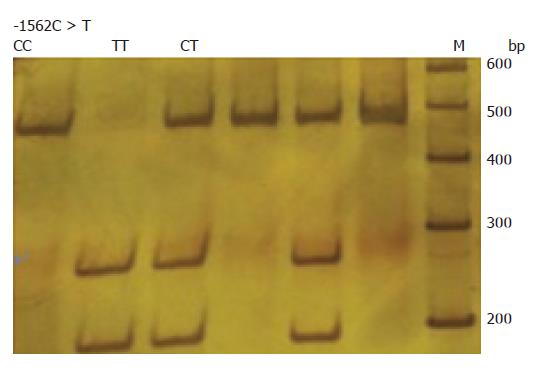
MMP-9 -1562C>T genotypes were determined by polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) assay. The primers used were 5′-GGCACATAGTAGGCCCTTTAA-3′(forward) and 5′-TCACTCCTTTCTTCCTAGCCA-3′(reverse)[11]. PCR was performed with a 20 μL volume containing 20 ng DNA template, 2.0 μL 10 × PCR buffer, 0.5 U Taq DNA polymerase, 20 pmol of each primer and 1.6 μL 2.5 mmol/L dNTP. Amplification was for 1 min at 94°C, followed by 35 cycles of 30 s at 94°C, 30 s at 57°C, and 30 s at 72°C, and a final step at 72°C for 1 min. PCR products were digested with Sph1 (Takara) and separated on 8% polyacrylamide gel electrophoresis. After electrophoresis, the homozygous C allele was represented by a DNA band at 442 bp, whereas the homozygous T allele was represented by a DNA band at 264 and 178 bp, and heterozygotes at 442, 264 and 178 bp (Figure 1).
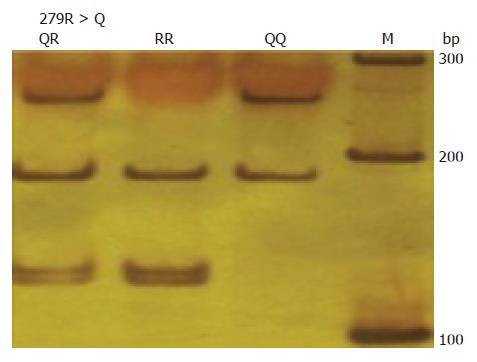
279R>Q genotypes were determined using a PCR-RFLP assay, as previously described. The primers used were 5′-GAGAGATGGGATGAACTG 3′(forward) and 5′-GTGGTGGAAATGTGGTGT-3′(reverse)[12]. PCR products were digested with Msp1 (Takara) and separated by electrophoresis on 8% polyacrylamide gels. The 279R allele has two restriction sites, represented by DNA bands at 187, 129 and 123 bp; the 279 Q allele has only one restriction site, represented by DNA bands at 252 and 187 bp (Figure 2).
Odds ratios (ORs) and 95% confidence intervals (CIs) were estimated by logistic regression analyses from comparison of genotypes between CRC patients and healthy controls, using SPSS version 13.0 (SPSS, Chicago, IL, USA), adjusting for the potential confounders such as age, sex, tobacco use, alcohol use, and BMI. Association of the genotype with clinicopathological parameters was evaluated by Fisher’s exact test. The χ2 test was used to assess Hardy-Weinberg equilibrium. In all cases, P < 0.05 was considered statistically significant.
Characteristics of the study population and the association with CRC are presented in Table 1. There were no significant differences in terms of distribution for age and gender between cases and controls (P = 0.325 and 0.951, respectively). However, cases tended to have a higher BMI (P < 0.001), and were more likely to smoke cigarettes (P < 0.001).
| Controls/cases | OR | 95% CI | |
| Sex | |||
| Male | 101/70 | 1 | |
| Female | 98/67 | 0.986 | (0.638-1.524) |
| Age (yr) | |||
| ≤ 60 | 98/60 | 1 | |
| > 60 | 101/77 | 1.245 | (0.804-1.928) |
| Smoking status | |||
| Non-smoker | 147/75 | 1 | |
| Smoker | 52/62 | 2.337 | (1.473-3.708)b |
| Alcohol duration (yr) | |||
| Never | 168/103 | 1 | |
| 1-15 | 16/14 | 1.427 | (0.669-3.046) |
| >15 | 15/20 | 2.175 | (1.066-4.437) |
| BMI (kg/m2) | |||
| 18.5-22.9 | 101/35 | 1 | |
| 23-24.9 | 38/54 | 4.101 | (2.329-7.220) |
| > 25 | 60/48 | 2.309 | (1.345-3.962)d |
The MMP-9 -1562C>T and 279R>Q polymorphisms genotype and allele distribution in cases and controls are shown in Table 2. The distribution of MMP-9 279R>Q and -1562C>T polymorphisms in cases and controls were all consistent with a Hardy-Weinberg equilibrium. In addition, there was no linkage disequilibrium between -1562C>T and 279R>Q polymorphisms (R2 = 0.009, D′ = 0.376).
