INTRODUCTION
Liver cancer is the 5th leading cancer type in the world[1,2]. Despite aggressive therapeutic approaches made in the past decades, the prognosis of liver cancer remains poor, 5-year mortality exceeds 95%[1-3]. It is thus necessary to seek other more rational approaches for treatment of liver cancer.
Endoglin is a homodimeric transmembrane glyco-protein, which was initially identified as a human leukemia-associated homodimer cell-membrane antigen[4]. Studies performed in different laboratories using various antibodies to endoglin have revealed endoglin expression and up-regulation in a wide range of tumor endothelia, but seldom found in the endothelia of normal tissues, suggesting that endoglin is highly related to tumor angiogenesis[5,6]. In addition, another study demonstrated that blockage of the endoglin pathway in human umbilical vein endothelial cells resulted in marked a inhibition of in vitro angiogenesis in combination with TGF-®, indicating that endoglin is a pro-angiogenic component in the endothelial cells[7]. Therefore, therapeutic approach targeting endoglin may potentially have the capability of avoiding system side-effects. It is thus conceivable to consider that passive immunotherapy with anti-endoglin mAb may potentially have the capability of inhibiting tumor growth and/or tumor metastasis through interference of the endoglin-related angiogenesis pathway. In our previous study, we have established a hybridoma cell line secreted monoclonal antibody (mAb) against murine endoglin, which has been demonstrated to have the capability of specifically staining with recombinant murine endoglin and tumor microvessel density by Western blot and immunohistochemistry[8]. In the current study, we produced and purified the mAb, and then passively transfused with the mAb in two murine hepatoma models to observe the therapeutic effects against hepatoma.
MATERIALS AND METHODS
Materials
Hybridoma cell line (mEDG) secreted mAb against murine endoglin was established by us[8]. Nude mice were purchased from the Animal Center of Central South University (Hunan, China). Hepa1-6 and H22 hepatoma cell lines were presented by State Key Laboratory of Biotherapy (Sichuan University, Chengdu, China) and stored by us. BALB/c and C57BL/6 mice were purchased from the Animal Center of Hainan province, China. EMAM and RPMI-1640 culture media were purchased from Gibco (USA). CM Affi-Gel blue gel kit was purchased from Bio-Rad (USA). mAb against CD31 and labeled streptavidin biotin reagents were purchased from Dako (USA). Alginate and FITC–dextran solution were purchased from Sigma (USA). In Situ Cell Death Detection kit (AP) was purchased from Roche (USA).
Production and purification of antibodies
The production and purification of the mAb against murine endoglin were carried out as previously described[8]. Briefly, the EMAM hybridoma cell line mEDG was cultured in complete EMAM medium supplemented with 100 mL/L fetal calf serum at 37°C in a humidified atmosphere containing 50 mL/L CO2 in air. Hybridoma cells grown at log-phase were collected and 5 × 106 cells (in 2 mL suspension) were intraperitoneally injected into nude mice. About one to two weeks later, the ascetic fluid was harvested, purified by affinity chromatography (CM Affi-Gel blue gel kit) following the manufacturer's instructions. In addition, for control observation, antibodies were also purified from the sera derived from the normal mice at 12 wk of age by affinity chromatography as previously performed by us[9].
Passive immunotherapy of hepatoma with anti-endoglin mAb
For the investigation of the therapeutic efficacy of the anti-endoglin mAb in anti-tumor activity in vivo, mice at 6 to 8 wk of age were firstly inoculated with 2 × 106 live tumor cells into the right flank and left untreated until palpable tumors of distinct size (about 4-6 mm in diameter) appeared in the mice. Then, the mice were randomly divided into three groups of ten mice each. Group 1 (anti-endoglin mAb therapy, anti-mAb) mice were administered intravenously with the purified anti-endoglin mAb (50 mg/kg) via tail vein, and then treated twice per week for 4 consecutive weeks. Group 2 (control therapy with the purified antibodies from normal mice at 12 wk of age, cont-Ab) mice were administered intravenously with the purified antibodies (50 mg/kg) from normal mice at 12 wk of age via tail vein, and then treated twice per week for 4 consecutive weeks, as did in group 1. Group 3 (untreated control, cont-NS) mice were injected with equal volume of normal saline (NS) without containing any antibodies. The Hepa1-6 hepatoma (Hepal-6) model was established in C57BL/6 mice, and the H22 hepatoma (H22) model was in BALB/c mice. All procedures were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee. The tumor size and survival rate were monitored at a three-day interval, and the tumor volume was calculated by the following formula: Tumor volume = 0.52 × length × width2.
Immunohistochemical detection of tumor microvessel density
To determine microvessel density (MVD), frozen sec-tions of tumor tissues were prepared, and subjected to immunohistochemical staining for CD31 as previously described[9-12]. Briefly, frozen sections were fixed in acetone, incubated, and stained with an antibody reactive to CD31. Sections were then stained with labeled streptavidin biotin reagents. Any endothelial cell or endothelial cluster positive for CD31 (purple staining) was considered to be a single countable microvessel.
Alginate encapsulation assay
Alginate-encapsulated tumor cell assays were performed as previously described[13]. Briefly, Hepal-6 or H22 hepatoma cells were resuspended in a 15 g/L sodium alginate solution and added dropwise into a swirling 37°C solution of 250 mmol/L calcium chloride. Alginate beads were formed containing approximately 1 × 105 tumor cells per bead. Mice were anesthetized, and four beads were subcutaneously implanted into an incision made on the dorsal side. Incisions were closed with surgical clamps. Thereafter, mice were grouped and treated with antibodies or NS as aforementioned. After 14 d, mice were injected intravenously with 100 μL of 100 mg/kg FITC-dextran solution. Beads were surgically removed and FITC-dextran was quantified against a standard curve of FITC-dextran as previously described[9-12].
Detection of tumor cell apoptosis in situ
To detect in situ tumor cell apoptosis, sections of tumor tissues from both Hepal-6 and H22 models were fixed with 10 g/L paraformaldehyde in PBS, and apoptotic cells were detected using TUNEL assay according to the manufacturer's instructions (In Situ Cell Death Detection kit, Roche, USA). Apoptosis rate was quantified in a blind fashion by two independent reviewers by determining the percentage of positively stained cell nuclei in 20 randomly chosen fields/section at 200 × magnification.
Statistical analysis
For comparison of individual time points, ANOVA and an unpaired Student's t-test were used. Kaplan-Meier method was used to plot survival curves and the statistical significance was determined by the log-rank test. P < 0.05 was considered statistically significant. Error bars represent the SD (standard deviation) unless otherwise indicated.
RESULTS
Inhibition of tumor growth
The tumor volume was monitored at a three-day interval. Tumors in both Hepal-6 (Figure 1A) and H22 (Figure 1B) models grew progressively in two control group mice treated with normal antibodies or NS, but a significant inhibition of tumor growth was observed in the mice passively transfused with anti-endoglin mAb (Figure 1). Compared with the control groups, tumor volume in the mice passively transfused with anti-endoglin mAb significantly decreased (P < 0.05) from d 21 or 24 after tumor cell injection (Figure 1).
Figure 1 Tumor volumes at different time-points in Hepal-6 (A) and H22 (B) models.
Cont-Ab: mice transfused with control antibodies; Cont-NS: mice transfused with NS; anti-mAb: mice transfused with anti-endoglin mAb. aP < 0.05, bP < 0.01. Data are shown as mean ± SD, n = 10 in each group.
Increased survival rate of tumor-bearing mice
The survival rate of the tumor-bearing mice was also monitored at a three-day interval. Compared with the control group mice treated with normal antibodies or NS, the survival rate of the mice passively transfused with anti-endoglin mAb significantly increased in both Hepal-6 (Figure 2A) and H22 (Figure 2B) models (P < 0.01 by log-rank test). Thus, significant increase in survival rate and inhibition of tumor growth in the two hepatoma models suggested that passive immunotherapy with an anti-endoglin mAb has significant therapeutic effects against murine hepatoma.
Figure 2 The survival rates at different time-points in Hepal-6 (A) and H22 (B) models.
Log-rank test, P < 0.01, n = 10 in each group.
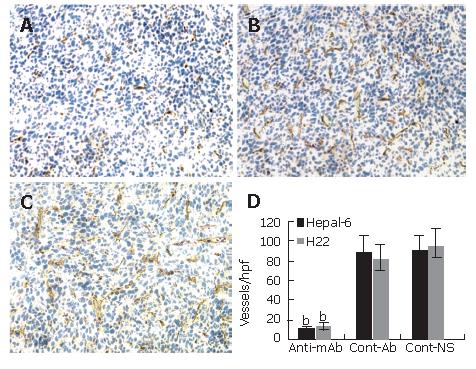
Inhibition of tumor angiogenesis in tumor tissues
Significantly more microvessels (purple color staining) were seen in the tumor tissue sections from the control mice (Figures 3B and C), while almost no such stained microvessels were seen in the tumor tissue sections from the mice passively transfused with anti-endoglin mAb (Figure 3A). The average number of microvessels per high-power field in both Hepal-6 and H22 hepatoma models (10.32 ± 3.51 and 12.85 ± 4.27, respectively) in the mice passively transfused with anti-endoglin mAb were significantly lower than those in the mice transfused with control antibodies (89.69 ± 15.06 and 80.56 ± 15.38, respectively) and the mice transfused with NS (89.12 ± 16.31 and 94.35 ± 17.89, respectively) (Figure 3D; P < 0.001).
Figure 3 Significant decrease in microvessel density (stained as purple color) in the tumor tissues from the mice transfused with anti-endoglin mAb (A) compared to the mice transfused with control antibodies (B) and the mice transfused with NS (C).
Quantitative analysis of microvessels in Hepal-6 and H22 models (D), the bars represent mean ± SD, bP < 0.001.
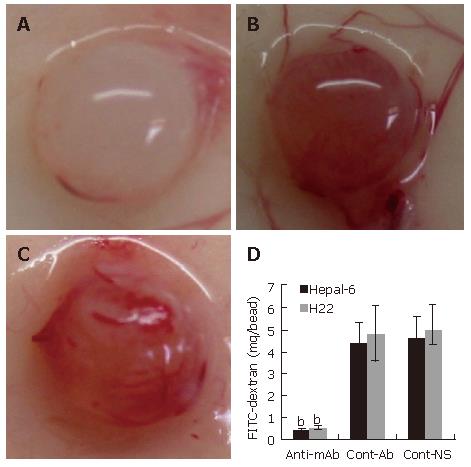
Inhibition of angiogenesis in alginate encapsulation beads in vivo
Inhibition of angiogenesis was further confirmed by using alginate encapsulation assay. Alginate implanted angiogenesis was quantified by measuring the uptake of FITC-dextran into beads. Compared with the two control groups (Figures 4B and C), a significant inhibition of angiogenesis was found on the beads from the mice passively transfused with anti-endoglin mAb (Figure 4A). FITC-dextran uptake (μg) was significantly decreased in both Hepal-6 and H22 hepatoma models (0.42 ± 0.07 and 0.51 ± 0.09, respectively) in the mice passively transfused with anti-endoglin mAb than that in the mice transfused with control antibodies (4.38 ± 0.94 and 4.79 ± 1.28, respectively) and the mice transfused with NS (4.56 ± 1.02 and 4.93 ± 1.15) (Figure 4D; P < 0.001).
Figure 4 Significant inhibition of angiogenesis in the tumor cell-encapsulated beads from the mice transfused with anti-endoglin mAb (A) than that in the tumor tissues from the mice transfused with control antibodies (B) and the mice transfused with NS (C).
Quantitative determination of FITC-dextran uptake in Hepal-6 and H22 models (D); the bars represent mean ± SD, bP < 0.001.
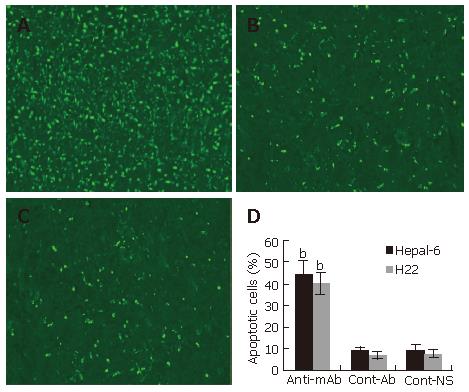
Increase in tumor cell apoptosis
To further investigate the anti-tumor mechanism induced by the passive transfusion with anti-endoglin mAb, a TUNEL assay was performed to observe the tumor cell apoptosis in situ. TUNEL assay revealed a marked increase in apoptosis in tumor tissues from both Hepal-6 and H22 hepatoma models (43.68% ± 7.32% and 39.54% ± 5.93%, respectively) in the mice passively transfused with anti-endoglin mAb than that in the mice transfused with control antibodies (8.43% ± 2.37% and 7.18% ± 1.96%, respectively) and the mice transfused with NS (9.02% ± 3.02% and 6.95% ± 2.39%, respectively) (Figure 5; P < 0.001).
Figure 5 Significantly increased apoptosis in the tumor tissues from the mice transfused with anti-endoglin mAb (A) compared to the mice transfused with control antibodies (B) and the mice transfused with NS (C).
Quantitative analysis of apoptotic cells in Hepal-6 and H22 models (D), the bars represent mean ± SD, bP < 0.001.
DISCUSSION
The generation of new blood vessels, or angiogenesis, is a complex multi-step process. Evidence suggests that the growth and persistence of solid tumors and their metastases are angiogenesis-dependent[14-17]. To date, a number of molecules that stimulate angiogenesis as well as those that inhibit this process have been elucidated[17,18]. In this regard, as a strategy for cancer therapy, anti-angiogenic therapy, which targets genetically stable endothelial cells, has several advantages over conventional tumor cell targeting in the therapy of solid tumors: (1) This approach may have the potential to circumvent the problem of acquired drug resistance[19,20]. The reasonable explanation is that drug resistant mutants are easily generated from tumor cells due to the genetic instability of tumor cells. On the contrary, genetically stable normal cells, such as vascular endothelial cells, would be far less adept at generating such mutants; (2) anti-angiogenic therapy may have the capability of overcoming the problem of tumor heterogeneity[21]. At present, tumor heterogeneity has become a major problem with the tumor cell-targeting therapy; (3) physiological barriers for the high-molecular weight drugs (such as antibodies and immunoconjugates) to penetrate into solid tumors will be also circumvented by targeting a tumor's vasculature rather than tumor cells themselves[22,23]. The possible reason is that, unlike tumor cells in the solid tumors, the vascular endothelial cells are directly accessible to circulating high-molecular weight drugs; (4) many thousands of dependent tumor cells will die of nutrient and/or oxygen deprivation if a capillary or a sector of the capillary bed fails[24,25]. Therefore, killing of only a minority of vascular endothelial cells of tumors may be sufficient to eradicate most malignant cells in tumor tissues; and (5) a single agent developed for anti-angiogenic therapy could be applied to various types of solid tumors and other angiogenesis-associated diseases.
A few lines of evidence have demonstrated that endoglin is one of the marker molecules of tumor angiogenesis, and is specifically expressed and up-regulated in tumor-associated angiogenic vasculatures, specially in tumor microvessels[1-3]. The level of endoglin in serum and tumor tissues detected by ELISA or immunohistochemistry correlated well with the prognosis of solid tumors[2,3,26]. Thus, a specific mAb against endoglin may be a good tool for diagnosis and treatment of solid tumors, including hepatoma.
In the current study, we used an anti-endoglin mAb established by us in our previous study to observe the anti-tumor activities by passive immunotherapy in two hepatoma models. We found that passive immunotherapy with anti-endoglin mAb could effectively suppress tumor growth, and prolong the survival rate of hepatoma-bearing mice. Angiogenesis was apparently inhibited within the tumor tissues, and the vascularization of alginate beads was also reduced in the mice passively transfused with anti-endoglin mAb. In addition, there were increased apoptotic cells within the tumor tissues from the mice passively transfused with anti-endoglin mAb. Based on the aforementioned findings, we may thus conclude the possibility that the therapeutic activities against hepatoma by passive immunotherapy with anti-endoglin mAb may result from the inhibition of tumor angiogenesis and the increase of tumor cell apoptosis, which may highly correlate with the blockage of endoglin-related signal pathway induced by anti-endoglin mAb.
The endothelium in normal vasculatures is considered the most quiescent because the turnover of the endothelial cells is very low[27-30]. However, the endothelial cells in the tumor tissues can undergo rapid proliferation which promote spurts of tumor angiogenesis due to hypoxia and secretion of various molecules that stimulate angiogenesis by the endothelial cells themselves and tumor cells. Therefore, a proliferation-associated antigen on endothelial cells may be a good candidate for anti-angiogenic therapy. Endoglin has been proven to be a proliferation-associated antigen on endothelial cells[7,31,32]. Previous studies demonstrated that anti-endoglin mAbs showed a highly restricted reactivity to proliferating endothelial cells, immature acute lymphoblastic leukemia (ALL) cells and myeloid/monocytic leukemia cells[4,33,34]. In the current study, we further found that passive immunotherapy with anti-endoglin mAb reached effectively anti-hepatoma activities in murine models. Thus, this study also demonstrated that passive immunotherapy with anti-endoglin mAb may be a potential approach for treatment of hepatoma and other solid tumors.