Published online Aug 21, 2007. doi: 10.3748/wjg.v13.i31.4242
Revised: April 23, 2007
Accepted: April 27, 2007
Published online: August 21, 2007
AIM: To investigate the role of gene variants and derived haplotypes of the CLOCK transcription factor in nonalcoholic fatty liver disease (NAFLD) and their relation with the disease severity.
METHODS: A total of 136 patients with NAFLD and 64 healthy individuals were studied. Liver biopsy was performed in 91 patients. Six tag SNPs showing a minor allele frequency > 10% (rs1554483 C/G; rs11932595 A/G; rs4580704 C/G; rs6843722 A/C; rs6850524 C/G and rs4864548 A/G) encompassing 117 kb of chromosome 4 and representing 115 polymorphic sites (r2 > 0.8) were genotyped.
RESULTS: rs11932595 and rs6843722 showed significant associations with NAFLD (empiric P = 0.0449 and 0.023, respectively). A significant association was also observed between clinical or histologic spectrum of NAFLD and rs1554483 (empiric P = 0.0399), rs6843722 (empiric P = 0.0229) and rs6850524 (empiric P = 0.00899) and between fibrosis score and rs1554483 (empiric P = 0.02697), rs6843722 (empiric P = 0.01898) and rs4864548 (empiric P = 0.02697). Test of haplotypic association showed that CLOCK gene variant haplotypes frequencies in NAFLD individuals significantly differed from those in controls (empiric P = 0.0097).
CONCLUSION: Our study suggests a potential role of the CLOCK polymorphisms and their haplotypes in susceptibility to NAFLD and disease severity.
-
Citation: Sookoian S, Castaño G, Gemma C, Gianotti TF, Pirola CJ. Common genetic variations in
CLOCK transcription factor are associated with nonalcoholic fatty liver disease. World J Gastroenterol 2007; 13(31): 4242-4248 - URL: https://www.wjgnet.com/1007-9327/full/v13/i31/4242.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i31.4242
During the past 20 years, obesity has reached worldwide epidemic proportions, not only among adults but also in young people, thus becoming a major contributor to the global health burden imposed by chronic disease and disability[1,2]. Nonalcoholic fatty liver disease (NAFLD) is one of the most common abnormalities observed in obese persons and refers to a wide spectrum of liver diseases[3,4], ranging from fatty liver alone to nonalcoholic steatohepatitis (NASH) with evidence of liver cell injury, a mixed inflammatory lobular infiltrate, and variable fibrosis[5]. Estimates based on imaging and autopsy studies suggest that about 20% to 30% of the adult population in Western countries have excess fat accumulation in the liver[6].
The pathogenesis of NAFLD is multifactorial[6] and, as a complex disease, the disorder develops from the interplay between genes and the environment[7,8]. While it is well known that environmental risk factors influence the development and progression of NAFLD[9], the contribution of genetic variation to the disease predisposition is still inconclusive despite the fact that several genes have been suggested as potential candidates for advanced NAFLD progression[10-14].
In addition, the ectopic accumulation of fat in the liver has been strongly associated with metabolic factors. For instance, insulin resistance, intra-abdominal fat and body fat distribution appear to play an important role in the pathogenesis of NAFLD[15]. In fact, the metabolic syndrome is a strong predictor of NAFLD[16].
In mammals, many physiological processes, including energy metabolism and food intake, are driven by a circadian timing system comprising a master pacemaker in the suprachiasmatic nuclei of the hypothalamus and peripheral oscillators in most body cells[17,18]. Besides, many transcripts that participate in common metabolic pathways, such as metabolism of glucose, cholesterol and fatty acids, show circadian rhythmicity[19,20].
A recent report by Turek et al[21] showed that homo-zygous circadian CLOCK (circadian locomoter output cycles protein kaput) mutant mice had a greatly altered diurnal feeding rhythm, were hyperphagic and obese, and developed a metabolic syndrome with hyperleptinemia, hyperlipidemia, hypoinsulinemia, hyperglycemia, and hepatic steatosis.
Given the results of the aforementioned studies, which showed that altered circadian rhythmicity resulted in pathophysiological changes resembling metabolic syndrome and fatty liver disease, the aim of this study was to investigate the role of gene variants and their predicted haplotypes of the linkage disequilibrium (LD) block of the CLOCK gene in NAFLD in a candidate gene case-control association study. Additionally, we tested the hypothesis of a relation between the gene variants and disease severity.