| Genotypes | Controls | Cases | OR1 (95% CI) |
| C-1562T | |||
| CC | 147 (73.9) | 104 (75.9) | 1.00 |
| CT | 47 (23.6) | 33 (24.1) | 0.877 (0.527-1.462) |
| TT | 5 (2.5) | 0 (0) | |
| T allele | 0.143 | 0.12 | |
| R279Q | |||
| 16 (8.0) | 12 (8.8) | 1.00 | |
| RQ | 85 (42.7) | 58 (42.3) | 0.936 (0.410-2.138) |
| RR | 98 (49.2) | 67 (48.9) | 0.961 (0.424-2.177) |
| R allele | 0.706 | 0.701 | |
| C-1562T and R279Q combinations | |||
| 279 QQ and -1562CC | 11 (5.5) | 9 (6.6) | 1.00 |
| 1-2 risk alleles2 | 155(77.9) | 109 (79.6) | 1.357 (0.474-3.886) |
| > 2 risk alleles | 33 (16.6) | 19 (13.9) | 1.227 (0.661-2.277) |
The frequency of MMP-9 -1562C>T and 279R>Q genotypes did not differ significantly between cases and controls (-1562CT+TT vs CC: OR=0.877; 95% CI = 0.527-1.462; P = 0.615; 279RQ vs QQ: OR = 0.936; 95% CI = 0.410-2.138; P = 0.875; RR vs QQ: OR=0.961; 95% CI = 0.424-2.177; P = 0.924). We examined the combined effects of the two polymorphisms among cases and controls, using 279R and -1562T as risk alleles. We found that neither the genotypes containing one or two risk alleles, nor those containing more than two risk alleles were associated with increased risk of CRC.
We also estimated the association between the -1562C>T and 279R>Q genotypes and clinicopathological findings among CRC patients (Table 3). Logistic regression analysis revealed that CRC patients with the -1562CT + TT genotype showed increased risk of lymph node metastasis (CT+TT vs CC; P = 0.022 ). Age, sex, TNM classification and external membrane invasion were not correlated with the -1562 C>T genotype. There was no significant difference between the 279R>Q polymorphism and clinicopathological features. However, the frequency of the MMP-9 279RR + RQ genotype was higher than the QQ genotype among CRC patients aged < 60 years.
| Parameters | C-1562T | R279Q | ||
| CC | CT+TT | RQ+RR | ||
| Age (yr) | ||||
| < 60 (n = 60) | 49 | 11 | 1 | 59 |
| ≥ 60 (n = 77) | 55 | 22 | 11 | 66c |
| Sex | ||||
| Male (n= 70) | 57 | 13 | 6 | 64 |
| Female (n = 67) | 47 | 20 | 6 | 61 |
| Lymph node metastasis | ||||
| N(-)(n = 87) | 71 | 16 | 8 | 79 |
| N(+)(n = 46) | 29 | 17a | 4 | 42 |
| TNM classification | ||||
| StageI(n = 26) | 21 | 5 | 3 | 23 |
| ≥ Stage II(n = 107) | 79 | 28 | 9 | 98 |
| External membrane invasion | ||||
| (+) (n = 102) | 76 | 26 | 9 | 93 |
| (-) (n = 31) | 24 | 7 | 3 | 28 |
Increased levels of MMP-9 have been found to be associated with CRC susceptibility[13]. However, despite of the strong rationale for this study, our results showed that the two MMP-9 polymorphisms did not enhance susceptibility to the development of CRC, although we did find that the MMP-9 -1562C>T polymorphism was associated with lymph node metastasis of CRC.
Consistent with this finding is a previous report of an absence of an association between the -1562C>T polymorphism and gastric cancer susceptibility; that report also found the -1562T allele to be associated with the invasive phenotype of gastric cancer[14]. Similarly, a Chinese study has suggested that the -1562C>T genotype distribution in CRC cancer patients and healthy controls was comparable, but an association between the -1562C allele and the invasive capability of CRC was not observed[15]. To explain this discrepancy, two points warrant consideration. First, the difference may be due to the different populations; all our subjects were drawn from a population pool in the northern part of China, whereas their subjects were not.
