Published online Aug 21, 2007. doi: 10.3748/wjg.v13.i31.4255
Revised: April 23, 2007
Accepted: April 26, 2007
Published online: August 21, 2007
AIM: To investigate whether Epigallocatechin-3-gallate (EGCG) can induce apoptosis of the gastric cancer cell line MKN45 and its apoptotic pathway.
METHODS: To determine this, apoptotic rates of MKN45 cells after EGCG treatment with or without caspase-3 inhibitor were evaluated by Annexin V-FITC + PI staining The influence of EGCG on the activity of caspase-3 in the MKN45 cells was determined by ELISA. By Rhodamine123 staining, the membrane potential change of the mitochondrion was also investigated, and mRNAs and protein expression of the bcl-2 family were analyzed by RT-PCR and Western blot.
RESULTS: EGCG can induce apoptosis of MKN45 cells in time- and dose-dependent manner. Eight hours after EGCG treatment, the activity of caspase-3 in the MKN45 increased, especially 12 h after treatment. The mitochondrial membrane potential was significantly weakened 4 h after EGCG insult. The mRNA and protein expression levels of pro-apoptotic members, such as Bax, Bid and Bad, were upregulated gradually as treated time increased. Moreover, the mRNA and protein expression levels of anti-apoptotic members, such as Bcl-xL and Bcl-2, were inhibited.
CONCLUSION: These data support that EGCG can induce apoptosis of the human gastric cancer cell line MKN45, and the effect is in a time- and dose-dependent manner. The apoptotic pathway triggered by EGCG in MKN45 is mitochondrial-dependent.
-
Citation: Ran ZH, Xu Q, Tong JL, Xiao SD. Apoptotic effect of Epigallocatechin-3-gallate on the human gastric cancer cell line MKN45
via activation of the mitochondrial pathway. World J Gastroenterol 2007; 13(31): 4255-4259 - URL: https://www.wjgnet.com/1007-9327/full/v13/i31/4255.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i31.4255
Epigallocatechin-3-gallate is the main monomer in green tea polyphenol; the others are Epigallocatechin (EGC), Epicatechin-3-gallate (ECG) and Epicatechin (EC). Many studies have shown green tea polyphenolic compounds could inhibit tumor growth and metastasis both in vivo and in vitro[1,2]. It has a wide range of target organs, such as skin, lung, esophagus, stomach, duodenum and colon, liver, pancreas, bladder and prostate without serious adverse effects to rodents and humans[3].
Recent studies have indicated that EGCG inhibits proliferation and induces apoptosis of several tumor cell lines in vitro and decreases the size of tumors in mice and rats[4,5]. However, the cell death mechanism of EGCG on gastric cancer cells is presently unknown. The present study will focus on the apoptosis inducing effect of EGCG on the human gastric cancer cells and its apoptotic pathway.
The human gastric cancer cell line MKN45 was kept at our institute. Cells were maintained in RPMI 1640 medium (Gibco BRL, USA) supplemented with 10% heat-inactivated fetal bovine serum (Gibco BRL, USA), penicillin (100 μg/mL) and streptomycin (100 U/mL) in a 5% CO2 atmosphere at 37°C. EGCG monomer (purity > 99%) was purchased from the Tea Research Institute, Chinese Academy of Agriculture. Annexin V-FITC + PI apoptosis detection kit was purchased from Biosea Corporation (Beijing, China). Caspase-3 cellular activity assay kit and caspase-3 inhibitor (caspase-3 inhibitor II) were purchased from Calbiochem (Germany). Rhodamine 123 fluorochrome was purchased from Molecular Probes (Germany). A rabbit monoclonal antibody to Bcl-xL, mouse monoclonal antibodies to bcl-2 and Bad, and rabbit polyclonal antibodies to Bax and Bid were purchased from Santa Cruz Biotech (USA).
Exponentially growing MKN45 cells were trypsinized and harvested in suspension, then cells were seeded onto 96 well plates at a density of 5 × 103 cells per well. Twenty-four hours later, fresh RPMI-1640 medium containing different concentrations of EGCG (40, 60, 80 μmol/L) was added at 100 μL per well and six replicate wells for each treatment. In EGCG treated groups (40 and 80 μmol/L), caspase-3 inhibitor II was added at a final concentration of 2 μmol/L. In the control group, equal volumes of RPMI-1640 medium were added. After being incubated for 12, 18, 24 and 48 h respectively, 20 μg MTT (5 mg/mL) was added to each well of one 96 well plate. Cells were further incubated for 4 h. Then, after discarding the medium, 100 μL DMSO was added and gently mixed for 20 min. Finally, the OD values were measured by an ELISA reader (Microplate Reader, Model 550, Bio Rad). Cell survival was calculated as OD value of EGCG exerted cells/OD value of mock treated cells × 100%.
According to the manufacturer’s instructions, exponentially growing MKN45 cells were trypsinized and harvested in suspension. Then, cells were seeded into 25 cm2 flasks at a density of 2 × 105 cells, 5 mL per flask. Twenty-four hours later, EGCG was added at final concentrations of 40, 60 and 80 μmol/L respectively. Caspase-3 inhibitor II was added in 40 μmol/L and 80 μmol/L EGCG treated groups, with a final concentration of 2 μmol/L. In the control group, equal volume of RPMI-1640 medium was added. After being incubated for 12, 18 and 24 h, cells were harvested by trypsinization and rinsed with cold PBS twice. After centrifugation (4°C, 1000 × g) for 10 min, cells were suspended by 200 μL binding buffer and then treated with 10 μL Annexin V-FITC and 5 μL propidium iodide (PI) for 15 min at room temperature. Flow cytometric analysis of cells was performed with an EpicsXL Coulter flow cytometer. Both the MTT test and cytometric analysis were repeated three times.
Exponentially growing MKN45 cells were seeded into 75 cm2 flasks at a density of 6 × 105 cells, 10 mL per flask. Twenty-four hours later, EGCG was added at different final concentrations of 40, 60 and 80 μmol/L respectively. Caspase-3 inhibitor II was added with a final concentration of 2 μmol/L in the 80 μmol/L EGCG treated group. In mock-treated cells, equal volumes of RPMI-1640 medium were added. After being incubated for 8, 12, 18 and 24 h, cells were harvested and rinsed with cold PBS twice. After centrifugation (4°C, 1000 × g) for 10 min, according to the manufacturer’s instructions, cells were suspended by 20 μL of cell lysis buffer and incubated on ice for 15-20 min. Then, cells were transferred into a sterile eppendorf tube and centrifuged at 10 000 × g for 10 min at 4°C. Ten microlitres of supernatant were collected and added to 96 well plates, then treated with 10 μL caspase-3 substrate AC-DEVD-ρNA, the reaction system was adjusted to 100 μL and measured by an ELISA reader (Microplate Reader, Model 550, Bio Rad) at 405 nm with a reference wavelength of 630 nm. Data were recorded at 10 min intervals for 60 to 120 min. Data presented as A405 versus time for each sample were plotted. Then, the activity of caspase-3 was calculated based upon the obtained slope of the line.
Activity (pmol/min) = slope (ΔA/min) × assay vol (μL) ×50 μmol/L /average A405 This experiment was repeated three times.
Exponentially growing MKN45 cells were seeded into 25 cm2 flasks at a density of 2 × 105 cells, 5 mL per flask. Twenty-four hours later, EGCG was added at different final concentrations of 40, 60, 80 μmol/L, respectively. In mock-treated cells, equal volumes of RPMI-1640 medium were added. After incubating 4, 8 and 12 h, 5 μmol/L Rhodamine 123 were added into the flasks, incubated at 37°C for 30 min, and cells were harvested by trypsinization and rinsed with cold PBS twice. After centrifugation (4°C 1000 × g) for 10 min, the cells were suspended in 200 μL binding buffer and then analyzed by flow cytometry (EpicsXL Coulter) with an excitation wavelength of 505 nm and emission wavelength of 534 nm. The experiment was repeated three times and the average values were used.
Total RNA was extracted from cells that had been treated with EGCG (80 μmol/L) for 0, 12, 24 and 36 h and subjected to RT-PCR. The primer sequences and amplification sizes were as follows: bax forward: 5'-TCTGACGGCAACTTCAACTG-3', bax reverse: 5'-CACTGTGACCTGCTCCAGAA-3' (299 bp product), bid forward: 5'-ACAGCATGGACCGTAGCATC-3', bid reverse: 5'-GTCCATCCCATTTCTGGCTA-3' (302 bp product), bad forward: 5'-CCCAGAGTTTGAGCCGAGTG-3', bad reverse: 5'-GCTGTGCTGCCCAGAGGTT-3' (314 bp product), bcl-2 forward: 5'-GGAGGATTGTGGCCTTCTTT-3', bcl-2 reverse: 5'-TCACTTGTGGCTCAGATAGGC-3' (287 bp product), bcl-xl forward: 5'-GAGGCAGGCGACGAGTTT-3', bcl-xl reverse: 5'-GACGGAGGATGTGGTGGA-3' (491 bp product), ß-actin forward: 5'-GGACTTCGAGCAAGAGATGG-3', β-actin reverse: 5'-ACATCTGCTGGAAGGTGGAC-3' (404 bp product). The PCR protocol for bcl-2 consisted of denaturation at 94°C for 5 min, 33 cycles of denaturation at 94°C for 30 s, annealing at 55.8°C for 1 min and extension at 72°C for 1 min, final extension at 72°C for 10 min. The protocols for ß-actin, bax, bid, bcl-xl and bad were the same except for annealing at 60°C, 62°C, 56.3°C, 56°C and 57°C for 1 min and amplification for 30, 35, 30, 32, and 32 cycles respectively. Amplification was exponential throughout all cycles. The PCR products were electrophoresed in a 2% agarose gel and stained with goldviewTM.
Cells were treated with 80 μmol/L EGCG for 0, 12, 24 and 36 h. At appropriate time, cells were lysed with lysis buffer (1% Triton X-100, 1 mmoi/L sodium ortho-vanadate, 4.9 mmol/L MgCl2, 1 mmol/L PMSF, 21 μg/mL aprotinin, and 0.5 μg/mL leupeptin). An equal amount of protein was resolved in SDS-polyacrylamide gel electrophoresis. The proteins were transferred to a PVDF membrane (Immobilon-P, Millipore, USA), incubated with primary antibody and then with horse-radish peroxidase conjugated secondary antibody. The primary antibodies were anti-Bcl-2, Bcl-xL, Bad, Bid and Bax (Santa Cruz Biotech, USA). The signals were visualized using the ECL enhanced chemiluminescence kits.
All data were expressed as mean ± SD. Statistical analysis was performed by using SAS 6.12, t-test, or correlation analysis where appropriate. A level of P < 0.05 was considered significant in all statistical tests.
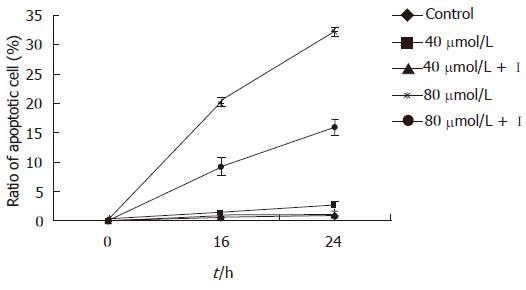
In the present study EGCG exhibited time-and dose-dependent apoptosis inducing effects on MKN45 cells. The ratio of apoptotic cells was (9.97 ± 1.16)% 12 h after 80 μmol/L EGCG treatment, which was significantly higher than that of mock-treated [(1.18 ± 0.27)%, P < 0.01]. After 18 h treatment, the apoptotic cells were markedly increased in all three concentrations of EGCG treated groups compared to that in mock-treated ones (P < 0.05). The result showed that terminal stage apoptotic cells increased obviously than early stage cells after 24 h treatment, and the cells were mainly in the terminal apoptotic stage and necrotic stage after being treated for 48 h (Table 1). However, the inhibitory effect of EGCG on MKN45 cells was significantly weakened after treatment of caspase-3 inhibitor (P < 0.05) (Figure 1). Meanwhile, the result of MTT was consistent with Annexin V-FITC + PI.
| Celldeath | Dose(μmol/L) | Time after treatment | |||
| 12 h | 18 h | 24 h | 48 h | ||
| Early | Control | 0.86 ± 0.19 | 0.70 ± 0.25 | 0.91 ± 0.18 | 0.96 ± 0.12 |
| stage | 40 | 1.04 ± 0.51 | 2.54 ± 1.79a | 1.72 ± 1.30 | 1.57 ± 0.63 |
| apoptotic | 60 | 1.91 ± 1.30 | 5.73 ± 0.81a | 5.69 ± 2.55a | 14.45 ± 3.38a |
| cells | 80 | 6.30 ± 0.16a | 14.93 ± 0.09a | 13.05 ± 0.21b | 4.09 ± 1.49a |
| Terminal | Control | 0.32 ± 0.25 | 0.10 ± 0.10 | 0.30 ± 0.16 | 0.55 ± 0.05 |
| stage | 40 | 0.65 ± 0.44 | 0.40 ± 0.26a | 0.50 ± 0.17a | 5.28 ± 2.29a |
| apoptotic | 60 | 2.00 ± 0.84 | 1.06 ± 0.10a | 2.49 ± 0.52b | 26.45 ± 2.32a |
| cells | 80 | 3.67 ± 1.36a | 8.48 ± 0.14b | 19.05 ± 1.06b | 62.90 ± 4.70b |
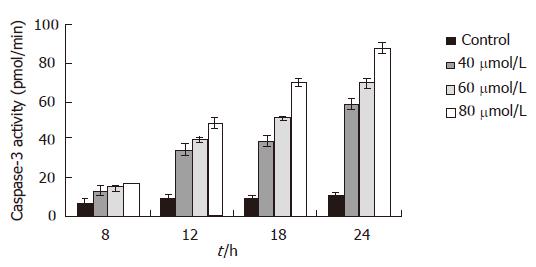
Eight hours after EGCG treatment, the activity of capsases-3 in MKN45 cells was increased, but only 80 μmol/L EGCG treated group was significantly increased (P < 0.05). It was gradually and obviously increased 12 h after EGCG insult in all three different concentrations (P < 0.01). After a 24 h treatment of 80 μmol/L EGCG, the activity of caspase-3 was 8.58 folds higher than that the of mock-treated. The influence of EGCG on the activity of caspase-3 in the MKN45 cells was in a time- and dose-dependent manner (Figure 2). However, after being treated with caspase-3 inhibitor, the caspase-3 activity was dramatically decreased. Moreover, after the treatment of caspase-3 inhibitor, the inhibitory effect of EGCG on MKN45 cells was significantly weakened, and the cell apoptotic rates were also dramatically decreased.
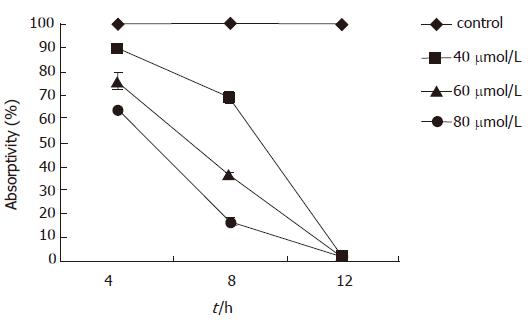
The mitochondrial membrane potential began to decline 4 h after EGCG treatment, which was positively correlated with time and dose. The mitochondrial potential was (89.50 ± 0.86)% 4 h after 40 μmol/L EGCG treatment, which was significantly lower than that of the mock-treated (P < 0.01). The absorptivity of Rhodamine 123 reached the lowest level [(1.36 ± 0.66)%] 12 h after 80 μmol/L EGCG treatment (Figure 3).
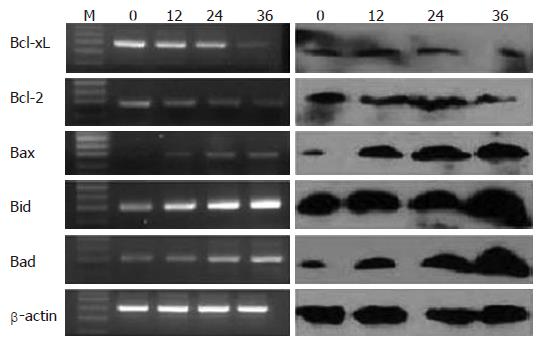
After 80 μmol/L EGCG treatment, a significant decrease of anti-apoptotic bcl-2 family of mRNAs and proteins, such as bcl-2 and Bcl-xL, were detected (P < 0.05). However, there was a significant increase of pro-apoptotic bcl-2 family of mRNAs and proteins, such as Bad, Bid and Bax (P < 0.05). The changes of these genes were time course-dependent, as show in Figure 4.
EGCG is the most abundant catechin in the green tea extract and has significant effects against tumorigenesis and tumor growth[6-8]. The biological activities of EGCG include antioxidative activities, inhibition of cell growth, and antiviral activities[9]. EGCG can also inhibit mutagenesis induced by carcinogens, inhibit transformation, tumour invasion and angiogenesis, induce cell growth arrest in G1 phase and cell apoptosis[10,11].
In our previous study, we have demonstrated that EGCG can inhibit the growth of different digestive tumor cells, and MKN45 was the most sensitive one among the gastric cancer cell lines tested[12]. EGCG can induce cancer cell apoptosis by various pathways. On the one hand, EGCG can directly bind to the Fas death receptor to initiate the caspase-8 activation and apoptosis[13]. On the other hand, EGCG can also induce apoptosis by the mitochondrion pathway. The apoptosis inducing effect of EGCG on human colon adenocarcinoma cells HT-29 depends on the mitochondrion to activate caspase-9 and caspase-3[14].
The present study has classified and counted apoptotic cells in the early stage, terminal stage and necrotic stage respectively by flow cytometry, in order to investigate the apoptosis inducing effect of EGCG on the human gastric cancer cell line MKN45. The result showed that apoptotic cells appeared 12 h after being treated by EGCG, and the apoptotic rates were in a time- and dose-dependent manner. The apoptotic cells were mainly in early stage after being insulted by EGCG from 12 to 24 h, and terminal stage apoptotic cells were increased gradually from 24 to 48 h after EGCG treatment. The terminal stage apoptotic cells and necrotic cells were predominant 48 h later, which was more significant in the 80 μmol/L EGCG treated group. The ratio of apoptotic cells in 80 μmol/L EGCG treated group was significantly higher than that of mock-treated 12 h after treatment. However, 18 h after treatment, the apoptotic cells were markedly increased in all three doses of EGCG treated groups compared to that in mock- treated ones (P < 0.05). So, it can be concluded that EGCG could induce apoptosis of MKN45 cells, and the apoptotic rates were in a time- and dose-dependent manner. However, after the treatment of caspase-3 inhibitor, the cell apoptotic rates were dramatically decreased. The survival rates of MKN45 cells after EGCG treatment detected by MTT simultaneously was consistent with a normal cell percentage detected by Annexin V-FITC + PI. Hence, it indicated that the inhibitory effect of EGCG on MKN45 cells was mediated by the apoptosis pathway and this apoptotic procedure was associated with the activation of caspase-3.
Caspases play a pivotal role in the initiation and execution of apoptosis induced by various stimuli. Among them, caspase-3 is a key caspase involved in apoptosis, which is a joint effector of the endogenous and exogenous pathways[15]. Activated caspase-3 can hydrolyze PARP in the nucleus, resulting in DNA repair block and apoptosis[16]. We use specific caspase-3 substrate to interact with caspase-3 which was activated in the apoptotic pathway, and to detect caspase-3 activity at different time and dose of EGCG treatment by the ELISA method. In order to investigate the upstream events of caspase-3 activation in this apoptotic pathway, we measured the change of mitochondrial membrane potential. The change of mitochondrial permeability is considered the earliest event in the cell apoptosis cascade. The mitochondrial membrane potential change results in the cytochrome C release to initiate caspase-9 and -3 activation and subsequent apoptosis[14,17]. By Rhodamine 123 staining, the change of mitochondrial membrane potential was investigated. In normal cells, mitochondria can absorb Rhodamine 123, and its absorptivity will decline while the membrane potential decreases. The present result showed that the mitochondrial membrane potential began to decline 4 h after EGCG treatment, and the absorptivity of Rhodamine 123 reached the lowest level 12 h after EGCG treatment. Whilst, the activity of caspase-3 in MKN45 cells was increased 8 h after EGCG insult, and was apparently increased 12 h after EGCG insult. When the caspase-3 inhibitor was added, however, the caspase-3 activity was dramatically decreased. The change of mitochondrial membrane potential was earlier than the increase of caspase-3 activity, therefore, it is speculated that the apoptotic pathway triggered by EGCG in MKN45 cells was dependent on the mitochondrion to activate caspase-3.
The Bcl-2 family of proteins, including Bad, Bax, Bid, Bcl-2, and Bcl-xL, are key regulators of mitochondria-mediated apoptosis[18,19]. Pro-apoptotic Bad and Bax induce apoptotic cell death by promoting mitochondrial release of cytochrome-c and Smac[20], Anti-apoptotic Bcl-2 and Bcl-xL inhibit mitochondria-mediated apoptosis and promote cell survival by preventing cytochrome-c or Smac release from the mitochondria. A shift in the balance between anti- and pro-apoptotic bcl-2 family proteins towards the expression and activation of Bad and Bax proteins could lead to mitochondria-dependent caspase activation and apoptotic cell death. Thus, it is possible that EGCG induces mitochondrial release of cytochrome-C and Smac by affecting the expression of the bcl-2 family of proteins. Our study demonstrated that anti-apoptotic genes were down-regulated and pro-apoptotic genes were up-regulated by EGCG treatment in the MKN45, the level of transcription conforms to the protein level. The ratio of Bcl/Bax gradually decreased as the treated time increased.
In conclusion, the present study demonstrated that EGCG could induce apoptosis of the human gastric cancer cell line MKN45, and the effect was in a time- and dose-dependent manner. The apoptotic pathway triggered by EGCG in MKN45 cells was dependent on mitochondrial and caspase-3 pathways, also controlled by the ratio of Bcl-2/Bax. This investigation provides further evidence for its clinical application in the therapy of gastric cancer.
S- Editor Zhu LH L- Editor Alpini GD E- Editor Li JL
| 1. | Leone M, Zhai D, Sareth S, Kitada S, Reed JC, Pellecchia M. Cancer prevention by tea polyphenols is linked to their direct inhibition of antiapoptotic Bcl-2-family proteins. Cancer Res. 2003;63:8118-8121. [PubMed] |
| 2. | Yang CS, Chung JY, Yang G, Chhabra SK, Lee MJ. Tea and tea polyphenols in cancer prevention. J Nutr. 2000;130:472S-478S. [PubMed] |
| 3. | Suganuma M, Okabe S, Sueoka N, Sueoka E, Matsuyama S, Imai K, Nakachi K, Fujiki H. Green tea and cancer chemoprevention. Mutat Res. 1999;428:339-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 139] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 4. | Lambert JD, Yang CS. Mechanisms of cancer prevention by tea constituents. J Nutr. 2003;133:3262S-3267S. [PubMed] |
| 5. | Brusselmans K, De Schrijver E, Heyns W, Verhoeven G, Swinnen JV. Epigallocatechin-3-gallate is a potent natural inhibitor of fatty acid synthase in intact cells and selectively induces apoptosis in prostate cancer cells. Int J Cancer. 2003;106:856-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 143] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 6. | Chung FL, Wang M, Rivenson A, Iatropoulos MJ, Reinhardt JC, Pittman B, Ho CT, Amin SG. Inhibition of lung carcinogenesis by black tea in Fischer rats treated with a tobacco-specific carcinogen: caffeine as an important constituent. Cancer Res. 1998;58:4096-4101. [PubMed] |
| 7. | Mukhtar H, Ahmad N. Green tea in chemoprevention of cancer. Toxicol Sci. 1999;52:111-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 102] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 8. | Moyers SB, Kumar NB. Green tea polyphenols and cancer chemoprevention: multiple mechanisms and endpoints for phase II trials. Nutr Rev. 2004;62:204-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 110] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 9. | Chung JY, Huang C, Meng X, Dong Z, Yang CS. Inhibition of activator protein 1 activity and cell growth by purified green tea and black tea polyphenols in H-ras-transformed cells: structure-activity relationship and mechanisms involved. Cancer Res. 1999;59:4610-4617. [PubMed] |
| 10. | Nihal M, Ahmad N, Mukhtar H, Wood GS. Anti-proliferative and proapoptotic effects of (-)-epigallocatechin-3-gallate on human melanoma: possible implications for the chemoprevention of melanoma. Int J Cancer. 2005;114:513-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 163] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 11. | Hofmann CS, Sonenshein GE. Green tea polyphenol epigallocatechin-3 gallate induces apoptosis of proliferating vascular smooth muscle cells via activation of p53. FASEB J. 2003;17:702-704. [PubMed] |
| 12. | Mittal A, Pate MS, Wylie RC, Tollefsbol TO, Katiyar SK. EGCG down-regulates telomerase in human breast carcinoma MCF-7 cells, leading to suppression of cell viability and induction of apoptosis. Int J Oncol. 2004;24:703-710. [PubMed] |
| 13. | Hayakawa S, Saeki K, Sazuka M, Suzuki Y, Shoji Y, Ohta T, Kaji K, Yuo A, Isemura M. Apoptosis induction by epigallocatechin gallate involves its binding to Fas. Biochem Biophys Res Commun. 2001;285:1102-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 87] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | Chen C, Shen G, Hebbar V, Hu R, Owuor ED, Kong AN. Epigallocatechin-3-gallate-induced stress signals in HT-29 human colon adenocarcinoma cells. Carcinogenesis. 2003;24:1369-1378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 174] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 15. | Islam S, Islam N, Kermode T, Johnstone B, Mukhtar H, Moskowitz RW, Goldberg VM, Malemud CJ, Haqqi TM. Involvement of caspase-3 in epigallocatechin-3-gallate-mediated apoptosis of human chondrosarcoma cells. Biochem Biophys Res Commun. 2000;270:793-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 85] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Li YH, Wang C, Meng K, Chen LB, Zhou XJ. Influence of survivin and caspase-3 on cell apoptosis and prognosis in gastric carcinoma. World J Gastroenterol. 2004;10:1984-1988. [PubMed] |
| 17. | Hibasami H, Komiya T, Achiwa Y, Ohnishi K, Kojima T, Nakanishi K, Akashi K, Hara Y. Induction of apoptosis in human stomach cancer cells by green tea catechins. Oncol Rep. 1998;5:527-529. [PubMed] |
| 18. | Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3451] [Cited by in RCA: 3569] [Article Influence: 170.0] [Reference Citation Analysis (0)] |
| 19. | Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305:626-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2418] [Cited by in RCA: 2543] [Article Influence: 121.1] [Reference Citation Analysis (0)] |
| 20. | Deng Y, Lin Y, Wu X. TRAIL-induced apoptosis requires Bax-dependent mitochondrial release of Smac/DIABLO. Genes Dev. 2002;16:33-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 357] [Article Influence: 15.5] [Reference Citation Analysis (0)] |