Published online Aug 21, 2007. doi: 10.3748/wjg.v13.i31.4177
Revised: March 23, 2007
Accepted: March 28, 2007
Published online: August 21, 2007
CT scan is regarded as the imaging modality of choice in patients with pancreaticobiliary ductal abnormalities. However, the axial orientation of the CT images provides only limited anatomical view of pancreaticobiliary ductal abnormalities. The technological advances of multi-detector row CT and three-dimensional image processing in workstations allows rapid image acquisition and a short postprocessing time. In particular, multiplanar reformations (MPR) and minimum intensity projections (MinIP) offer rapid and accurate images of the anatomy and abnormalities of the pancreaticobiliary tree. Moreover, MPR and MinIP help determine the relationship between the pancreaticobiliary ductal anatomy and the surrounding structures. This pictorial review illustrates the wide spectrum of images obtained by the MPR and MinIP of the anomalies and disorders of the pancreaticobiliary tree.
- Citation: Kim HC, Yang DM, Jin W, Ryu CW, Ryu JK, Park SI, Park SJ, Shin HC, Kim IY. Multiplanar reformations and minimum intensity projections using multi-detector row CT for assessing anomalies and disorders of the pancreaticobiliary tree. World J Gastroenterol 2007; 13(31): 4177-4184
- URL: https://www.wjgnet.com/1007-9327/full/v13/i31/4177.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i31.4177
Conventional CT scan does not provide adequate information about the pancreaticobiliary ductal anatomy and its abnormalities because the orientation of these ducts is not suitable for axial images. For this reason, endoscopic retrograde cholangiopancreatography (ERCP) and percutaneous transhepatic cholangiography (PTC) have been used as the most sensitive and specific diagnostic modalities and moreover these techniques have therapeutic potential as well[1]. However, ERCP and PTC are more invasive and time-consuming compared to CT scan. As a non-invasive modality, MR cholangiopancreatography (MRCP) has recently become a well-established diagnostic tool for assessing the pancreaticobiliary tree[2].
Multi-detector row CT (MDCT) is a major advance in the field of diagnostic imaging because it allows a fast table speed, and when combined with thin slices, permits data collection that is well suited for workstation analysis. Cholangiopancreatographic images can be produced using a workstation with advanced postprocessing techniques such as multiplanar reformations (MPR) and minimum intensity projections (MinIP). The MPR images using MDCT gives rapid assessment of the pancreaticobiliary ducts along different planes without loosing information about the surrounding structures. MinIP images display the voxel with the lowest attenuation number within a slab for an arbitrary projection selected by the operator. By using the MinIP technique, the fluid density, as contained in the pancreaticobiliary duct, is picked up from the contrast-enhanced vessel together with that of the enhanced hepatic and pancreatic parenchyma[3,4]. The combined use of MPR and MinIP techniques significantly improves the images of the pancreatic and bile ducts and their site of confluence compared with those obtained by the axial CT.
In this review, we examine the use of MPR and MinIP in the assessment of the anomalies and disorders of the pancreaticobiliary tree, and correlate the results with those obtained with ERCP, MRCP and PTC. The specific topics addressed include choledocholithiasis, cholangiocarcinoma, gallbladder carcinoma with obstructive jaundice, pancreatitis, pancreatic adenocarcinoma, pancreatic cystic neoplasms and congenital anomalies of the pancreaticobiliary tree. We also discuss the limitations of using MPR and MinIP in the assessment of anomalies and disorders of the pancreaticobiliary tree.
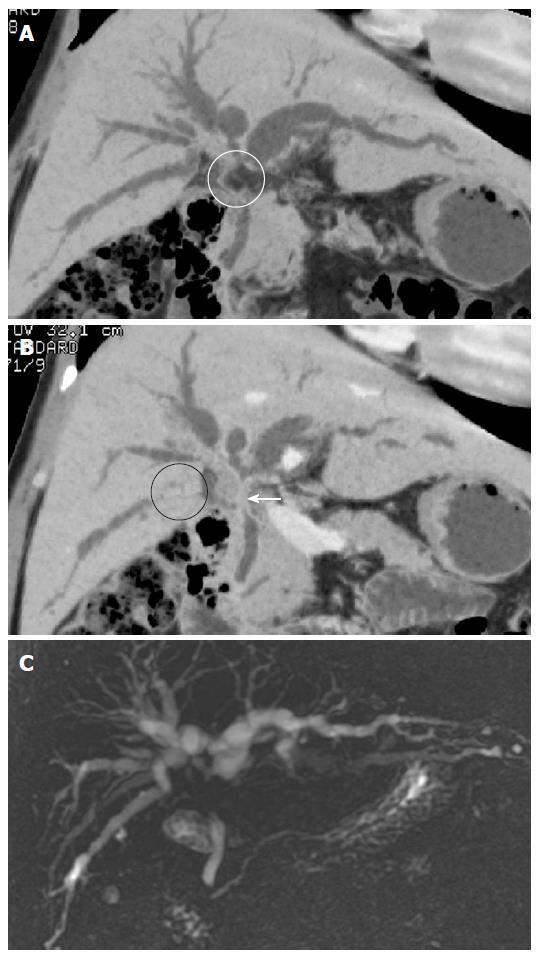
CT scans were performed using a MDCT scanner (LightSpeed Ultra, GE Medical System, Milwaukee, WI). The precontrast scan of the upper abdomen was performed to screen for gallbladder or biliary duct stones. The postcontrast scan of the pancreaticobiliary system was performed after a delay of 35 s for the arterial phase and a delay of 70 s for the portal venous phase after starting infusion of 120-150 mL of nonionic contrast material at a rate of 3 mL/s through the antecubital vein. Biliary contrast material was not used. For the postcontrast scans, the technical parameters included detector row configuration of 8 mm × 1.25 mm, collimation of 1.25 mm, a slice thickness of 5 mm, a table speed of 33.5 mm/rotation, a pitch of 1.675 and a rotation time of 0.8 s. When the axial images revealed pancreaticobiliary abnormalities, the arterial and portal venous phase images were additionally reconstructed at 1.25 mm intervals. The reconstructed images were then transferred to a dedicated workstation and the coronal, sagittal and oblique planes of the MPR and MinIP images were created by radiologists who had 5-7 years’ experience with MPR and MinIP techniques. The oblique angles for the MPR images were selected to follow the course of the pancreaticobiliary duct (Figure 1A). Curved MPR images were also obtained interactively by tracing a curved path through the imaging volume along the course of the pancreaticobiliary duct (Figure 1B). The MinIP technique was performed using different slab thicknesses based on the dilatation of the pancreaticobiliary duct (Figure 1C).
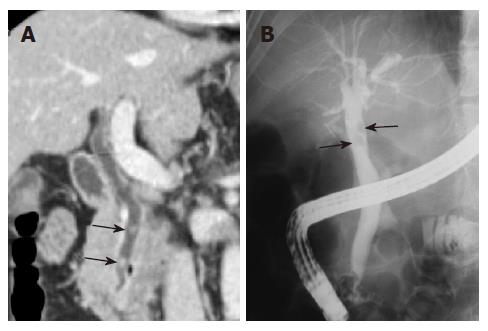
Although CT scan is not the imaging study of choice in patients with a clinical suspicion of choledocholithiasis, bile duct stones can often be detected by this technique in obstructive jaundice and acute upper abdominal pain. Detection of bile duct stones on CT depends on the density of the stone, the presence of bile duct dilatation and CT parameters such as slice thickness, reconstruction interval, pitch, kVp, and use of a contrast agent[5]. Especially, the ability to detect stones using CT scan is strongly influenced by the attenuation of the stones. Approximately 20% of the stones have isoattenuation with bile, and these stones are usually not detected by CT. MRCP has an excellent detection rate for bile duct stones, with a specificity of 92%-100%[6]. However, MRCP is an expensive technique, and is not suitable for patients with a pacemaker or a metallic prosthesis. Although helical CT cholangiography using intravenous biliary contrast agents has provided good results in detecting choledocholithiasis, this technique can result in serious allergic reactions and moreover has a low spatial resolution.