Second, the association between the -1562C>T polymorphism and the risk of lymph node metastasis of CRC is consistent with the biological function of MMP-9. MMP-9 is up-regulated in various human cancer types. The over-expression of MMP-9 is positively correlated with the depth of invasion, lymphatic and venous invasion, and lymph node metastasis, such as in CRC[7], gastric carcinoma[16], and prostate cancer[17]. Lymph node metastasis is considered as the most important prognostic factor of CRC[18,19]. In addition, the 5-year survival rate of patients with CRC with lymph node metastasis is worse than that in those without lymph node metastasis[20]. We postulated that the promoter polymorphisms could increase the risk of lymph node metastasis by affecting the expression of MMP-9.
The -1562 C>T polymorphism is located within an important regulatory element that appears to be a binding site for a transcription repressor protein. DNA-protein interaction is abolished by the C-to-T substitution at the polymorphic site, which results in a higher promoter activity of the T-allelic promoter[21]. It is suggested that the MMP-9 promoter-1562C>T polymorphism appears to regulate gene expression in an allele-specific manner.
A surprising finding was that the frequency of the MMP-9 279RR + RQ genotype was higher than that of the QQ genotype among patients younger than 60 years (P < 0.05). The results indicate that the MMP-9 279R allele may lead to a younger age of onset of CRC. The 279R>Q polymorphism is located in the catalytic domain of the MMP-9 gene, and within one of the fibronectin type-II like repeats required for binding the enzyme to its substrate elastin[22]. The polymorphism led to the substitution of a positively charged amino acid (arginine) by an uncharged amino acid (glutamine). These factors may contribute to the mechanism of our findings.
The major limitation of our study is the relatively small sample size, which could have resulted in a less precise estimation of the association between MMP-9 polymorphisms and CRC susceptibility. Another weakness is that the study population was limited to native northern Chinese in Shenyang city, Liaoning province, and thus the results may not apply to other populations.
In conclusion, our study provides evidence of a connection between MMP-9 -1562C>T polymorphisms and increased risk of developing CRC. Our results are consistent with a report of the multiple functions of MMP-9 in cancer[23], and especially with its role in tumor cell migration and invasion[24].
S- Editor Zhu LH L- Editor Knapp E E- Editor Wang HF
| 1. | Sounni NE, Noel A. Membrane type-matrix metalloproteinases and tumor progression. Biochimie. 2005;87:329-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 101] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 2. | Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8:221-233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2317] [Cited by in RCA: 2170] [Article Influence: 120.6] [Reference Citation Analysis (0)] |
| 3. | Gum R, Lengyel E, Juarez J, Chen JH, Sato H, Seiki M, Boyd D. Stimulation of 92-kDa gelatinase B promoter activity by ras is mitogen-activated protein kinase kinase 1-independent and requires multiple transcription factor binding sites including closely spaced PEA3/ets and AP-1 sequences. J Biol Chem. 1996;271:10672-10680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 288] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 4. | Herszenyi L, Hritz I, Pregun I, Sipos F, Juhasz M, Molnar B, Tulassay Z. Alterations of glutathione S-transferase and matrix metalloproteinase-9 expressions are early events in esophageal carcinogenesis. World J Gastroenterol. 2007;13:676-682. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Pellikainen JM, Ropponen KM, Kataja VV, Kellokoski JK, Eskelinen MJ, Kosma VM. Expression of matrix metalloproteinase (MMP)-2 and MMP-9 in breast cancer with a special reference to activator protein-2, HER2, and prognosis. Clin Cancer Res. 2004;10:7621-7628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 246] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 6. | Sun WH, Sun YL, Fang RN, Shao Y, Xu HC, Xue QP, Ding GX, Cheng YL. Expression of cyclooxygenase-2 and matrix metalloproteinase-9 in gastric carcinoma and its correlation with angiogenesis. Jpn J Clin Oncol. 2005;35:707-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | Illemann M, Bird N, Majeed A, Sehested M, Laerum OD, Lund LR, Danø K, Nielsen BS. MMP-9 is differentially expressed in primary human colorectal adenocarcinomas and their metastases. Mol Cancer Res. 2006;4:293-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 79] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 8. | Daniel P, Wagrowska-Danilewicz M, Danilewicz M, Stasikowska O, Malecka-Panas E. Transforming growth factor beta 1 and metalloproteinase-9 overexpression in colorectal cancer (CC) and adenoma. Int J Colorectal Dis. 2007;22:1165-1172. [PubMed] |
| 9. | Wang Y, Fang S, Wei L, Wang R, Jin X, Wen D, Li Y, Guo W, Wang N, Zhang J. No association between the C-1562T polymorphism in the promoter of matrix metalloproteinase-9 gene and non-small cell lung carcinoma. Lung Cancer. 2005;49:155-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Ye S. Polymorphism in matrix metalloproteinase gene promoters: implication in regulation of gene expression and susceptibility of various diseases. Matrix Biol. 2000;19:623-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 242] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 11. | Awakura Y, Ito N, Nakamura E, Takahashi T, Kotani H, Mikami Y, Manabe T, Kamoto T, Habuchi T, Ogawa O. Matrix metalloproteinase-9 polymorphisms and renal cell carcinoma in a Japanese population. Cancer Lett. 2006;241:59-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Hu Z, Huo X, Lu D, Qian J, Zhou J, Chen Y, Xu L, Ma H, Zhu J, Wei Q. Functional polymorphisms of matrix metalloproteinase-9 are associated with risk of occurrence and metastasis of lung cancer. Clin Cancer Res. 2005;11:5433-5439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 13. | Wilson S, Wakelam MJ, Hobbs RF, Ryan AV, Dunn JA, Redman VD, Patrick F, Colbourne L, Martin A, Ismail T. Evaluation of the accuracy of serum MMP-9 as a test for colorectal cancer in a primary care population. BMC Cancer. 2006;6:258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Matsumura S, Oue N, Nakayama H, Kitadai Y, Yoshida K, Yamaguchi Y, Imai K, Nakachi K, Matsusaki K, Chayama K. A single nucleotide polymorphism in the MMP-9 promoter affects tumor progression and invasive phenotype of gastric cancer. J Cancer Res Clin Oncol. 2005;131:19-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 83] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 15. | Xu EP, Huang Q, Lu BJ, Xing XM, Lai MD. [The correlation between polymorphisms of matrix metalloproteinase-2 and -9 genes and colorectal cancer of Chinese patients]. Zhonghua YiXue YiChuanXue ZaZhi. 2006;23:78-81. [PubMed] |
| 16. | Zheng H, Takahashi H, Murai Y, Cui Z, Nomoto K, Niwa H, Tsuneyama K, Takano Y. Expressions of MMP-2, MMP-9 and VEGF are closely linked to growth, invasion, metastasis and angiogenesis of gastric carcinoma. Anticancer Res. 2006;26:3579-3583. [PubMed] |
| 17. | Ishimaru H, Kageyama Y, Hayashi T, Nemoto T, Eishi Y, Kihara K. Expression of matrix metalloproteinase-9 and bombesin/gastrin-releasing peptide in human prostate cancers and their lymph node metastases. Acta Oncol. 2002;41:289-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Gennari L, Doci R, Rossetti C. Prognostic factors in colorectal cancer. Hepatogastroenterology. 2000;47:310-314. [PubMed] |
| 19. | Sarli L, Bader G, Iusco D, Salvemini C, Mauro DD, Mazzeo A, Regina G, Roncoroni L. Number of lymph nodes examined and prognosis of TNM stage II colorectal cancer. Eur J Cancer. 2005;41:272-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 235] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 20. | Matsumoto K, Nakayama Y, Inoue Y, Minagawa N, Katsuki T, Shibao K, Tsurudome Y, Hirata K, Nagata N, Itoh H. Lymphatic microvessel density is an independent prognostic factor in colorectal cancer. Dis Colon Rectum. 2007;50:308-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 21. | Zhang B, Ye S, Herrmann SM, Eriksson P, de Maat M, Evans A, Arveiler D, Luc G, Cambien F, Hamsten A. Functional polymorphism in the regulatory region of gelatinase B gene in relation to severity of coronary atherosclerosis. Circulation. 1999;99:1788-1794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 421] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 22. | Zhang B, Henney A, Eriksson P, Hamsten A, Watkins H, Ye S. Genetic variation at the matrix metalloproteinase-9 locus on chromosome 20q12.2-13.1. Hum Genet. 1999;105:418-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 111] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 23. | Nelson AR, Fingleton B, Rothenberg ML, Matrisian LM. Matrix metalloproteinases: biologic activity and clinical implications. J Clin Oncol. 2000;18:1135-1149. [PubMed] |
| 24. | Björklund M, Koivunen E. Gelatinase-mediated migration and invasion of cancer cells. Biochim Biophys Acta. 2005;1755:37-69. [PubMed] |