A total of 136 consecutive unrelated patients of self-reported European ancestry with features of NAFLD (36 males and 100 females), including ultrasonographic (USG) examinations suggestive of fatty infiltration[22] performed in all the cases by the same operator, were included in this study. Secondary causes of steatosis, including alcohol abuse (≥ 30 g alcohol daily for men and ≥ 20 g for women), total parenteral nutrition, hepatitis B and hepatitis C virus infection, and the use of drugs known to precipitate steatosis, were excluded in all cases. By using standard clinical and laboratory evaluation as well as liver biopsy features when applicable, autoimmune liver disease, metabolic liver disease, Wilson’s disease, and α-1-antitrypsin deficiency were likewise ruled out in all patients.
For the evaluation of the disease severity, NAFLD cases were classified as follows: fatty liver with persistently normal liver function test (FL-NLFT); fatty liver with persistently abnormal liver function test (FL-ALFT); and NASH proven through biopsy as described below. Patients were defined to have abnormal liver function test in the presence of at least one of the following criteria: (1) elevated serum alanine (ALT) and/or aspartate aminotransferase (AST), defined as > 41 U/L; (2) gamma-glutamyl-transferase (GGT) > 50 U/L; and (3) alkaline phosphatase (FA) > 250 UI/L.
Additionally, 64 healthy individuals (22 males and 42 females) with the same demographic background and who underwent the annual health examination during the same study period were included in the study as a control group. All healthy controls were subjected to USG examination. None of them evidenced fatty change, biochemical abnormalities or features indicative of metabolic syndrome.
Health examinations included anthropometric measure-ments, a questionnaire on health-related behaviours, and biochemical determinations. Body mass index (BMI) was calculated as weight/height2 (kg/m2) and was used as the index for relative weight. Additionally, waist and hip circumferences were also assessed. Blood was drawn from fasting subjects who had lain in a supine resting position for at least 30 min. Serum insulin, total cholesterol, HDL and LDL-cholesterol, triglycerides, plasma glucose and liver function tests were measured by standard clinical laboratory techniques. Homeostasis Model Assessment (HOMA) was used to evaluate an insulin resistance index and was calculated as fasting serum insulin (μU/mL) × fasting plasma glucose (mmol/L)/22.5. Insulin resistance was defined as HOMA index ≥ 2.4[23]. Elevated blood pressure was defined as systolic arterial blood pressure (SABP) ≥ 130 mmHg and/or diastolic arterial blood pressure (DABP) ≥ 85 mmHg or receipt of anti-hypertensive medications. All the investigations performed in this study were conducted in accordance with the guidelines of the Declaration of Helsinki. Written consent from individuals was obtained in accordance with the procedures approved by the Ethical Committee of our institution.
A percutaneous liver biopsy (LB) was performed in 91 patients with fatty changes in the liver on USG plus persistently abnormal liver function tests (in at least 3 different determinations in a follow-up for 6 months period). LB was performed under ultrasound guidance with a modified 1.4-mm diameter Menghini needle (Hepafix, Braun, Germany) on an outpatient basis. Liver biopsy specimens were routinely fixed in 40 g/L formaldehyde (pH 7.4), embedded in paraffin and stained with hematoxylin and eosin, Masson trichrome and silver impregnation for reticular fibers. All biopsies were read by the same liver pathologist who was blinded to patient details. The diagnosis of NASH was confirmed by liver histology, and grading of necroinflammatory activity or staging of fibrosis was scored according to the system developed by Brunt et al[24]. NASH was defined as steatosis plus any stage of fibrosis or as steatosis plus lobular inflammation plus ballooning degeneration[6].
The genetic analyses were done on genomic DNA extracted from white blood cells by a standard method as previously described[25].
In order to assess the contribution of Clock gene variants to NAFLD, we selected tag SNPs (tSNPs) by using an aggressive tagging approach to construct single marker and multi-marker tests to capture alleles of interest[26] and the phase II genotyping data from the HapMap project for Caucasians from the CEU dataset with a minor allele frequency (MAF) ≥ 0.10 and a minimum r2 of 0.8.
Genotyping was performed by a high-throughput genotyping method involving PCR amplification of genomic DNA with two-tailed allele-specific primers that introduce priming sites for universal energy-transfer-labeled primers as previously described[27].
The PLINK software was used for assessing association between SNPs and affection status and quantitative traits as well as for testing Hardy-Weinberg equilibrium and LD measures. SNP haplotype analysis was performed by both WHAP[28] and Haploview[29] softwares. These tools were also used to obtain haplotype frequencies. Control for multiple testing was done by permutation testing of individual traits to obtain an empirical P-value.
Differences in genotype frequencies between cases and controls were analyzed using PLINK and WHAP softwares as previously described. Multi-marker haplotype test was performed by Haploview software[29].
Phenotypic quantitative data were expressed as mean ± SE. For univariate analysis, differences between groups were assessed by Student’s t test on log-transformed variables. The conservative Bonferroni procedure was used for multiple testing corrections. We used the CSS/Statistica program package, StatSoft V 6.0 (Tulsa, USA) to perform these analyses.
Table 1 shows the clinical features, anthropometric variables and laboratory findings of the cases and controls. NAFLD patients were older and had most of the risk factors of the metabolic syndrome: elevated BMI, waist-hip ratio, waist circumference, fasting insulin level, and HOMA index. Of 136 patients, 45 (33%) were classified as having FL-NLFT, 35 (25.7%) as FL-ANFT, and 56 (41.2%) as NASH proven through biopsy.
| Variables | Control group | NAFLD patients | EmpiricP value |
| Number of subjects | 64 | 136 | |
| Age, yr | 46.14 ± 1.64 | 55.58 ± 1.06 | 0.001 |
| Smoking habit, cigarettes/d | 1.58 ± 0.52 | 1.30 ± 0.75 | NS |
| Physical activity, h/wk | 1.4 ± 0.30 | 1.4 ± 0.32 | NS |
| Drinking habits, g alcohol/d | 1.72 ± 0.64 | 0.75 ± 0.42 | NS |
| BMI, kg/m2 | 25.61 ± 0.64 | 36.02 ± 3.11 | 0.001 |
| Waist circumference, cm | 84.13 ± 2.14 | 103.07 ± 1.42 | 0.001 |
| Waist-hip ratio | 0.84 ± 0.01 | 0.91 ± 0.007 | 0.001 |
| SABP, mmHg | 121.64 ± 1.87 | 124.05 ± 2.11 | NS |
| DABP, mmHg | 75.86 ± 1.32 | 78.38 ± 1.49 | NS |
| Fasting plasma glucose, mmol/L | 4.73 ± 0.07 | 5.74 ± 0.22 | 0.050 |
| Fasting plasma insulin, pmol/L | 46.1 ± 3.5 | 94.5 ± 6.9 | 0.001 |
| HOMA Index | 1.42 ± 0.12 | 3.44 ± 0.30 | 0.001 |
| Total cholesterol, mmol/L | 5.59 ± 0.17 | 5.44 ± 0.15 | NS |
| HDL cholesterol, mmol/L | 1.27 ± 0.09 | 1.20 ± 0.05 | NS |
| LDL-cholesterol, mmol/L | 3.22 ± 0.29 | 3.11 ± 0.14 | NS |
| Uric acid, μmol/L | 220 ± 51 | 244 ± 27 | NS |
| Triglycerides, mmol/L | 1.74 ± 0.13 | 2.02 ± 013 | NS |
| ALT, UI/L | 24.37 ± 5.2 | 49.25 ± 5.32 | 0.080 |
| AST, UI/L | 26.4 ± 2.0 | 36.72 ± 2.27 | 0.080 |
| γGT, UI/L | 36.50 ± 6.8 | 53.69 ± 5.32 | NS |
| AF, UI/L | 221.87 ± 13.0 | 228.90 ± 11.2 | NS |
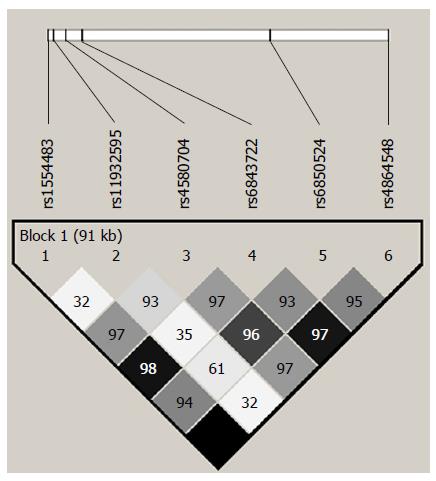
To diminish the burden of genotyping the multiple variants present in the CLOCK gene, we selected 6 tagSNPs showing a minor allele frequency (MAF) > 10% (rs1554483 C/G; rs11932595 A/G; rs4580704 C/G; rs6843722 A/C; rs6850524 C/G and rs4864548 G/A) encompassing 117 kb in chromosome 4. The 6 tagSNPs represent 115 polymorphic sites with an r2 > 0.8 considering the HapMap project data (Figure 1); Table 2 illustrates the tSNPs description. Test results from the Tagger algorithm showed the aforementioned single-marker tags and 8 multi-marker tags capturing all SNPs of MAF ≥ 5% (Table 3). The distribution of the genotypes was in Hardy-Weinberg equilibrium (data not shown).
| NCBI SNPreference1 | Location in theCLOCK gene | Hetero-zygosity | dsSNPallele | Minorallele | MAF |
| rs1554483 | Intron 12 | 0.494 | C/G | G | 0.491 |
| rs11932595 | Intron 10 | 0.478 | A/G | G | 0.288 |
| rs4580704 | Intron 8 | 0.395 | C/G | G | 0.311 |
| rs6843722 | Intron 7 | 0.483 | A/C | C | 0.476 |
| rs6850524 | Intron 1 | 0.490 | C/G | C | 0.3557 |
| rs4864548 | Upstream | 0.490 | G/A | A | 0.478 |
| Single tagSNP | Associated | Frequency | OR (95% CI) | Empiric |
| allele | P value | |||
| rs1554483 | G | 0.491 | 1.51 (0.93-2.45) | 0.0599 |
| rs11932595 | A | 0.712 | 1.65 (1.10-2.47) | 0.0449 |
| rs4580704 | C | 0.688 | 1.03 (0.68-1.55) | 0.99 |
| rs6843722 | C | 0.476 | 1.70 (1.05-2.76) | 0.0239 |
| rs6850524 | G | 0.643 | 1.40 (0.85-2.29) | 0.1458 |
| rs4864548 | A | 0.478 | 1.54 (0.95-2.50) | 0.0779 |
| Multi-marker tagSNP test | Associated haplotype | Frequency | OR (95% CI) | Empiric P value |
| rs11932595, rs6850524 | AC | 0.315 | 0.88 (1.55-1.41) | 0.91 |
| GC | 0.039 | 0.32 (0.10-0.94) | 0.099 | |
| rs4580704, rs4864548 | CA | 0.477 | 1.55 (0.99-2.44) | 0.1066 |
| GG | 0.307 | 0.90 (0.56-1.46) | 0.8764 | |
| CG | 0.212 | 0.62 (0.37-1.07) | 0.119 | |
| rs4580704, rs6843722 | CA | 0.213 | 0.58 (0.34-0.98) | 0.0792 |
| rs4580704, rs6850524 | GC | 0.300 | 0.90 (0.56-1.47) | 0.939 |
| rs6850524, rs4864548 | GG | 0.171 | 0.81 (0.46-1.45) | 0.806 |
In univariate analysis, after the multiple comparison correction by permutation tests, there were significant differences in the allele frequency of the rs11932595 and rs6843722 between the control group and NAFLD patients (empiric P = 0.0449 and 0.0239, respectively). The test results for the multi-marker analysis showed that rs11932595/rs6850524 (haplotype GC) and rs4580704/rs6843722 (haplotype CA) were significantly associated with NAFLD (nominal P = 0.0424 and 0.0373, respectively). However, permutation testing to correct for the multiple hypothesis yielded a non-significant empirical P value for these comparisons (P = 0.099 and 0.0792, respectively) (Table 3).
When we tested the hypothesis of a relation between the gene variants and the clinical and histologic spectrum of NAFLD (disease severity), a significant association was observed with rs1554483 (empiric P = 0.039), rs6843722 (empiric P = 0.0229) and rs6850524 (empiric P = 0.00899). Moreover, in the multi-marker analysis, a significant association was observed for rs11932595/rs6850524 (haplotype AC, empiric P = 0.04858 and haplotype GC, empiric P =0.0143) and for rs4580704/rs4864548 (haplotype CA, empiric P = 0.0248).
Besides, when we analyzed the allele frequencies of tSNPs in patients with NASH, we observed a significant association with the overall fibrosis score of the disease and rs1554483 (empiric P = 0.02697), rs6843722 (empiric P = 0.01898) and rs4864548 (empiric P = 0.02697). However, no association was observed between the necroinflammatory grade and the tSNPs.
Using the WHAP software, we further evaluated the LD pattern for the aforementioned tSNPs. The estimation of haplotypes from genotype data showed 5 possible combinations covering 94.8% of the common haplotypes with a frequency higher than 0.01 (Table 4). In the omnibus test of haplotypic association, we observed that CLOCK gene variant haplotypes frequencies in NAFLD individuals significantly differed from those in control subjects (empiric P = 0.0097). Haplotypes GACCGA and CGCACG explain much of the global effect (Table 4).
| Haplotype | Control group | NAFLD patients | OR (95% CI) | Empiric P |
| (n = 128) | (n = 272) | level | ||
| GACCGA | 0.275 | 0.433 | 1.82 (1.22-2.70) | 0.00562 |
| CAGACG | 0.363 | 0.309 | 0.89 (0.54-1.49) | 0.316 |
| CGCAGG | 0.160 | 0.147 | 0.84 (0.46-1.53) | 0.737 |
| GGCCGA | 0.118 | 0.088 | 0.73 (0.35-1.54) | 0.371 |
| CGCACG | 0.084 | 0.024 | 0.27 (0.09-0.83) | 0.00902 |
Trying to dissect the association signal, we performed an analysis to control for individual markers one by one. We observed that no single tag SNP could explain the entire omnibus test results. By analyzing paired haplotypes, we observed that most of the paired haplotypes composed by tSNPs rs1554483, rs11932595, rs6850524 and rs4864548 were significantly associated with NAFLD (data not shown). Furthermore, the haplotype GAGA of these four tSNPs was significantly associated with NAFLD (OR: 2.13, 95% CI: 1.33-3.44, nominal P = 0.0011, empirical P = 0.00849).
We examined the genetic influence of the gene variants and haplotypes of the LD block of the CLOCK transcription factor in a case-control study of NAFLD, and found that, in the analysis of individual markers, rs11932595 and rs6843722 were significantly associated with the disease being rs11932595A and rs6843722C allele carriers 1.6-fold more likely to have NAFLD in comparison with non-carriers. Moreover, we observed that CLOCK gene variant haplotypes frequencies in NAFLD individuals significantly differed from those in control subjects, indicating that either the GACCGA haplotype confers a 2-fold increase in the risk of suffering from NAFLD. Conversely, the CGCACG haplotype seems to confer a 3-fold protection against NAFLD, although the results from less frequent haplotypes should be taken with caution. By analyzing paired haplotypes, we observed that most of the paired haplotype composed by tSNPs rs1554483, rs11932595, rs6850524 and rs4864548 were significantly associated with NAFLD. Furthermore, the haplotype GAGA of these four tSNPs was significantly associated with NAFLD, indicating that subjects carrying this haplotype are more than twice as likely to have NAFLD in comparison with non-carriers.
Additionally, we evaluated the role of the gene variants in the disease severity and observed a significant association between the clinical or histological spectrum of NAFLD and the rs1554483, rs6843722 and rs6850524 tag SNPs.
Finally, the haplotype analysis revealed a significant association with the NAFLD, while no specific association signal for any single tSNPs could explain this positive result. This observation seems to indicate the presence of un-typed causal variants associated with the disease.
To our knowledge, the potential contribution of CLOCK to the NAFLD susceptibility and severity has not been elucidated in humans and our study is probably the first to provide evidence of association to the disease. However, previous evidence in a mice model carrying a loss-of-function mutation in the CLOCK gene supports our findings as the animals develop adipocyte hypertrophy and excessive accumulation of fat in the liver[21].
The CLOCK gene encodes a basic helix-loop-helix transcription factor that is essential for the circadian rhythm[30]. The circadian clock system located within the hypothalamic suprachiasmatic nucleus orchestrates the cascade of events in the central nervous system (CNS) that controls timing of food intake and metabolic processes as well as other physiological rhythms[31]. Circadian and metabolic processes are also regulated by CLOCK genes in peripheral tissues, particularly those involved in nutrient homeostasis such the liver[32]. Furthermore, in the mammalian circadian hierarchy, the liver may be primarily coupled to the CNS through rhythmic behaviour, such as feeding[32].
Despite the fact that the molecular mechanisms underlying our observations are unknown, one possible explanation is that the gene variants in the CLOCK gene may lead to altered gene expression not only in the CNS but also in the liver.
Recently published findings about the role of gene variation on gene expression strengthen the hypothesis that variation in DNA sequence may contribute to individual differences in quantitative traits and disease susceptibility[33]. Interestingly, among 1097 genes and about 2 million SNPs tested for differences in expression phenotypes, CLOCK is listed as showing significant differences among ethnic groups, mostly owing to genetic variation in conserved non-coding regions[34].
It is not clear why some persons develop simple steatosis, whereas others progress to severe cirrhosis. However, the fact that NASH is observed only in a fraction of patients with NAFLD clearly suggests a genetic predisposition. We observed that when we divided the NAFLD patients into categories of cases according to the clinical or histologic spectrum, a significant association was seen between the disease progression and the above-mentioned tSNPs (rs1554483, rs6843722 and rs6850524). Furthermore, tSNPs were significantly associated with the fibrosis score of the disease.
Although association does not necessarily mean a causal-relation, in this case, several lines of evidence support the relation between the CLOCK gene variants and NAFLD severity. For instance, a comprehensive and quantitative approach surveying the diurnal expression profiles of 49 mouse nuclear receptors (NRs) in white and brown adipose tissue, and liver and skeletal muscle revealed that 25 of 45 expressed NRs showed a rhythmic cycle[35]. Hence, the dynamic circadian changes in NRs expression may offer a logical explanation for our findings. Some of these NRs have already been reported to be involved in the pathogenesis of NASH (i.e. nuclear peroxisome proliferator-activated receptors, PPARs) or in promoting liver fibrosis (i.e. retinoic acid and farnesoid X receptor)[36]. Not surprisingly, it was shown that the retinoid receptors can interact with CLOCK and an ortholog of CLOCK in a hormone-dependent manner[37]. Additionally, PPARγ is one of the metabolic transcription factors closely related to the coupled feedback pathway of the circadian oscillators[38].
Lastly, activation of hepatic stellate cells (HSC) from lipid-storing pericytes to myofibroblastic cells constitutes a major cellular event in the genesis of liver fibrosis. It has recently been shown that putative adipogenic transcription factors are expressed in quiescent HSC. Many of these factors are under the control of PPARγ and sterol response element-binding proteins (SREBP-1)[39]. Interestingly, among the transcription factors of the CLOCK machinery, SREBP was shown to play an important role in the circadian organization of the liver transcriptome[40].
In summary, genetic factors contribute to virtually every human disease by conferring susceptibility or resistance, affecting the severity or progression of disease, and interacting with environmental factors that modify disease course and expression. Our study suggests a potential role of the CLOCK gene variants and their related haplotypes in increased susceptibility to NAFLD and disease progression. Understanding how the reported variants located in different introns can affect the Clock function may require additional studies. However, it is worth mentioning that even robust genetic findings cannot always be readily explained at a functional level; however they are seeds for future research[41].
We hope our study can serve as a primer since further research is needed to confirm and extend the current findings in a larger population so as to reveal the intimate mechanism by which the CLOCK variants may lead to the mentioned phenotype.
The pathogenesis of NAFLD is multifactorial and, as a complex disease, the disorder develops from the interplay between genes and environment. In mammals, many physiological processes are driven by a circadian timing system comprising a master pacemaker in the suprachiasmatic nuclei of the hypothalamus and peripheral oscillators in most body cells. Besides, many transcripts that participate in common metabolic pathways, such as metabolism of glucose, cholesterol and fatty acids, show circadian rhythmicity
The present study examined the genetic influence of the gene variants and haplotypes of the LD block of the CLOCK transcription factor in a case-control study of nonalcoholic fatty liver disease. The role of common polymorphisms in the abovementioned gene as indicators of disease risk is discussed.
It is known that while the majority of patients with risk factors, such as obesity or insulin resistance, develop hepatic steatosis, only a minority progress to a more advanced liver disease (steatohepatitis or cirrhosis). Therefore, it is important to be able to identify individuals with fatty liver for intervention before this condition progresses. Recent advances in functional genomics are providing us great opportunities to decipher the interconnecting networks of genes, proteins, and metabolites. The use of candidate gene approach found CLOCK gene variants to be associated with an increased risk of NASH and also an important role in the progression of the disease.
The elucidation of genetic risk factors for nonalcoholic fatty liver disease will allow development of susceptibility testing for disease prediction, particularly important for high-risk individuals.
Linkage disequilibrium (LD) is a term used in the study of population genetics for the non-random association of alleles at two or more loci, not necessarily on the same chromosome. Tag SNPs is a representative SNPs in a region of the genome with high linkage disequilibrium. It is possible to identify genetic variation without genotyping every SNP in a chromosomal region. Tagger is a tool for the selection and evaluation of tag SNPs from genotype data, such as that from the International HapMap Project. Aggressive tagging approach is searching for multimarker predictors as effective surrogates for single tag SNPs using the web-based service of the Tagger computer program. r2 is the minimal coefficient of determination at which all alleles are to be captured. WHAP program is a method for testing SNP haplotype associations with qualitative and quantitative traits in samples of individuals with or without parental genotype data. Basic-helix-loop-helix is a protein structural motif that characterizes a family of transcription factors.
This study is well conceived, well done and well presented.
S- Editor Liu Y L- Editor Kumar M E- Editor Liu Y
| 1. | Desapriya E. Obesity epidemic. Lancet. 2004;364:1488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, Natale S, Vanni E, Villanova N, Melchionda N. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1907] [Cited by in RCA: 1914] [Article Influence: 87.0] [Reference Citation Analysis (0)] |
| 4. | Bellentani S, Saccoccio G, Masutti F, Crocè LS, Brandi G, Sasso F, Cristanini G, Tiribelli C. Prevalence of and risk factors for hepatic steatosis in Northern Italy. Ann Intern Med. 2000;132:112-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 842] [Cited by in RCA: 862] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 5. | Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2357] [Cited by in RCA: 2341] [Article Influence: 90.0] [Reference Citation Analysis (0)] |
| 6. | Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology. 2003;37:1202-1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1488] [Cited by in RCA: 1477] [Article Influence: 67.1] [Reference Citation Analysis (0)] |
| 7. | Chakravarti A, Little P. Nature, nurture and human disease. Nature. 2003;421:412-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 80] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Juran BD, Lazaridis KN. Genomics and complex liver disease: Challenges and opportunities. Hepatology. 2006;44:1380-1390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Thomas EL, Brynes AE, Hamilton G, Patel N, Spong A, Goldin RD, Frost G, Bell JD, Taylor-Robinson SD. Effect of nutritional counselling on hepatic, muscle and adipose tissue fat content and distribution in non-alcoholic fatty liver disease. World J Gastroenterol. 2006;12:5813-5819. [PubMed] |
| 10. | Day CP. Genes or environment to determine alcoholic liver disease and non-alcoholic fatty liver disease. Liver Int. 2006;26:1021-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 120] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 11. | Namikawa C, Shu-Ping Z, Vyselaar JR, Nozaki Y, Nemoto Y, Ono M, Akisawa N, Saibara T, Hiroi M, Enzan H. Polymorphisms of microsomal triglyceride transfer protein gene and manganese superoxide dismutase gene in non-alcoholic steatohepatitis. J Hepatol. 2004;40:781-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 174] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 12. | Merriman RB, Aouizerat BE, Bass NM. Genetic influences in nonalcoholic fatty liver disease. J Clin Gastroenterol. 2006;40 Suppl 1:S30-S33. [PubMed] |
| 13. | Piao YF, Li JT, Shi Y. Relationship between genetic polymorphism of cytochrome P450IIE1 and fatty liver. World J Gastroenterol. 2003;9:2612-2615. [PubMed] |
| 14. | Song J, da Costa KA, Fischer LM, Kohlmeier M, Kwock L, Wang S, Zeisel SH. Polymorphism of the PEMT gene and susceptibility to nonalcoholic fatty liver disease (NAFLD). FASEB J. 2005;19:1266-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 189] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 15. | Utzschneider KM, Kahn SE. Review: The role of insulin resistance in nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2006;91:4753-4761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 555] [Cited by in RCA: 639] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 16. | Hamaguchi M, Kojima T, Takeda N, Nakagawa T, Taniguchi H, Fujii K, Omatsu T, Nakajima T, Sarui H, Shimazaki M. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann Intern Med. 2005;143:722-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 785] [Cited by in RCA: 806] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 17. | Mendoza J. Circadian clocks: setting time by food. J Neuroendocrinol. 2007;19:127-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 177] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 18. | Wijnen H, Young MW. Interplay of circadian clocks and metabolic rhythms. Annu Rev Genet. 2006;40:409-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 257] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 19. | Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, Schultz PG, Kay SA, Takahashi JS, Hogenesch JB. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109:307-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1770] [Cited by in RCA: 1794] [Article Influence: 78.0] [Reference Citation Analysis (0)] |
| 20. | Delaunay F, Laudet V. Circadian clock and microarrays: mammalian genome gets rhythm. Trends Genet. 2002;18:595-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 69] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308:1043-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1793] [Cited by in RCA: 1886] [Article Influence: 94.3] [Reference Citation Analysis (0)] |
| 22. | Mendler MH, Bouillet P, Le Sidaner A, Lavoine E, Labrousse F, Sautereau D, Pillegand B. Dual-energy CT in the diagnosis and quantification of fatty liver: limited clinical value in comparison to ultrasound scan and single-energy CT, with special reference to iron overload. J Hepatol. 1998;28:785-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 108] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 23. | Haffner SM, Kennedy E, Gonzalez C, Stern MP, Miettinen H. A prospective analysis of the HOMA model. The Mexico City Diabetes Study. Diabetes Care. 1996;19:1138-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 379] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 24. | Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467-2474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2702] [Cited by in RCA: 2878] [Article Influence: 110.7] [Reference Citation Analysis (0)] |
| 25. | Kawasaki ES. Sample preparation from blood, cells, and other fluids. PCR Protocols: A Guide to Methods and Application. San Diego: Academic Press 1990; 146-152. [DOI] [Full Text] |
| 26. | de Bakker PI, Yelensky R, Pe'er I, Gabriel SB, Daly MJ, Altshuler D. Efficiency and power in genetic association studies. Nat Genet. 2005;37:1217-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1358] [Cited by in RCA: 1385] [Article Influence: 69.3] [Reference Citation Analysis (0)] |
| 27. | Myakishev MV, Khripin Y, Hu S, Hamer DH. High-throughput SNP genotyping by allele-specific PCR with universal energy-transfer-labeled primers. Genome Res. 2001;11:163-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 224] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 28. | Purcell S, Daly MJ, Sham PC. WHAP: haplotype-based association analysis. Bioinformatics. 2007;23:255-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 128] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 29. | Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10996] [Cited by in RCA: 11841] [Article Influence: 563.9] [Reference Citation Analysis (0)] |
| 30. | King DP, Zhao Y, Sangoram AM, Wilsbacher LD, Tanaka M, Antoch MP, Steeves TD, Vitaterna MH, Kornhauser JM, Lowrey PL. Positional cloning of the mouse circadian clock gene. Cell. 1997;89:641-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1086] [Cited by in RCA: 1022] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 31. | Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3160] [Cited by in RCA: 3177] [Article Influence: 138.1] [Reference Citation Analysis (0)] |
| 32. | Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M. Entrainment of the circadian clock in the liver by feeding. Science. 2001;291:490-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1257] [Cited by in RCA: 1292] [Article Influence: 53.8] [Reference Citation Analysis (0)] |
| 33. | Stranger BE, Forrest MS, Dunning M, Ingle CE, Beazley C, Thorne N, Redon R, Bird CP, de Grassi A, Lee C. Relative impact of nucleotide and copy number variation on gene expression phenotypes. Science. 2007;315:848-853. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1398] [Cited by in RCA: 1332] [Article Influence: 74.0] [Reference Citation Analysis (0)] |
| 34. | Spiegel K, Knutson K, Leproult R, Tasali E, Van Cauter E. Sleep loss: a novel risk factor for insulin resistance and Type 2 diabetes. J Appl Physiol (1985). 2005;99:2008-2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 735] [Cited by in RCA: 724] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 35. | Yang X, Downes M, Yu RT, Bookout AL, He W, Straume M, Mangelsdorf DJ, Evans RM. Nuclear receptor expression links the circadian clock to metabolism. Cell. 2006;126:801-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 716] [Cited by in RCA: 732] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 36. | Gaemers IC, Groen AK. New insights in the pathogenesis of non-alcoholic fatty liver disease. Curr Opin Lipidol. 2006;17:268-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 37. | McNamara P, Seo SB, Rudic RD, Sehgal A, Chakravarti D, FitzGerald GA. Regulation of CLOCK and MOP4 by nuclear hormone receptors in the vasculature: a humoral mechanism to reset a peripheral clock. Cell. 2001;105:877-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 353] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 38. | Staels B. When the Clock stops ticking, metabolic syndrome explodes. Nat Med. 2006;12:54-55; discussion 55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 110] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 39. | She H, Xiong S, Hazra S, Tsukamoto H. Adipogenic transcriptional regulation of hepatic stellate cells. J Biol Chem. 2005;280:4959-4967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 250] [Article Influence: 11.9] [Reference Citation Analysis (1)] |
| 40. | Brewer M, Lange D, Baler R, Anzulovich A. SREBP-1 as a transcriptional integrator of circadian and nutritional cues in the liver. J Biol Rhythms. 2005;20:195-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 65] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 41. | Craddock N, Owen MJ, O'Donovan MC. The catechol-O-methyl transferase (COMT) gene as a candidate for psychiatric phenotypes: evidence and lessons. Mol Psychiatry. 2006;11:446-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 155] [Article Influence: 8.2] [Reference Citation Analysis (0)] |