INTRODUCTION
Crohn’s disease is characterized by mononuclear cell infiltration of affected bowel segments, consisting primarily of macrophages and T helper cells, and can affect both the small and large bowel. Apart from genetic and environmental factors T cells seem to play a pathogenic role since anti-IL-12 monoclonal antibodies (mAb) have a beneficial effect in Crohn’s disease and such animal models probably via apoptosis induction of activated T helper cells[1,2]. However, CD4 T cell depletion also leads to impaired immune defence towards various pathogens, e.g. in AIDS. Similarly, although to a lesser extent, currently used drugs for the treatment of Crohn’s disease impair immune reactions towards infections, e.g. steroids or azathioprine. More recent therapies, i.e. anti-TNF-α monoclonal antibodies (mAb) or anti-IL-12 mAb, can even lead to reactivation of latent infections such as tuberculosis[3,4]. Therefore, a major problem of all previously described highly effective Crohn’s disease therapies is the impaired immune defence towards infections.
Animal models of small bowel inflammation as in Crohn’s disease are scarce and have limitations. For example TNFΔARE mice have ileitis but do not have clinical signs of bowel inflammation[5]. Others, such as the SAMP1/Yit model are not widely available[6]. One model of small bowel inflammation with similarities to Crohn’s disease is the murine Toxoplasma gondii (T. gondii) infection which - in susceptible mice (C57BL/6) - leads initially to a T helper 1 (Th1) mediated small bowel inflammation[7-10]. Mice die because of the severe intestinal inflammation within 10 d. In contrast, athymic mice, which do not have T cells, or IL-12 deficient mice survive longer without this intestinal inflammation but eventually die due to severe generalized infection[8,11,12]. This nicely demonstrates that both, chronic small bowel inflammation and defence towards pathogens, can be studied in this model.
CD2 is a T cell surface molecule expressed on virtually all T cells, thymocytes, natural killer cells and, only in mice, on B cells. CD2 plays a pivotal role in the immunological synapse initiating T cell/APC contact even before T cell receptor recognition of peptide-MHC complex[13-15]. For mucosal immunology, CD2 plays an even more important role, since lamina propria lymphocytes proliferate via CD3/T cell receptor complex much less than via CD2, both in vitro and in vivo[16,17]. CD2 deficient mice have a normal phenotype, a normal leukocyte composition and can mediate robust immune responses[18,19]. Recent in vitro experiments suggest that T cells from CD2-/- mice may be defective in proliferation and cytokine production[20]. However, CD2-/- mice did not show a general immunosuppression or an increased tumor incidence as demonstrated by normal cellular immune responses upon infection with lymphocytic choriomeningitis virus or Pneumocystis jiroveci[19,21].
Our previous work demonstrated that an anti-CD2 mAb effectively prolonged survival in transfer colitis without affecting immune defence against infection[22]. In order to clarify the role of CD2 in further detail, we now report on T. gondii infection in CD2 deficient mice. Surprisingly CD2 deficient mice infected with T. gondii not only had less intestinal immunopathology, but also improved control of T. gondii infection. To the best of our knowledge this is the first example where a defined deficiency both enhances defence towards inflammation and at the same time helps to control an infection.
MATERIALS AND METHODS
Animals
Wildtype mice (WT) on a C57BL/6 background were obtained from the Research Institute for Experimental Medicine (FEM), Berlin, Germany. CD2 deficient (CD2-/-) mice on a C57BL/6 background were obtained from Taconic, NY, USA. Mice were bred under specific pathogen free (SPF) conditions at the Research Institute for Experimental Medicine (FEM), Berlin, Germany, and were used at 8 to 12 wk of age. Mice were kept in polycarbonate cages and had free access to sterile standard chow and water.
