Published online Jun 14, 2007. doi: 10.3748/wjg.v13.i22.3128
Revised: March 20, 2007
Accepted: April 4, 2007
Published online: June 14, 2007
AIM: To analyze the clinical manifestations and the effectiveness of therapy in patients with orthotopic liver transplantation (OLT)-associated hepatic artery stenosis (HAS) and non-anastomosis bile duct stricture.
METHODS: Nine cases were diagnosed as HAS and non-anastomosis bile duct stricture. Percutaneous transluminal angioplasty (PTA) was performed in four HAS cases, and expectant treatment in other five HAS cases; percutaneous transhepatic bile drainage, balloon dilation, stent placement were performed in all nine cases.
RESULTS: Diffuse intra- and extra-bile duct stricture was observed in nine cases, which was associated with bile mud siltation and biliary infection. Obstruction of the bile duct was improved obviously or removed. Life span/follow-up period was 13-30 mo after PTA of four HAS cases, 6-23 mo without PTA of other five cases.
CONCLUSION: Progressive, non-anastomosis, and diffuse bile duct stricture are the characteristic manifestations of HAS and non-anastomosis bile duct stricture after OLT. These are often associated with bile mud siltation, biliary infection, and ultimate liver failure. Interventional therapy is significantly beneficial.
- Citation: Zhao DB, Shan H, Jiang ZB, Huang MS, Zhu KS, Chen GH, Meng XC, Guan SH, Li ZR, Qian JS. Role of interventional therapy in hepatic artery stenosis and non-anastomosis bile duct stricture after orthotopic liver transplantation. World J Gastroenterol 2007; 13(22): 3128-3132
- URL: https://www.wjgnet.com/1007-9327/full/v13/i22/3128.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i22.3128
The pathogenesis of bile duct obstruction following orthotopic liver transplantation (OLT) is multifactorial, and is associated with significant morbidity and mortality[1]. With the improvement of pipeline anastomosis methods and techniques, the rate of bile duct anastomosis stricture and bile duct leak caused by these techniques has declined, and non-anastomosis bile duct stricture has become the major bile duct complication after OLT. Hepatic artery stricture (HAS) is an important cause of non-anastomosis bile duct stricture following OLT. The use of interventional therapy for resolving blood vessel and bile duct complications associated with liver transplantation has been indicated. However, the application of such techniques for both the hepatic artery and bile duct within an individual patient is rare. Here, we retrospectively analyzed the treatment (including dual intervention) and outcome of nine patients diagnosed with both HAS and non-stoma bile duct stricture following OLT.
From September 2003 to October 2006, 82 of 643 OLT patients had bile duct complications, 9 of which were diagnosed both HAS and non-stoma bile duct stricture (Table 1). Eight of these patients were male and 1 female. The average age was 45.1 (range, 32-65) years. The indications for liver transplantation were hepatic cirrhosis in four patients, hepatocellular cancer in two patients, recurrent hepatitis in two patients, and hepatic giant hemangioma in one patient. The OLT modus operandi was improved piggyback liver transplantation. End-to-end HA anastomosis was performed in 7, and blood vessel bypass in 2 patients. Seven patients received choledochoch-oledochostomy, and 2 choledochojejunostomy.
| Case | Sex | Age | Primary disease | Hepatic artery | Bile duct | ||||
| Anastomosis Manifestation Time afterOLT (mo) | Anastomosis Manifestation Time afterOLT (mo) | ||||||||
| 1 | M | 41 | Hepatic cirrhosis | End-to-end | Stoma stricture | 6 | End-to-end | Diffuse stricture | 7 |
| 2 | M | 51 | HCC | End-to-end | Stoma stricture | 3 | End-to-end | Diffuse stricture | 4 |
| 3 | M | 42 | Hemangiomas | End-to-end | Stoma stricture | 4 d | End-to-end | Diffuse stricture | 10 |
| 4 | M | 32 | Hepatic cirrhosis | End-to-end | Stoma stricture | 2 | End-to-end | Diffuse stricture | 21 |
| 5 | M | 65 | HCC | End-to-end | HA occlusion | 15 | End-to-end | Diffuse stricture | 17 |
| 6 | M | 37 | Hepatic cirrhosis | End-to-end | Stoma stricture | 12 | End-to-end | Diffuse stricture | 15 |
| 7 | M | 40 | Re-hepatitis | Bypass | Stoma stricture | 3 | R-Y | Diffuse stricture | 4 |
| 8 | F | 59 | Re-hepatitis | Bypass | Stoma stricture | 1 | R-Y | Diffuse stricture | 3 |
| 9 | M | 39 | Hepatic cirrhosis | End-to-end | Stoma stricture | 9 | End-to-end | Diffuse stricture | 11 |
HAS was diagnosed by color Doppler flow image (CDFI), computed tomography (CT), magnetic resonance imaging (MRI) and digital subtraction angiography (DSA). DSA is the gold standard. The criteria for CDFI were an intra-hepatic arterial blood flow resistance index ≤ 0.5 and an acceleration time of ≥ 0.08 s. HAS was defined by CT and MR angiography when a stricture ratio ≥ 50% was evident. Diagnosis by DSA was indicated when HAS was ≥ 40%, and when the pressure difference between pre- and post-stricture was ≥ 5 cmH2O.
Percutaneous transluminal angioplasty (PTA) or expectant treatment was used to treat stenotic HA.
Non-stoma bile duct stricture was diagnosed by physical examination, liver function tests and image analysis. Direct cholangiography is the gold standard. If diffuse intra- and extra-hepatic bile duct stricture, especially intra-hepatic and hepatic hilum bile duct stricture, but few bile duct expansions were shown by direct cholangiography, a non-stoma bile duct stricture could be diagnosed.
Interventional therapy consisted of percutaneous transhepatic bile drainage (PTBD), percutaneous transhepatic bile duct balloon dilation and/or stent placement, and removal of bile duct mud via basket extraction, and administration of antibiotics.
The following parameters were considered to indicate effective outcome: HAS ratio < 50%; pressure difference between pre- and post-stricture ≤ 3 cm H2O; decreased fever, jaundice, abdominal pain; and alkaline phosphatase (ALP), γ-glutamyltransferase (GGT), total bilirubin (TB) and direct bilirubin (DB) levels decreased by > 50%.
HAS was found in eight patients, and one hepatic artery occlusion in one patient. Non-anastomosis and diffuse bile duct stricture was seen in nine patients, associated with bile mud siltation, biliary infection, and two patients aggravated to ultimate liver failure (Table 2).
| Case | Hepatic artery | Bile duct | Survival/Follow-upperiod (mo) | ||||||
| Time afterOLT (mo) | Treatmen | Frequency | Outcome | Time afterOLT (mo) | Treatment | Frequency | Outcome | ||
| 1 | 6 | Stent | 1 | Disengaged (stent occlusion 3 mo later) | 7 | PTBD | 1 | Smooth drainage | 21 |
| 2 | 8 | Stent | 1 | Disengaged | 8 | PTBD/balloon | 4/2 | Smooth drainage | 17 |
| 3 | 4 d | Stent | 1 | Disengaged | 10 | Stent/PTBD | 1/4 | Removal 5 mo later | 18 |
| 4 | 2 | Balloon | 1 | Disengaged | 21 | PTBD | 3 | Smooth drainage | 30 |
| 5 | 15 | Expectant | 0 | Occlusion | 17 | PTBD/balloon | 3/2 | Smooth drainage | 23/died of liver failure |
| 6 | 12 | Expectant | 0 | Stricture | 15 | PTBD/balloon | 1/1 | Smooth drainage | 18 |
| 7 | 3 | Expectant | 0 | Stricture | 4 | PTBD | 2 | Smooth drainage | 6 |
| 8 | 1 | Expectant | 0 | Stricture | 3 | PTBD/balloon /bile mud removal | 8/1 | Smooth drainage | 9/third OLT |
| 9 | 10 | Expectant | 0 | Stricture | 11 | PTBD/balloon | 2/1 | Smooth drainage | 13 |
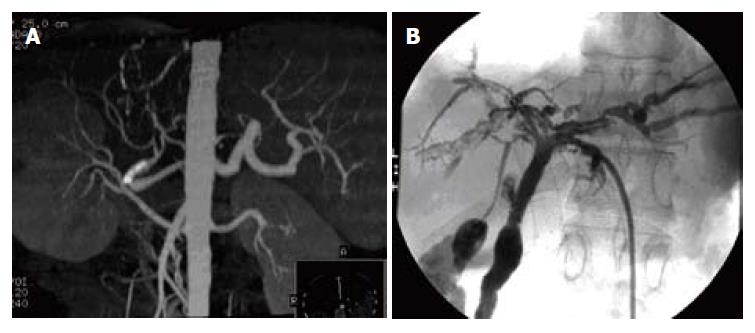
PTA was successfully performed in 4 HAS patients (Figures 1A-C and 2A). Stents were inserted in 3 of these 4 individuals while balloon dilation was performed in the other. Stricture was disengaged after PTA. Stent re-occlusion was detected by CDFI in one patient 3 mo after intervention, and a bypass circuit was established around the stent. Blood flow was normal in the other three patients.
Diffuse stricture of the hepatic hilum and intra-hepatic bile duct was evident in 3 patients (Figures 1D, E and 2B). In an another case, common bile duct stoma stricture was initially diagnosed. This evolved to diffuse stricture of the hepatic hilum and intra-hepatic bile duct 3 mo later. PTBD was initiated in all 4 patients. Simultaneous balloon dilation of the left and right hepatic and common bile duct, and further stent placement were performed in one patient. Bile duct clysis with heparinate and physiological saline was performed regularly. Chronically implanted bile duct drainage tubes were replaced every 1 to 2 mo. Bile duct drainage tube was retained for a long time.
Following intervention, symptoms related to fever, jaundice, abdominal pain improved markedly in all 4 patients. GGT, ALP, TB and DB levels decreased by more than 50% in all patients, and in two patients, fell to the normal reference range. Post-intervention survival/follow-up period ranged from 17 to 30 mo.
Four of the remaining 5 patients were diagnosed with HAS; one with HA occlusion. PTA was not performed in any of these patients. Expectant treatment was performed in each case. Hepatic artery stenosis or occlusion was apparent in all of these individuals.
Common bile duct stoma, which evolved to diffuse intra- and extra-hepatic stricture, was confirmed in all 5 patients. PTBD was performed in all cases. Simultaneous balloon dilation of the bile duct was undertaken in 4 patients, while bile mud was removed via net-basket extraction in the other. Bile duct clysis with heparinate and physiological saline was performed regularly. Chronically implanted bile duct drainage tubes were replaced every 1 to 2 mo.
Following intervention, symptoms related to fever, jaundice, abdominal pain improved markedly in all 5 patients. GGT, ALP, TB and DB levels decreased by more than 50% in all patients. Post-intervention survival/follow-up period ranged from 6, 9, 13 and 18 to 23 mo. Liver failure occurred in two patients, one of whom received another OLT, while the other died.
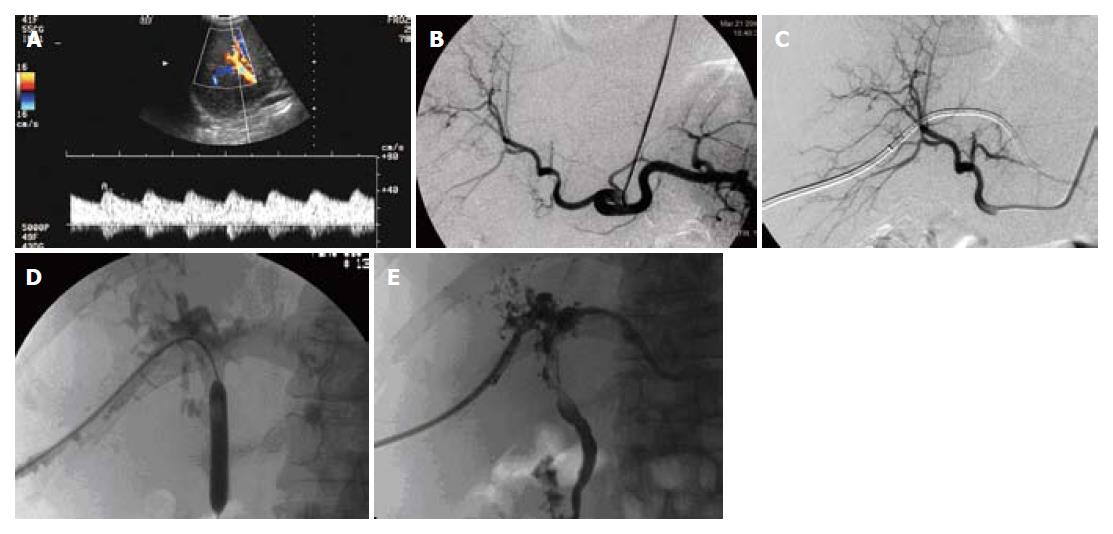
Case 2 (Figure 1) was a 51-year-old male. OLT was performed due to liver cirrhosis and hepatic cell carcinoma.
HAS was confirmed by CDFI (Figure 1A) and CT scanning 3 mo after OLT. However, no interventional therapy was initiated due to the lack of signs and symptoms. Hepatic arteriography revealed hepatic arterial stoma stricture 8 mo after OLT (degree 70%, length 5 mm) (Figure 1B). Balloon dilation and stent placement were performed, and stricture was subsequently eliminated (Figure 1C). Hepatic artery blood flow was smooth, and remained so for 12 mo follow-up period as determined by CDFI.
Common bile duct stoma stricture was diagnosed by CDFI 4 mo after OLT. Expectant therapy was chosen. However, this evolved to diffuse stricture of the hepatic hilum and intra-hepatic bile duct 4 mo later. PTBD and bile duct balloon dilation were performed. The drainage tube was retained and replaced every month.
GGT,ALP, TB,and DB levels proceeded to decrease after intervention, with TB value stabilizing at 100 ng/mL. The patient was discharged with drainage in situ, and remained symptom-free at 17 mo follow-up.
During the process of liver transplantation, there are periods of unequal cold and warm ischemia, which may lead to ischemia-reperfusion injury. When the bile duct becomes ischemic, epithelial cells produce less ATP, leading to calcium overload and subsequent membrane disorganization. This may cause endothelial xerochase, ablation, full-thickness mucosal necrosis/perforation, and fiber formation. These changes can promote bile duct stricture[2]. Ostroff et al[3] have suggested that if the time interval between HA and portal vein blood flow recovery is excessive, the bile system may be exposed to long-term warm ischemic conditions. Partial blood flow restoration at this point may further result in HA contraflow, and induce the formation of microthrombi in the HA, leading to consequent bile duct stricture following reconstruction. This theory implies that if portal vein and HA blood flow is restored simultaneously, the extent of ischemic injury to the bile duct should be lessened. In our cohort of patients, the time interval between HA and portal vein reopening was 5 to 16 min. This cannot indicate that the time of HA anastomosis definitively relates to non-stoma bile duct stricture following OLT.
The pathogenesis of non-stoma bile duct stricture following OLT is not clear. According to the causes of non-stoma bile duct stricture, it can be divided into two types: ischemic and immunologic. The possible causes of ischemic type non-stoma bile duct stricture include HAS, hepatic artery thrombosis, long donor liver cold/warm ischemia time[4]. Hintze et al[4] thought that the breakdown of bile duct blood supply and damage of the hepatic artery could increase the rate of non-stoma bile duct stricture following OLT. Zheng et al[5] reported that 3 of 5 hepatic artery thromboses and 8 HAS patients had hepatic hilum and intra-hepatic bile duct stricture, accounting for 45.5%, which exceeded 23.5% of which without earlier period poor blood supply in the hepatic artery among 32 cases of bile duct complication. Stange et al[6] reported that more than 50% of patients with HAS following OLT also had cholangitis, non-stoma bile duct necrosis, or liver abscess. Close to half of these patients required further transplantation. All 9 cases presented in the current report had both HAS or hepatic artery thrombosis and non-stoma bile duct stricture, suggesting that HAS is an important mediator of non-stoma bile duct stricture.
Through prompt PTA operation, HAS could be eliminated, normal blood flow in hepatic artery could be recovered, bile duct blood supply could be improved, therefore, the non-stoma bile duct stricture could be delayed or avoided following OLT[7]. When HAS was not eliminated in a timely manner, the probability of non-stoma bile duct stricture occurrence was increased. In our study, through PTA in four HAS patients following OLT, the time of bile duct stricture occurrence was delayed, symptoms of fever and abdominal pain improved significantly, the values of hemobilirubin decreased obviously and survival or follow-up period was prolonged, thereby emphasizing the importance of timely and correct interventional therapy for HAS.
The treatment of bile duct stricture following OLT has been transitioned from pure operation to non-operation[8]. Indeed 90% of bile duct obstruction can be improved through balloon expansion, endoprosthesis placement and bile duct drainage[9].
Interventional bile duct therapy was associated with minimal trauma, intact bypass circuitry, and bile duct blood supply. Repeated interventional bile duct therapy can prolong graft survival time, delay or avoid re-transplantation. Symptomatic improvements were noted in all 9 patients with regard to fever, jaundice, abdominal pain and biliary infection. Overall quality of life was also improved. Hence, it is apparent that interventional therapy for non-stoma bile duct stricture has a great clinical value, and could be considered a first line treatment option.
However, patients with HAS and non-stoma bile duct stricture had a relatively poorer prognosis. Presumably this was due to HAS-associated bile duct capillary embolism, leading to diffuse bile duct stricture and bile mud siltation, biliary infection and liver failure. Safdar et al[10] studied 57 patients with bile leakage after liver transplantation and discovered an incidence of 28% HAS necessitating re-transplantation. Dong et al[11] reported that two patients underwent re-transplantation for treatment of non-stoma bile duct stricture. The fact that two of our patients progressed to liver failure demonstrates that sometimes intervention therapy cannot cure non-stoma bile duct stricture radically. Accordingly, intervention therapy for non-stoma bile duct stricture has its own limitation.
Single bile duct stoma stricture was presumably caused by the anastomosis technique, stoma hydroncus or scarring, and was not associated with HAS. Balloon dilation and stent placement were found to be effective methods of treatment.
In conclusion, progressive, diffuse, intra- and extra-hepatic bile duct strictures were the characteristic manifestations of HAS with non-stoma bile duct stricture following OLT, while HAS could be the cause of non-stoma bile duct stricture. These were associated with biliary mud siltation, biliary infection, and ultimately liver function failure. Our data demonstrate that intervention therapy for HAS and bile duct stricture is of significant value in ameliorating these pathologies and symptoms, and should be applied first.
S- Editor Liu Y L- Editor Kumar M E- Editor Wang HF
| 1. | Pfau PR, Kochman ML, Lewis JD, Long WB, Lucey MR, Olthoff K, Shaked A, Ginsberg GG. Endoscopic management of postoperative biliary complications in orthotopic liver transplantation. Gastrointest Endosc. 2000;52:55-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 178] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 2. | Stange B, Settmacher U, Glanemann M, Nüssler NC, Bechstein WO, Neuhaus P. Hepatic artery thrombosis after orthotopic liver transplantation. Transplant Proc. 2001;33:1408-1409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Ostroff JW. Post-transplant biliary problems. Gastrointest Endosc Clin N Am. 2001;11:163-183. [PubMed] |
| 4. | Hintze RE, Adler A, Veltzke W, Abou-Rebyeh H, Felix R, Neuhaus P. Endoscopic management of biliary complications after orthotopic liver transplantation. Hepatogastroenterology. 1997;44:258-262. [PubMed] |
| 5. | Zheng SS, Xu X, Liang TB, Chen HY, Wang WL, Wu J. [Biliary complications following early hepatic arterial insufficiency in liver transplantation]. Zhonghua YiXue ZaZhi. 2005;85:1665-1669. [PubMed] |
| 6. | Stange BJ, Glanemann M, Nuessler NC, Settmacher U, Steinmüller T, Neuhaus P. Hepatic artery thrombosis after adult liver transplantation. Liver Transpl. 2003;9:612-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 265] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 7. | Nishida S, Kato T, Levi D, Naveen M, Thierry B, Vianna R, Selvaggi G, Buitorago E, Al-Niami A, Nakamura N. Effect of protocol Doppler ultrasonography and urgent revascularization on early hepatic artery thrombosis after pediatric liver transplantation. Arch Surg. 2002;137:1279-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 66] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Pawlak J, Wróblewski T, Małkowski P, Nyckowski P, Zieniewicz K, Grzelak I, Alsharabi A, Michałowicz B, Krawczyk M, Karwowski A. Vascular complications related to liver transplantation. Transplant Proc. 2000;32:1426-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Xu MD, Yao LQ, He YF, He GJ, Gao WD, Zhou PH, Zhong YS, Fan J, Qing XY. Value of endoscopic management of bile complications after liver transplantation. Zhongguo Shiyong Waike Zazhi. 2005;25:341-343. |
| 10. | Safdar N, Said A, Lucey MR, Knechtle SJ, D'Alessandro A, Musat A, Pirsch J, McDermott J, Kalayoglu M, Maki DG. Infected bilomas in liver transplant recipients: clinical features, optimal management, and risk factors for mortality. Clin Infect Dis. 2004;39:517-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Dong JH, Zhang LD, Wang SG, Bie P, Yang ZY. [Prophylaxis and management of ischemic-type biliary lesion after orthotopic liver transplantation]. Zhonghua YiXue ZaZhi. 2006;86:1236-1239. [PubMed] |
| 12. | Righi D, Cesarani F, Muraro E, Gazzera C, Salizzoni M, Gandini G. Role of interventional radiology in the treatment of biliary strictures following orthotopic liver transplantation. Cardiovasc Intervent Radiol. 2002;25:30-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |