Published online Aug 21, 2007. doi: 10.3748/wjg.v13.i31.4264
Revised: April 2, 2007
Accepted: April 11, 2007
Published online: August 21, 2007
AIM: To evaluate the efficacy and safety of entecavir (ETV) in hepatitis Be antigen (HBeAg)-positive chronic hepatitis B (CHB) patients who had not received a nucleoside analogue and who had failed in lamivudine (LVD) therapy.
METHODS: Sixty-one patients were divided into three groups. Forty-two patients who had not received a nucleoside analogue were randomized into two groups: group A (n = 21) received LVD 100 mg/d and group B (n = 21) received ETV 0.5 mg/d. The remaing 19 patients treated with LVD (n = 19), who switched to ETV 1.0 mg/d served as group C. All patients were treated for 48 wk. HBV DNA levels were measured with polimerase-chain-reaction (PCR) analysis. Liver function tests, HBV serology and safety assessments were also conducted.
RESULTS: Significantly more patients in group B (52.1% and 71.4%) had undetectable HBV DNA levels than in groups A (35.8% and 38%; P < 0.0001) and C (10.6% and 21.1%, P < 0.0001) at week 24 and 48, respectively. At wk 48, ALT levels were normalized in more patients in group B (85.7%) than in groups A (76.2%) and C (74%).
CONCLUSION: ETV had a significantly higher response rate than LVD in patients with HBeAg-positive CHB who had not previously received a nucleoside analogue; ETV can effectively inhibit the replication of HBV DNA and normalize the levels of ALT in refractory CHB patients treated with LVD; and ETV is safe in clinical application.
- Citation: Ren FY, Piao DM, Piao XX. A one-year trial of entecavir treatment in patients with HBeAg-positive chronic hepatitis B. World J Gastroenterol 2007; 13(31): 4264-4267
- URL: https://www.wjgnet.com/1007-9327/full/v13/i31/4264.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i31.4264
More than 400 million people worldwide are chronically infected with hepatitis B virus (HBV)[1]. Suppression of HBV replication is a principal goal of long-term hepatitis B therapy[2]. Lamivudine (LVD) is the first potentially non-cytotoxic oral nucleoside analogue approved for the treatment of chronic hepatitis B. Studies of long-term LVD treatment in patients with HBeAg-positive chronic hepatitis B have found that maintenance of virologic suppression is associated with improved histologic findings in the liver[3,4]. However, prolonged treatment of chronic HBV infection with LVD is frequently associated with the development of mutations in the HBV polymerase gene conferring resistance to LVD[5-7]. The emergence of LVD resistance may result in a rebound in viral load, ALT elevation and progression of hepatic disease[8-11]. Preclinical and phase III studies suggested ETV is a novel hepatitis B antiviral agent with potent antiviral activity against the HBV and LVD-resistant HBV[12-14].
The present study was designed to assess the efficacy and safety of ETV in patients with HBeAg-positive chronic hepatitis B (CHB), and compare the ETV and LVD therapy in patients treated with a nucleoside analogue.
This trial involves 61 adult patients from Yan Ban area in Jilin Province with HBeAg-positive chronic hepatitis B enrolled between February 2006 to March 2006 who received antiviral agents with activity against hepatitis B, or LVD. Inclusion and exclusion criteria for the study followed the study of HBeAg-positive patients reported by Chang et al[12]. The patients aged between 19 and 68 years and had HBeAg-positive chronic hepatitis B and compensated liver function: a total serum bilirubin level of 2.5 mg per deciliter (42.8 μmol/L)or less; a prothrombin time not more than three seconds longer than normal or a ratio not greater than 1.5; a serum albumin level of at least 3.0 g per deciliter; and no histrory of variceal bleeding or hepatic encephalopathy. Eligible patients also had detectable hepatitis B surface antigen (HBsAg) for at least 24 wk before screening, evidence of HBV DNA by any commercial assay at least 4 wk before screening, and a serum alanine aminotransferase level 1.3-10.0 times that of the upper limit of normal at screening. Exclusion criteria included co-infection with hepatitis C, D, or the human immunodeficiency virus, the presence of other of liver diseases; use of interferon, thymosin, antiviral agents with activity against hepatitis B within 24 wk before randomization; prior LVD therapy lasting more than 12 wk; an alpha fetoprotein level greater than 100 ng/mL; a history of ascites requiring diuretics or paracentesis; and previous treatment with ETV and Adefovir (ADV).
The study was conducted in compliance with the Helsinki Declaration and approved by the local regulatory bodies. All patients provided written informed consent.
The 61 patients were divided into three groups. Forty-two patients who had not received a nucleoside analogue were randomized into two groups: group A (n = 21) received LVD 100 mg/d and group B (n = 21) received ETV 0.5 mg/d. The remaing 19 refratory patients treated with LVD served as group C (n = 19), who switched to ETV 1.0 mg/d. HBV DNA levels were measured by PCR analysis at week 2, 4, 8, 12, 16, 20, 24, 28, 32, 36, 40, 44, and 48. ALT, HBV serology and safety assessments were also conducted. All patients were treated for 48 wk.
At 24 wk and/or 48 wk, the reduction in HBV DNA level and the proportion of patients with undetectable HBV DNA, HBeAg loss, HBeAg seroconversion (HBeAg loss and the appearance of HBe antibody), and normalization of ALT were evaluated. Serum HBV DNA was measured using the PCR analysis. Liver function tests were performed by standard methods.
Measures of safety included adverse events, hematologic measurement, chemical measurement, vital signs, and deaths. The severity of adverse events was graded based on a three-point scale (mild, moderate, and severe), and causality was determined by the investigator. The safety was assessed throughout the treatment. Hepatitis flares during treatment were defined with elevation of the alanine aminotransferase level by more than twice the baseline level and more than 10 times the upper normal limit.
Baseline characteristics of the patients were presented as means ± SE. Statistical analyses were conducted according to the intention-to-treat procedure. The Chi-square test or the Fisher’s exact test was used to compare differences in proportion between treatment groups. Continuous data were analyzed using Kruskall-Wallis test and Mann-Whithey test. A two-tailed P value of less than 0.05 was considered statistically significant.
All treatments were well matched with regard to baseline characteristics (Table 1). Of the 61 patients, 60 completed the treatment, while the remaining one drop out the study, and one group A patient withdrew at 32 wk.
| Group A | Group B | Group C | |
| Characteristics | (LVD, 100 mg/d) | (ETV, 0.5 mg/d) | (ETV, 1.0 mg/d) |
| Patients (n) | 21 | 21 | 19 |
| Age ( yr) | 31 ± 12 | 33 ± 10 | 31 ± 11 |
| Sex ( M/F ) | 11/10 | 12/9 | 11/8 |
| HBV DNA (log copies/mL) | 8.49 ± 1.10 | 8.52 ± 1.02 | 8.60 ± 0.90 |
| ALT (IU/L) | 201.6 ± 178.2 | 211.2 ± 144.7 | 198.9 ± 169.5 |
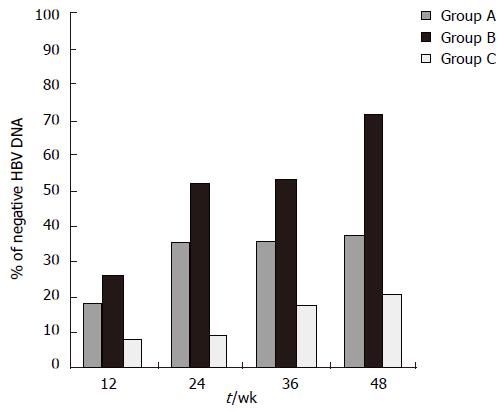
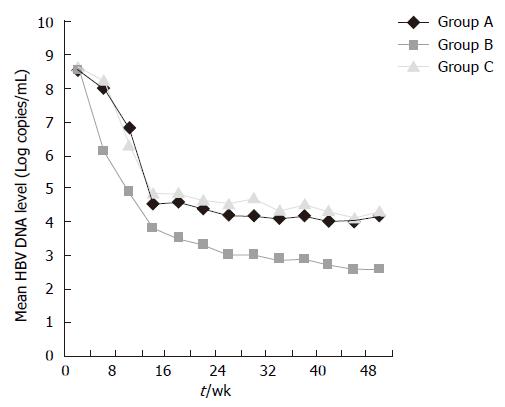
At wk 24, significantly more patients in group B (52.1%) had undetectable HBV DNA levels than in groups A (35.8%; P < 0.0001) and C (10.6%, P < 0.0001). HBV DNA was negative at wk 48 in 8 (38%) patients treated with LVD (group A) and in 15 (71.4%) patients treated with ETV 0.5 mg/d (group B), and 4 (21.1%) patients switched to ETV 1.0 mg/d (group C). HBV DNA became negative at wk 12, 4 and 12, respectively in the three groups. Significantly more patients in group B had undetectable HBV DNA levels (71.4%) by PCR analysis than in group A (38%; P < 0.0001) (Figure 1). HBV DNA levels in group B fell throughout the treatment (Figure 2). The mean reduction in the serum HBV DNA levels at wk 48 was also greater in group B (5.9 log copies/mL) than in groups A (4.2 log copies/mL, P < 0.001) and C (4.3 log copies/mL).
At wk 48, the rate of HBeAg seroconversion in group B (15%) was similar to group A (18%); and it was 6% in group C.
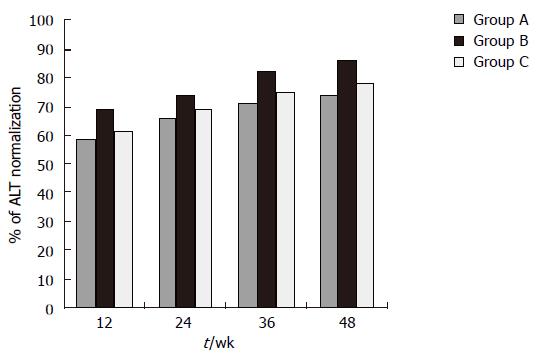
At wk 48, significantly more patients achieved normalization of ALT levels in group B (85.7%) than in groups A (76.2%) and C (74%). A mild flare-up without bilirubin alteration was observed only in one group A patient (Figure 3).
The frequency of adverse events during treatment was similar among the three groups. The most frequent adverse events were headache, upper respiratory tract infection, nasopharyngitis, cough, pyrexia, upper abdominal pain, fatigue, and diarrhea, most of which were of mild-to-moderate severety. No hepatocellular carcinoma, liver decompensation of death was recorded (Table 2). One virologic rebound due to resistance occurred in group C.
| Adverse events | Group A | Group B | Group C |
| Patients (n) | 21 | 21 | 19 |
| Headache | 7 | 6 | 7 |
| Upper respiratory tract infection | 3 | 3 | 4 |
| Nasopharyngitis | 4 | 3 | 4 |
| Cough | 2 | 2 | 1 |
| Pyrexia | 3 | 3 | 4 |
| Upper abdominal pain | 2 | 2 | 1 |
| Fatigue | 5 | 6 | 5 |
| Diarrhea | 7 | 6 | 6 |
| Death | 0 | 0 | 0 |
| ALT > 2 × baseline > and 10 × ULN | 4 | 1 | 2 |
Therapeutic options against hepatitis B virus remain a major clinical challenge. The goal of teatment is HBV DNA suppression, normalization of ALT levels and reduction in liver necroinflammation. Currently, Nucleoside analogs such as LVD, ADV and ETV are well tolerated and lower and normalize the serum HBV DNA and ALT levels[3,4,12-18]. However, the efficacy and safety profile of LVD, ADV and ETV had different clinical situations in the treatment of hepatitis B.
In this study, ETV was associated with suppression of viral replication in patients who had undetectable levels of HBV DNA 48 wk after the start of treatment and with the magnitude of reduction in the level of HBV DNA. ALT levels became normal in more patients after treatment with ETV than with LVD, which was in agreement with a previous study of patients with HBeAg-positive chronic hepatitis B[12]. Although this suggests that ETV may be more effective than LVD in preventing adverse clinical outcomes among patients with HBeAg-positive chronic hepatitis B, a longer surveillance is necessary. In patients with LVD-refractory HBeAg-positive chronic hepatitis B, we found that the switching to ETV can effectively inhibit the raplication of HBV DNA and normalize the levels of ALT. These results were in agreement with previous studies reported by Sherman M et al, and Chang TT et al[14,15].
The tolerability and safety profiles of ETV therapy for patients who had not previously received a nucleoside analogue and for patients who switched to ETV were similar to those reported in patients with HBeAg-positive CHB, and there were no unexpected adverse effects[12-15]. On the basis of its extensive use in both chronic hepatitis B and HIV infection, LVD is established as a well-tolerated antiviral agent. However, continued surveillance is necessary to confirm its long-term safety.
Liaw et al[19] showed that for patients with HBeAg-negative or HBeAg-positive chronic hepatitis B who had cirrhosis or advanced fibrosis, treatment with LVD slows the progression of liver disease, presumably by suppressing the viral replication and decreasing the resultant necroinflammatory response. ETV, The response rates of ETV were significantly higher than LVD in patients who had not previously received a nucleoside analogue, which demonstrated benefit as a primary therapy for HBeAg-positive chronic hepatitis B. The advent of more potent antiviral agents for the treatment of chronic hepatitis B offers the potential to control HBV DNA replication and to arrest or halt the progression of liver disease.
S- Editor Liu Y L- Editor Ma JY E- Editor Liu Y
| 1. | Lai CL, Ratziu V, Yuen MF, Poynard T. Viral hepatitis B. Lancet. 2003;362:2089-2094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 595] [Cited by in RCA: 590] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 2. | Liaw YF, Leung N, Guan R, Lau GK, Merican I. Asian-Pacific consensus statement on the management of chronic hepatitis B: an update. J Gastroenterol Hepatol. 2003;18:239-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 79] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 3. | Lai CL, Chien RN, Leung NW, Chang TT, Guan R, Tai DI, Ng KY, Wu PC, Dent JC, Barber J. A one-year trial of lamivudine for chronic hepatitis B. Asia Hepatitis Lamivudine Study Group. N Engl J Med. 1998;339:61-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1381] [Cited by in RCA: 1347] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 4. | Dienstag JL, Schiff ER, Wright TL, Perrillo RP, Hann HW, Goodman Z, Crowther L, Condreay LD, Woessner M, Rubin M. Lamivudine as initial treatment for chronic hepatitis B in the United States. N Engl J Med. 1999;341:1256-1263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1054] [Cited by in RCA: 1008] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 5. | Lau DT, Khokhar MF, Doo E, Ghany MG, Herion D, Park Y, Kleiner DE, Schmid P, Condreay LD, Gauthier J. Long-term therapy of chronic hepatitis B with lamivudine. Hepatology. 2000;32:828-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 270] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 6. | Liaw YF, Leung NW, Chang TT, Guan R, Tai DI, Ng KY, Chien RN, Dent J, Roman L, Edmundson S. Effects of extended lamivudine therapy in Asian patients with chronic hepatitis B. Asia Hepatitis Lamivudine Study Group. Gastroenterology. 2000;119:172-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 532] [Cited by in RCA: 519] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 7. | Chang TT, Lai CL, Chien RN, Guan R, Lim SG, Lee CM, Ng KY, Nicholls GJ, Dent JC, Leung NW. Four years of lamivudine treatment in Chinese patients with chronic hepatitis B. J Gastroenterol Hepatol. 2004;19:1276-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 196] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 8. | Liaw YF. Impact of YMDD mutations during lamivudine therapy in patients with chronic hepatitis B. Antivir Chem Chemother. 2001;12 Suppl 1:67-71. [PubMed] |
| 9. | Liaw YF, Chien RN, Yeh CT, Tsai SL, Chu CM. Acute exacerbation and hepatitis B virus clearance after emergence of YMDD motif mutation during lamivudine therapy. Hepatology. 1999;30:567-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 403] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 10. | Dienstag JL, Goldin RD, Heathcote EJ, Hann HW, Woessner M, Stephenson SL, Gardner S, Gray DF, Schiff ER. Histological outcome during long-term lamivudine therapy. Gastroenterology. 2003;124:105-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 602] [Cited by in RCA: 574] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 11. | Mutimer D. Hepatitis B virus infection: resistance to antiviral agents. J Clin Virol. 2001;21:239-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Chang TT, Gish RG, de Man R, Gadano A, Sollano J, Chao YC, Lok AS, Han KH, Goodman Z, Zhu J. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N Engl J Med. 2006;354:1001-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1107] [Cited by in RCA: 1089] [Article Influence: 57.3] [Reference Citation Analysis (0)] |
| 13. | Lai CL, Shouval D, Lok AS, Chang TT, Cheinquer H, Goodman Z, DeHertogh D, Wilber R, Zink RC, Cross A. Entecavir versus lamivudine for patients with HBeAg-negative chronic hepatitis B. N Engl J Med. 2006;354:1011-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 915] [Cited by in RCA: 909] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 14. | Sherman M, Yurdaydin C, Sollano J, Silva M, Liaw YF, Cianciara J, Boron-Kaczmarska A, Martin P, Goodman Z, Colonno R. Entecavir for treatment of lamivudine-refractory, HBeAg-positive chronic hepatitis B. Gastroenterology. 2006;130:2039-2049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 312] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 15. | Chang TT, Gish RG, Hadziyannis SJ, Cianciara J, Rizzetto M, Schiff ER, Pastore G, Bacon BR, Poynard T, Joshi S. A dose-ranging study of the efficacy and tolerability of entecavir in Lamivudine-refractory chronic hepatitis B patients. Gastroenterology. 2005;129:1198-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 197] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 16. | Marcellin P, Chang TT, Lim SG, Tong MJ, Sievert W, Shiffman ML, Jeffers L, Goodman Z, Wulfsohn MS, Xiong S. Adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B. N Engl J Med. 2003;348:808-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1060] [Cited by in RCA: 1019] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 17. | Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, Chang TT, Kitis G, Rizzetto M, Marcellin P, Lim SG, Goodman Z, Wulfsohn MS. Adefovir dipivoxil for the treatment of hepatitis B e antigen-negative chronic hepatitis B. N Engl J Med. 2003;348:800-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 780] [Cited by in RCA: 735] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 18. | Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, Chang TT, Kitis G, Rizzetto M, Marcellin P, Lim SG, Goodman Z, Ma J. Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B. N Engl J Med. 2005;352:2673-2681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 422] [Cited by in RCA: 408] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 19. | Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, Tanwandee T, Tao QM, Shue K, Keene ON, Dixon JS, Gray DF, Sabbat J. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521-1531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1739] [Cited by in RCA: 1740] [Article Influence: 82.9] [Reference Citation Analysis (0)] |