Infection with Toxoplasma gondii
C57BL/6 (n = 8) or CD2-/- mice (n = 16) were infected with Toxoplasma gondii by gavage with 100 cysts of the ME49 strain as previously described[8]. Cysts were obtained from brains of NMRI mice that had been infected intraperitoneally with 10 cysts for 2-3 mo, as previously described[23]. Mice were sacrificed on d 7 or 8 of infection (8 control mice and 8 CD2-/- mice), when WT mice showed severe signs of disease. Serum, spleen, mLN and ileum of each mouse were obtained.
To determine the outcome of infection in the CD2-/- mice that survived the acute stage of infection, we investigated the time to death and the cause of death during the chronic stage of infection. Therefore, for the remaining mice cumulative survival was determined, histological scores and parasite load were compared by Mann-Whitney-U-test. These experiments were repeated twice.
Isolation of spleen cells, mLN cells and colon culture
Single cell suspensions were prepared as described above using a 70 μm mesh cellstrainers (BD Biosciences, Germany). Cells were washed and cultured in complete medium (RPMI 1640 medium containing 10% fetal calf serum (FCS), 100 U/mL penicillin/streptomycin, 3 mmol/L glutamine, and 50 μmol/L β-mercaptoethanol). The cell viability was always greater than 90% as determined by trypan blue dye exclusion.
Total colon culture was performed as previously described[24]. Briefly, segments of colon (1 cm in length) were cut, opened longitudinally and washed. Tissue was cultured in serum free medium. Cytokine concentrations were determined in the 24 h supernatant by ELISA. Protein concentrations of the homogenate were quantified by a Bradford assay as previously described[25].
Cytokine assay
Isolated spleen cells and mLN cells (1 × 106 cells/mL in 24-well plates (NUNC, Germany)) were stimulated with coated αCD3 mAb 145-2C11 (10 μg/mL) and soluble αCD28 mAb 37.51 (1 μg/mL) or Toxoplasma lysate antigen (TLA). Supernatants were collected 48 hours after beginning of the culture and examined for cytokine secretion (IL-2, IL-4, IL-6, IL-10, TNF-α, and IFN-γ) by sandwich ELISA. Antibodies (purified and biotinylated) as well as recombinant protein standards for IL-2, IL-4, IL-6, IL-10, TNF -α and IFN-γ (OptEIA-set BD Pharmingen, Germany) were used according to the manufacturer’s instructions.
Histological examination and microscopic scoring
Groups of 2-4 mice were killed by CO2 asphyxiation at 7 or 8 d after peroral infection with T. gondii (strain ME49). Tissue samples of the ileum were fixed in 4% formalin, embedded in paraffin and sections (5 μm) were stained with hematoxylin and eosin for histology. The degree of inflammation was blindly assessed by two investigators using a scoring system which was modified for the original score as described by Heimesaat et al[26] from 0-5 (0, normal; 1, edematous blubbing; 2, transsudate, intact epithelium; 3, cellular shedding into lumen; 4, beginning disintegration of epithelial layer; 5, complete destruction, necrosis).
Further samples were stained by immunoperoxidase method with rabbit anti-T. gondii IgG antibody as reported previously[27] and the number of parasites per cm ileum was determined as previously described[8].
In chronically infected CD2-/- mice the number of cysts in brain, lever, heart and lung were additionally specified.
Statistical analysis
Statistical analysis was carried out using SPSS for Windows. Survival was analyzed using Kaplan-Meier analysis. For other comparisons the Mann-Whitney U test was used. Values were expressed as mean (95% confidence intervals) and standard error of mean (SEM). A P-value of less than 0.05 was considered significant.
RESULTS
T. gondii infected CD2-/- mice survive longer
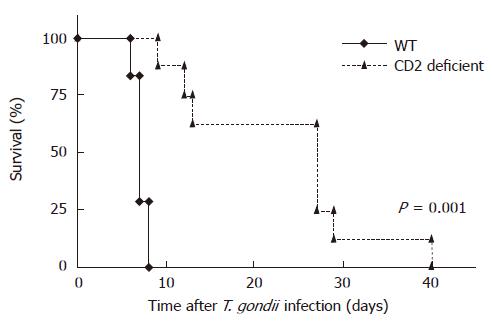
To examine the role of CD2 in T. gondii mediated ileitis, WT and CD2-/- mice were orally infected with 100 cysts of T. gondii. As shown in Figure 1 survival after oral infection was significantly increased in CD2 deficient mice compared to WT mice (23.5 vs 7.1 d, P = 0.001). While all infected WT mice died between 7 and 9 d after infection, none of CD2-/- mice died within this period of time. At the same time WT mice lost significantly more weight than CD2-/- mice (d 7 post infection (p.i.): WT (mean ± SE of the original body weight): 81.7 ± 0.6% vs CD2-/-: 84.7 ± 0.7%; P = 0.01).
Figure 1 Prolonged survival of CD2-/- mice (n = 8) vs WT mice (n = 7) after infection (p.
o.) with 100 cysts of T. gondii (strain ME49) (P = 0.001).
CD2-/- mice show decreased intestinal inflammation and improved parasite control in T. gondii infection
Because of the remarkable difference in mortality between WT and CD2-/- mice, and since mortality during acute T. gondii infection can result either from uncontrolled inflammation[28] or increased parasite replication[29] we also examined the small intestines of infected WT and CD2-/- mice at d 7 or 8 p.i. in order to determine histological changes.
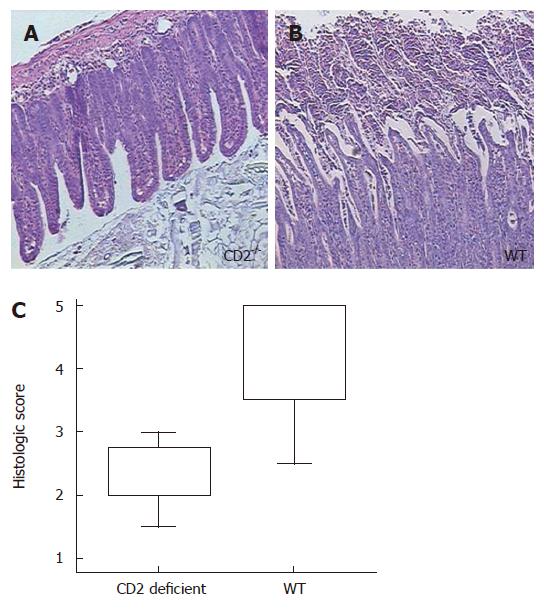
Severe necrosis of the villi and mucosal cells was particularly observed in the small intestine of WT mice. In contrast, CD2-/- mice showed decreased ileal inflammation (score of WT vs CD2-/- mice: 4.3 vs 2.3, P = 0.02; Figure 2).
Figure 2 CD2 deficiency improves ileal Th1 immunopathy after Toxoplasma gondii infection with less necrosis compared to WT mice (P = 0.
002). The histology of the ileum is shown at 7 d after infection. While CD2-/- mice show a nearly normal histological appearance of the ileum (A), the ileum of WT mice (B) show disintegration of epithelial layer, cellular shedding into the lumen and massive crypt destruction. The box blot on the (C) summarizes the examination of the ilea of 8 CD2-/- and 8 WT mice giving the median, upper and lower quartile, maximum and minimum.
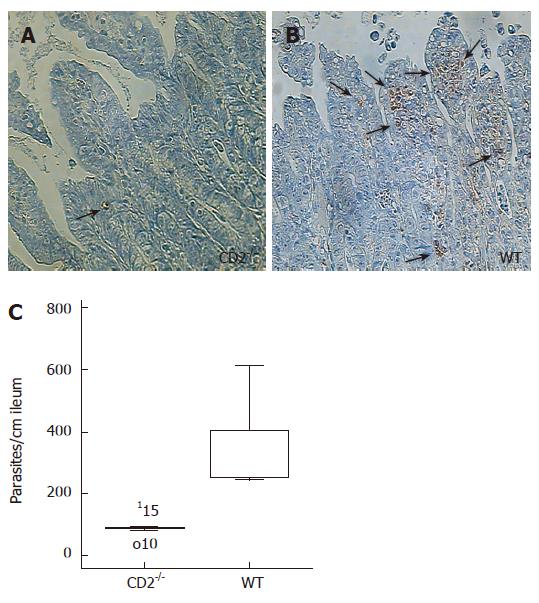
CD2-/- showed significantly lower parasite load in the ileum compared to WT mice (88 ± 12 vs 349 ± 58, P < 0.01; Figure 3). Collectively, these data indicate that CD2-/- but not WT mice control both the ileal Th1 type inflammation as well as the parasite infection.
Figure 3 CD2 deficiency reduces parasite load in Toxoplasma gondii infection.
Staining of pseudcysts in the ileum of T. gondii infected CD2-/- (A) and WT mice (B). One week after infection CD2-/- mice show significantly less pseudocysts. (C) Box blot analysis of pseudocysts in the ileum of CD2-/- (n = 5) and WT mice (n = 6), (P = 0.006). 115 and o10 depict counted parasite number outside the box.
To determine the outcome of infection in the CD2-/- mice that survived the acute stage of infection (7-10 d p.i.), we investigated the time to death and the cause of death between 11 and 23 d after infection. At the time of death, the ilea of CD2-/- mice showed only moderate signs of inflammation (mean score 2.8 ± 0.13). Only small amounts of parasites were detected in the terminal ileum and the brain (ileum: 10.5 ± 3.3; brain: 179.6 ± 91.4) whereas liver, heart and lungs displayed no sign of inflammation and parasite burden (not shown).
Lymphocytes of mLN of T. gondii infected CD2-/- mice produce less proinflammatory cytokines
IFN-γ was previously found to be a key cytokine in the process of T. gondii-induced ileal necrosis. Therefore, IFN-γ concentrations of supernatants of small intestinal cultures, mLN lymphocytes (local) as well as sera and splenocytes (systemic) were studied in infected CD2-/- compared to infected WT mice.
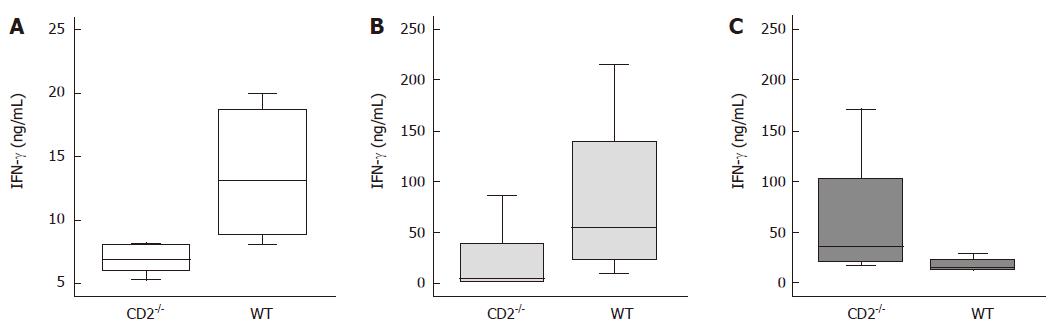
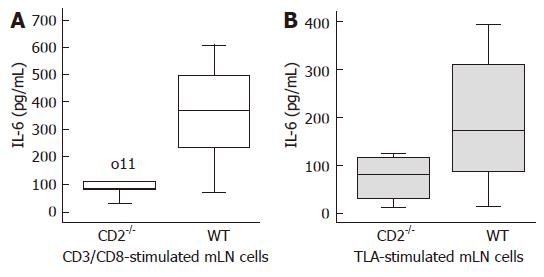
As shown in Figure 4, the decrease in inflammation and parasite load in CD2-/- mice compared to WT mice was paralleled by decreased production of IFN-γ from TLA-stimulated lymphocytes of mLN (P = 0.039) and lower IFN-γ levels in the serum (P = 0.009). Surprisingly, TLA-stimulated CD2-/- splenocytes produced higher levels of IFN-γ (P = 0.021). Similarly, a reduction of IL-6 production by anti-CD3/CD28 stimulated lymphocytes (P = 0.011) and to a lesser extent by TLA stimulated lymphocytes of mLN (P < 0.05) of CD2-/- mice was found compared to WT cells (Figure 5).
Figure 4 Decreased intestinal IFN-γ production in CD2-/- mice upon Toxoplasma gondii infection.
Production of IFN-γ by lymphocytes and concentration in sera obtained from CD2-/- or WT mice infected with the ME49 strain of T. gondii. Sera (A) and supernatants of stimulated mLN lymphocytes (B) of CD2-/- mice contain significantly less IFN-γ than appropriate probes of WT mice (P = 0.009, P = 0.039). Supernatants of stimulated splenocytes (C) of CD2-/- mice produce significantly more IFN-γ than appropriate cells of WT mice (P = 0.021).
Figure 5 Decreased intestinal IL-6 production in CD2-/- mice upon T.
gondii infection. The mLN lymphocytes were stimulated with CD3/CD28 (A) or TLA (B) for 48 h. Stimulated mLN lymphocytes of CD2-/- mice produce significantly less IL-6 vs WT mice (P = 0.011, P < 0.05). o11 depicts a measured IL-6 concentration outside the box.
No significant differences were found with regard to production of IL-2, IL-4, IL-10, and TNF-α in all organs and sera.
DISCUSSION
In this study we demonstrate for the first time that a defined T cell adhesion receptor deficiency, i.e. CD2 deficiency, can improve survival and simultaneously enhance infection control in a pathogen-induced model of Crohn’s disease, i.e. T. gondii induced enteritis. This stands in contrast to almost all currently used Crohn’s disease medications including immunosuppressive drugs and “biologicals”.
Peroral application of 100 T. gondii cysts to susceptible mice (i.e. C57BL/6 mice) leads to severe immunopathology of the small intestine which has never been observed in humans[8,10,30]. CD4+ T cells but not the parasite itself play a central effector role and lead to a cytokine storm with lethal consequences[8,31-34]. Importantly, the small bowel inflammation also occurs in areas where no tachyzoites are detectable demonstrating that this disease is not directly linked to the presence of parasites although initially induced by them. The dominance of Th1 cytokines, the intestinal inflammation at sites away from tachyzoites, the involvement of resident enteric bacteria, and the effectiveness of anti-inflammatory substances (e.g. anti-TNF-α mAb) makes this murine disease a model for Crohn’s disease[8-11,23,26,34-36].We here describe that pathogen-induced intestinal pathology was less severe in mice deficient in CD2. This finding supports our previous studies using blocking anti-CD2 mAb that ameliorated transfer colitis in mice and that did not aggravate T. gondii infection[22]. In contrast to the mAb studies we found reduced production of IFN-γ and IL-6 by mLN while the blocking anti-CD2 mAb only reduced IL-2 production by splenocytes and intestinal T cells. Interestingly, we found not only anti-inflammatory effects in CD2-deficient mice but also reduced parasite loads in CD2-deficient mice. In principle there are two possible explanations for our observation of reduced pathology and increased resistance against parasite replication:
(1) In the early phase of infection CD2 deficiency might improve immune defence against T. gondii leading to less severe intestinal inflammation. Since macrophages can be activated via the CD2/CD58 (humans) or CD2/CD48 (mice) pathway the lack of CD2 might lead to a weaker stimulation of antigen presenting cells (APC). As a result, APCs as well as activated T cells produce less IL-6 and IFN-γ. Since IL-6 enhances intracellular replication of T. gondii[33] fewer parasites are available at early stages for the induction of intestinal pathology.
(2) CD2 deficiency disturbs the immunological synapse which is required for antigen presentation[13-15]. This implies that higher antigen concentrations are needed to mount a similar T cell response[37]. Since at least 20 cysts must be given to C57BL/6 mice in order to induce intestinal pathology this might well explain that the inflammatory stimulus in CD2 deficient mice is not sufficient[7]. Secondly, lower IFN-γ and IL-6 production can be observed leading to less severe small bowel inflammation.
In both settings the finding of reduced IFN-γ and IL-6 production by mLN could explain the less severe ileitis seen in CD2 deficient mice. Particularly, IL-6 has been shown in recent years to be a key cytokine in mediating bowel inflammation[38-41]. Most recently, it was shown that particularly IL-17 secreting T cells (Th17 cells) are responsible for the production of IL-6 and that application of mAb to IL-6 or its receptor (IL-6R) ameliorates colitis, both in mice and in humans[40-43]. In addition, IL-6 itself induces vice versa IL-17 production leading to further inflammation[44].
The role of IFN-γ in Crohn's disease is less clear. For many years this cytokine was considered to be the most important cytokine in the pathogenesis of this Th1 mediated disease. However, the IFN-γ or -receptor deficiency does not prevent TNBS-induced colitis[45,46]. In IL-10 deficient mice, another Crohn’s disease model, mAb directed at IFN-γ can prevent enterocolitis but do not affect established enterocolitis[47]. In contrast, anti-IFN-γ mAb given after colitis induction ameliorate transfer colitis[48]. Similarly, anti-IFN-γ mAb given 5 d after T. gondii infection decreases intestinal pathology[8]. When CD2 deficient mice were infected with T. gondii we observed lower IFN-γ serum levels as well as decreased IFN-γ production by TLA-stimulated lymphocytes of mLN which might explain decreased intestinal inflammation. It remains to be shown, why IFN-γ concentrations were lower in serum and mLN compared to wildtype mice. In contrast, TLA-stimulated CD2-/- splenocytes produced higher IFN-γ levels possibly explaining less parasite burden. Beaman et al[33] showed that pre-treatment of T. gondii infected macrophages with IFN-γ resulted in active killing of parasites. It is, however, unclear, how CD2 deficiency enhances splenic IFN-γ responses. To some extent the strong Th1 responsiveness in the spleen compared to a Th2 responsiness in the mLN after T. gondii infection was previously described by Chardes et al[35].
Although it was originally reported that mice deficient in CD2 show no obvious immunological phenotype[18], quantitative analysis led to the conclusion that the responses of peripheral T cells to specific antigens and proliferation were impaired in the absence of CD2[20,37,49]. Segregation of receptor and counter-receptors into supramolecular clusters into the immunological synapse (IS) may promote optimal signalling by causing CD2 clustering[14,15,50]. Therefore, CD2 deficiency probably leads to a defective IS or to insufficient T cell activation in T. gondii infected, CD2-/- mice. This could cause a milder immune reaction which might protect T. gondii infected CD2-/- mice of dying in the acute phase of infection. How this improves immune surveillance against T. gondii is more difficult to explain.
Particularly, this improved resistance against parasite replication in infected CD2-/- mice is vitally important, because conventional IBD therapies like treatment with steroids or azathioprine decrease inflammation, but also decrease immune surveillance against infectious pathogens. Also new biologicals like infliximab or anti-IL-12 mAb are able to inhibit inflammation, but they impair immune surveillance at least as much as conventional immunosuppressives[3,51]. The results shown in this study using CD2 deficient mice demonstrate for the first time that chronic intestinal inflammation can be suppressed without suppressing infection control.
In summary, our results argue that CD2 is involved in immune defence and regulation of intestinal inflammatory processes.