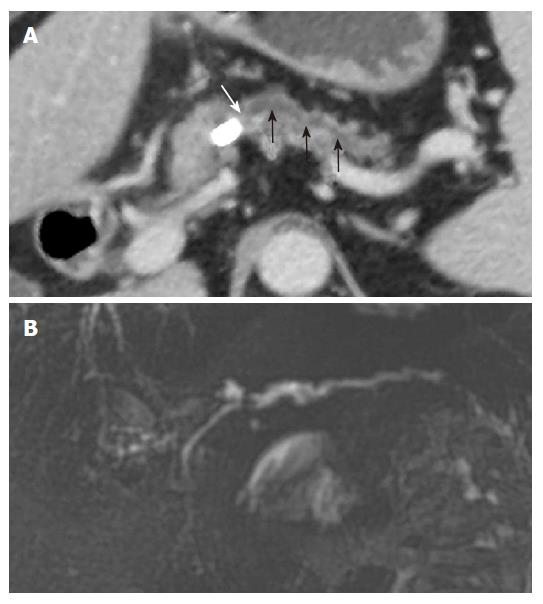
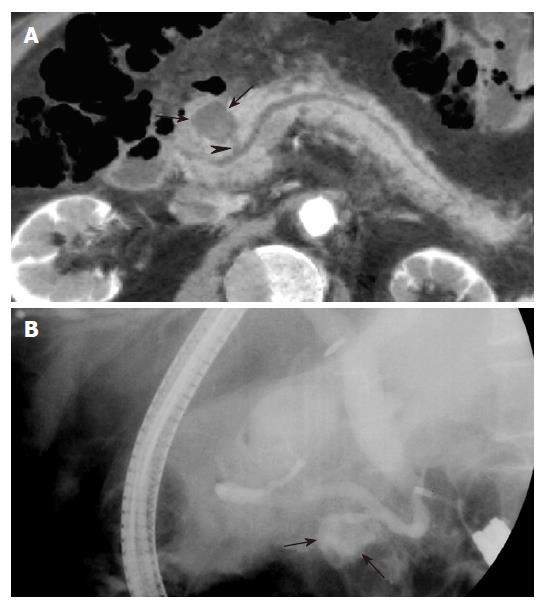
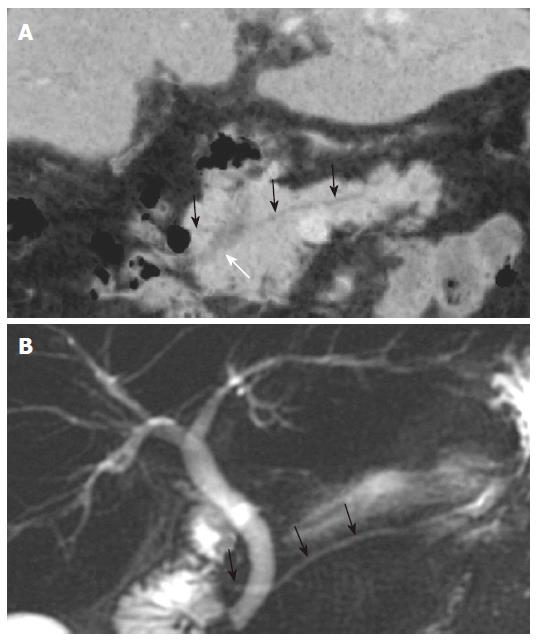
The MPR images using MDCT greatly improves the ability to detect biliary stones, since the images increase the conspicuity of stones within the bile duct. Coronal oblique reformatted MPR images can readily identify stones within the common bile duct (Figure 2). Because a MinIP image shows only the minimum pixel value along a projection within the range of a slab thickness, stones are most clearly visualized when the slab thickness does not exceed the size of the stone in the extrahepatic bile ducts[7]. In the case of small stones when the slab thickness of MinIP is wider than the size of the stone, the small stone may not be demonstrated in the MinIP images (Figure 3).
Cholangiocarcinoma, an adenocarcinoma that arises from the bile duct epithelium, is the most common tumor of the biliary tract. It is usually classified as extrahepatic, peripheral intrahepatic, hilar intrahepatic cholangiocarcinoma. MRCP is useful in depicting the severity of intrahepatic duct dilatation as well as the site and extent of the mass. Furthermore, MRCP can delineate the bile duct segments that are not opacified at ERCP or PTC. CT cholangiography using an intravenous biliary contrast agent can readily depict the bile duct. Yet it is not effective in patients with high-grade bile duct obstruction because of poor excretion of the biliary contrast material[8]. MDCT cholangiography using direct opacification of the biliary tree via a biliary drainage tube is feasible for defining the extent of ductal involvement in patients with hilar cholangiocarcinoma[9]. However, it can only be performed in selected patients who have a biliary drainage tube.
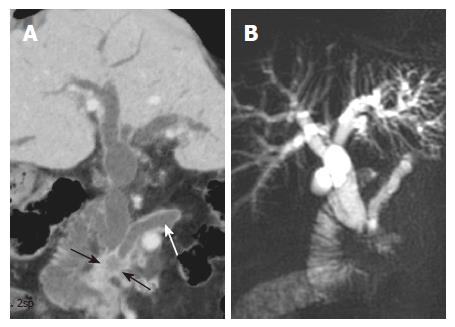
Patients with cholangiocarcinoma that involves the extrahepatic bile ducts or the hilum usually have obstructive jaundice. In these patients, CT is often the initial diagnostic modality because of its versatility and availability. The high speed and thin-section acquisition capability of multislice helical CT, and the high-quality MPR and MinIP images can better demonstrate the anatomy of the biliary tree[7]. These modalities provide an excellent overview of the cholangiographic images in cholangiocarcinoma involving the common bile duct and the biliary hilum (Figure 4). Therefore, combined evaluation with MPR and MinIP images, in addition to the axial images, is useful in evaluating obstructive biliary diseases[7,10].
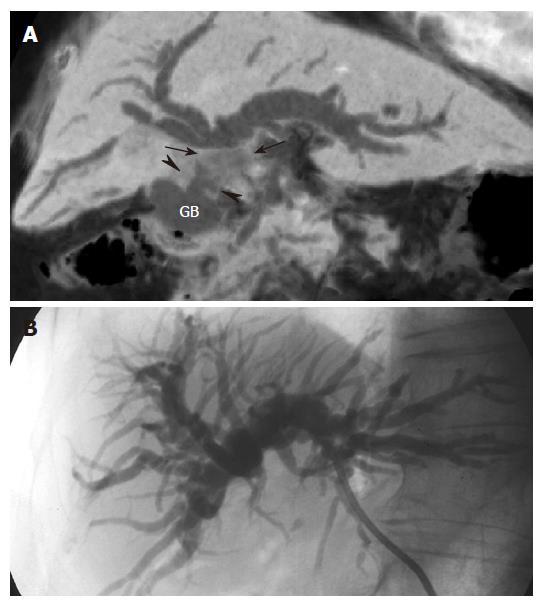
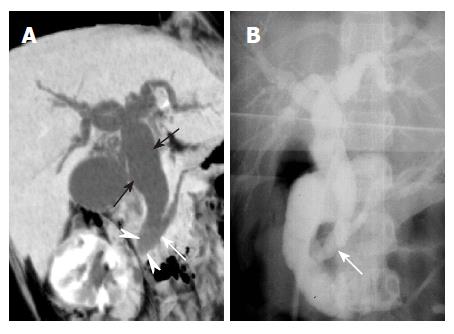
Extension of cancer from the gallbladder, duodenum or stomach can entrap and cause stenosis of the bile duct[1]. Obstructive jaundice is a common feature of gallbladder carcinoma, since the tumor spreads along the cystic duct to the extrahepatic bile duct. Lymph node enlargement and intraductal extension of the tumor results in biliary obstruction[11]. Although ERCP and PTC can demonstrate the malignant obstruction of the bile duct, gallbladder carcinoma itself is not visualized. By contrast, the relationship between gallbladder carcinoma and the obstructed bile duct is nicely demonstrated on the MinIP images (Figure 5).
Pancreatitis is the most common benign disease involving the pancreas. The pancreatic duct in acute pancreatitis is typically smooth and has a normal caliber, but it may be compressed by the edematous pancreas[12]. As a result, some of the pancreatic ducts in patients with acute pancreatitis may not be seen on MPR or MinIP images. By contrast, in patients with chronic pancreatitis, the main pancreatic duct and its side branches are dilated and have irregular contours. In addition, there may be intraductal calculi and stricture formation[13]. MPR can demonstrate these abnormalities and the images approach those obtained with ERCP and MRCP (Figure 6).
Pancreatic pseudocysts are localized collections of pancreatic fluid, debris and blood, that are commonly associated with acute and chronic pancreatitis, but are also seen after pancreatic trauma and in patients with pancreatic adenocarcinoma. Pseudocysts can have various locations and sizes. A communication between the pancreatic duct and pseudocyst is seen in less than 50% patients with ERCP[14]. Although ERCP has high specificity for the detection of a communication between the pseudocyst and the pancreatic duct, MinIP can also depict the ductal communication with pseudocyst (Figure 7).
Pancreatic adenocarcinoma originates from the pancreatic duct. The majority are located in the head of the pancreas (60%-65%), 20% occur in the body, 10% in the tail and 5% to 10% involve the entire gland[15]. The characteristic finding is stenosis of pancreaticobiliary duct, with obstruction of the pancreatic duct, the common bile duct or both, resulting in the “double duct” sign[15]. MRCP readily depicts pancreatic duct narrowing, upstream dilatation and the presence of a mass, and has been found to be as sensitive as ERCP.
However, CT is the imaging modality of choice when making the initial diagnosis and for staging of pancreatic adenocarcinoma[16]. Pancreatic adenocarcinoma usually appears as a hypoattenuating mass on CT, and can be distinguished from the normal enhancing pancreatic parenchyma during the parenchymal phase of enhancement. Pancreatic adenocarcinoma involving the head commonly obstructs the distal common bile duct. The MinIP images can readily demonstrate the relationship of pancreatic carcinoma with the pancreatic and the common bile ducts, which may enhance the visualization of tumor from the surrounding normal parenchyma (Figure 8).
Pancreatic cystic neoplasms are classified as serous cystadenoma, mucinous cystic neoplasm and intraductal papillary mucinous neoplasm (IPMN)[16]. In serous cystadenoma and mucinous cystic neoplasms, it is not common to find a communication between the neoplasm and the pancreatic duct. The imaging characteristics of IPMN vary depending on the type of tumor. The main duct type may be diffuse or there may be extensive dilatation of a segment of the pancreatic duct or of the entire pancreatic duct. The branch duct type usually occurs in the uncinate process, but can also occur in the body and tail of the pancreas. The tumor may appear as a cluster of small cysts with lobulating margin or as a single unilocular cyst that is commonly mistaken for other cystic neoplasms. Thus, a communication between the tumor and the pancreatic duct may be the main feature of IPMN imaging that distinguishes this condition from other cystic neoplasms[17]. MRCP is considered as an excellent imaging modality for determining whether a pancreatic cystic lesion communicates with the main pancreatic duct and for the assessment of ductal involvement[18]. MPR and MinIP images using MDCT technique not only improves the detection rate of pancreatic cystic lesions, but can also enhance the diagnostic capability by depicting the anatomic relationship between the pancreatic duct and the cystic lesion (Figure 9A). Like MRCP, curved MPR can display the ductal anatomy and the communication between the branching duct type of IPMN and the main pancreatic duct (Figure 9B).
During radiological evaluation, it is not uncommon to encounter a wide spectrum of anomalies of the pancreaticobiliary tree that may have gone undetected until adulthood. The most common congenital anomalies are choledochal cyst, anomalous pancreaticobiliary ductal union, aberrant biliary duct and pancreatic divisum. Rare entities include choledochocele and annular pancreas. Although relatively uncommon, the increased association of these anomalies with cholangitis, gallstones, cholangiocarcinoma and pancreatitis makes their recognition clinically important[19].
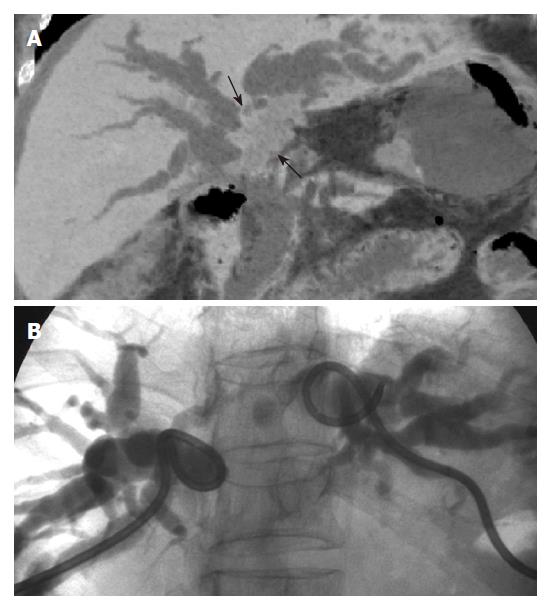
Choledochal cyst constitutes a spectrum of embryonic malformations of the pancreaticobiliary duct that result in biliary tree dilatation. An anomalous pancreaticobiliary ductal union with a long common channel or dysfunction of the sphincter of Oddi has been proposed to have a causative role in the formation of choledochal cyst[20]. On axial CT, choledochal cyst appears as cystic dilatation of the extrahepatic or intrahepatic bile ducts. Cholangiography is necessary to confirm the diagnosis and to define the ductal anatomy. MRCP allows adequate evaluation of the pancreaticobiliary duct; it confirms the diagnosis and delineates the anatomy noninvasively[21]. MinIP with MDCT provides detailed images of the anomalous pancreaticobiliary ductal union in the coronal plane[21], which mimic the findings seen on a cholangiogram (Figure 10).
Pancreatic divisum is the most common congenital anomaly involving the pancreatic ductal system and is seen in 5%-14% of the population[22]. In this anomaly, the ducts of the dorsal and ventral pancreas fail to fuse. On axial images, pancreas divisum is recognized easily by the presence of the dorsal pancreatic duct anterior and superior to the distal common bile duct. Alternatively, there is a dominant dorsal duct sign[22]. Although the diagnosis of pancreatic divisum is mostly established by ERCP, MRCP also has been found to be highly accurate[23]. However, with the increased use of MDCT and high quality workstations, MinIP images readily depict the anomalous duct of pancreatic divisum (Figure 11). It appears that MinIP reduces the partial volume effect and thus permits visualization of the non-dilated pancreatic ducts within the hyperattenuating pancreatic parenchyma.
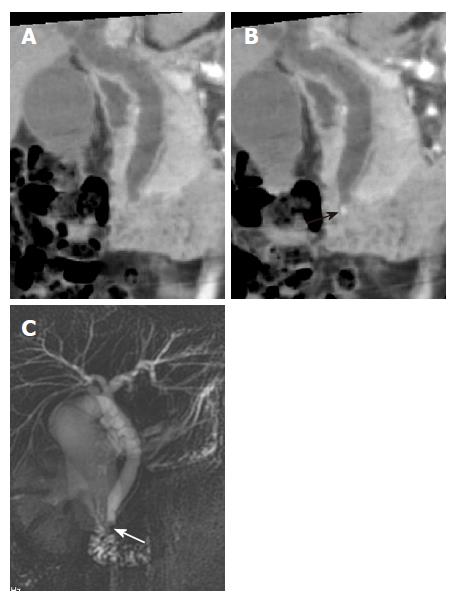
MPR and MinIP images using multislice helical CT do not depict the non-dilated peripheral bile ducts or small pancreatic ducts, which are not seen on the 2D axial images. The ability to visualize the ducts on MinIP images depends on the selection of an appropriate slab thickness[7]. A thick slab may contain structures with attenuation lower than that of the biliary and pancreatic juices. Therefore, focal disruption of the bile duct or pancreatic duct may be seen (Figure 12A). Conversely, a slab that is too thin might exclude a portion of the duct, which may result in loss of the projection effect of a single image (Figure 12B). Additionally, a tortuous bile duct or pancreatic duct may not be demonstrated in a single image. Although a tortuous structure such as the pancreaticobiliary tree is well visualized by curved MPR, it may not provide adequate anatomic overview of the pancreaticobiliary duct along with the surrounding structures[24]. In addition, curved MPR images are highly dependent on the accuracy of the operator who draws the curve, and artifactual lesions may be created or eccentric lesions may not be demonstrated.
MPR and MinIP images with MDCT provide useful information in patients with anomalies and disorders of the pancreaticobiliary tree. The images obtained correlate well with studies such as MRCP, ERCP and PTC. Despite some limitations, these techniques are useful noninvasive imaging techniques for cholangiographic and pancreatographic studies. In particular, MPR and MinIP are very helpful in clarifying complex anatomic relationships between the pancreaticobiliary tree and the surrounding structures.
S- Editor Liu Y L- Editor Anand BS E- Editor Ma WH
| 1. | Baron RL, Tublin ME, Peterson MS. Imaging the spectrum of biliary tract disease. Radiol Clin North Am. 2002;40:1325-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Georgopoulos SK, Schwartz LH, Jarnagin WR, Gerdes H, Breite I, Fong Y, Blumgart LH, Kurtz RC. Comparison of magnetic resonance and endoscopic retrograde cholangiopancreatography in malignant pancreaticobiliary obstruction. Arch Surg. 1999;134:1002-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Rao ND, Gulati MS, Paul SB, Pande GK, Sahni P, Chattopadhyay TK. Three-dimensional helical computed tomography cholangiography with minimum intensity projection in gallbladder carcinoma patients with obstructive jaundice: comparison with magnetic resonance cholangiography and percutaneous transhepatic cholangiography. J Gastroenterol Hepatol. 2005;20:304-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Raptopoulos V, Prassopoulos P, Chuttani R, McNicholas MM, McKee JD, Kressel HY. Multiplanar CT pancreatography and distal cholangiography with minimum intensity projections. Radiology. 1998;207:317-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Anderson SW, Lucey BC, Varghese JC, Soto JA. Accuracy of MDCT in the diagnosis of choledocholithiasis. AJR Am J Roentgenol. 2006;187:174-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Hallal AH, Amortegui JD, Jeroukhimov IM, Casillas J, Schulman CI, Manning RJ, Habib FA, Lopez PP, Cohn SM, Sleeman D. Magnetic resonance cholangiopancreatography accurately detects common bile duct stones in resolving gallstone pancreatitis. J Am Coll Surg. 2005;200:869-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 55] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Kim HC, Park SJ, Park SI, Park SH, Kim HJ, Shin HC, Bae WK, Kim IY, Lee HK. Multislice CT cholangiography using thin-slab minimum intensity projection and multiplanar reformation in the evaluation of patients with suspected biliary obstruction: preliminary experience. Clin Imaging. 2005;29:46-54. [PubMed] |
| 8. | Fleischmann D, Ringl H, Schöfl R, Pötzi R, Kontrus M, Henk C, Bankier AA, Kettenbach J, Mostbeck GH. Three-dimensional spiral CT cholangiography in patients with suspected obstructive biliary disease: comparison with endoscopic retrograde cholangiography. Radiology. 1996;198:861-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 73] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 9. | Kim HJ, Kim AY, Hong SS, Kim MH, Byun JH, Won HJ, Shin YM, Kim PN, Ha HK, Lee MG. Biliary ductal evaluation of hilar cholangiocarcinoma: three-dimensional direct multi-detector row CT cholangiographic findings versus surgical and pathologic results--feasibility study. Radiology. 2006;238:300-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Zandrino F, Benzi L, Ferretti ML, Ferrando R, Reggiani G, Musante F. Multislice CT cholangiography without biliary contrast agent: technique and initial clinical results in the assessment of patients with biliary obstruction. Eur Radiol. 2002;12:1155-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Levy AD, Murakata LA, Rohrmann CA. Gallbladder carcinoma: radiologic-pathologic correlation. Radiographics. 2001;21:295-314; questionnaire, 549-555. [PubMed] |
| 12. | Leyendecker JR, Elsayes KM, Gratz BI, Brown JJ. MR cholangiopancreatography: spectrum of pancreatic duct abnormalities. AJR Am J Roentgenol. 2002;179:1465-1471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 13. | Remer EM, Baker ME. Imaging of chronic pancreatitis. Radiol Clin North Am. 2002;40:1229-1242, v. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 50] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Nealon WH, Townsend CM, Thompson JC. Preoperative endoscopic retrograde cholangiopancreatography (ERCP) in patients with pancreatic pseudocyst associated with resolving acute and chronic pancreatitis. Ann Surg. 1989;209:532-58; discussion 532-58;. [PubMed] |
| 15. | Fulcher AS, Turner MA. MR pancreatography: a useful tool for evaluating pancreatic disorders. Radiographics. 1999;19:5-24; discussion 41-44; quiz 148-149. [PubMed] |
| 16. | Nino-Murcia M, Tamm EP, Charnsangavej C, Jeffrey RB. Multidetector-row helical CT and advanced postprocessing techniques for the evaluation of pancreatic neoplasms. Abdom Imaging. 2003;28:366-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Procacci C, Megibow AJ, Carbognin G, Guarise A, Spoto E, Biasiutti C, Pistolesi GF. Intraductal papillary mucinous tumor of the pancreas: a pictorial essay. Radiographics. 1999;19:1447-1463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 100] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 18. | Irie H, Honda H, Aibe H, Kuroiwa T, Yoshimitsu K, Shinozaki K, Yamaguchi K, Shimada M, Masuda K. MR cholangiopancreatographic differentiation of benign and malignant intraductal mucin-producing tumors of the pancreas. AJR Am J Roentgenol. 2000;174:1403-1408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 117] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 19. | Rizzo RJ, Szucs RA, Turner MA. Congenital abnormalities of the pancreas and biliary tree in adults. Radiographics. 1995;15:49-68; quiz 147-148. [PubMed] |
| 20. | Kim MJ, Han SJ, Yoon CS, Kim JH, Oh JT, Chung KS, Yoo HS. Using MR cholangiopancreatography to reveal anomalous pancreaticobiliary ductal union in infants and children with choledochal cysts. AJR Am J Roentgenol. 2002;179:209-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 62] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Sugiyama M, Haradome H, Takahara T, Abe N, Tokuhara M, Masaki T, Mori T, Hachiya J, Atomi Y. Anomalous pancreaticobiliary junction shown on multidetector CT. AJR Am J Roentgenol. 2003;180:173-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Soto JA, Lucey BC, Stuhlfaut JW. Pancreas divisum: depiction with multi-detector row CT. Radiology. 2005;235:503-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Bret PM, Reinhold C, Taourel P, Guibaud L, Atri M, Barkun AN. Pancreas divisum: evaluation with MR cholangiopancreatography. Radiology. 1996;199:99-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 160] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 24. | Desser TS, Sommer FG, Jeffrey RB. Value of curved planar reformations in MDCT of abdominal pathology. AJR Am J Roentgenol. 2004;182:1477-1484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |