Published online Apr 7, 2007. doi: 10.3748/wjg.v13.i13.1962
Revised: February 18, 2007
Accepted: March 19, 2007
Published online: April 7, 2007
AIM: To determine whether Lactobacillus plantarum can modify the deleterious effects of tumor necrosis factor-α (TNF-α) on intestinal epithelial cells.
METHODS: Caco-2 cells were incubated with TNF-α alone or in the presence of L. plantarum. Transepithelial electrical resistance was used to measure epithelial barrier function. Interleukin 8 (IL-8) secretion by intestinal epithelial cells was measured using an ELISA. Cellular lysate proteins were immunoblotted using the anti-extracellular regulated kinase (ERK), anti-phospho-ERK and anti-IκB-α.
RESULTS: A TNF-α-induced decrease in transepithelial electrical resistance was inhibited by L. plantarum. TNF-α-induced IL-8 secretion was reduced by L. plantarum. L. plantarum inhibited the activation of ERK and the degradation of IκB-α in TNF-α-treated Caco-2 cells.
CONCLUSION: Induction of epithelial barrier dysfunction and IL-8 secretion by TNF-α is inhibited by L. plantarum. Probiotics may preserve epithelial barrier function and inhibit the inflammatory response by altering the signal transduction pathway.
-
Citation: Ko JS, Yang HR, Chang JY, Seo JK.
Lactobacillus plantarum inhibits epithelial barrier dysfunction and interleukin-8 secretion induced by tumor necrosis factor-α. World J Gastroenterol 2007; 13(13): 1962-1965 - URL: https://www.wjgnet.com/1007-9327/full/v13/i13/1962.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i13.1962
Probiotics are defined as living microorganisms that exert beneficial effects on human health[1]. They are effective in shortening the duration of infectious diarrhea in children, and preventing antibiotics-associated diarrhea[2,3]. Probiotics have been shown to prevent a relapse of postoperative pouchitis in ulcerative colitis[4].
Tumor necrosis factor-α (TNF-α) is a proinflamma-tory cytokine and plays a central role in intestinal inflammation in Crohn disease. TNF-α levels in serum, stool and intestinal tissues are elevated in patients with Crohn’s disease[5,6]. In Crohn’s disease, the elevation in epithelial permeability of the ileal mucosa may be mediated by TNF-α[7]. Treatment with anti-TNF-α antibody is effective in cases of intractable Crohn’s disease[8].
As disturbance of the intestinal microflora plays an important role in the pathogenesis of murine experimental colitis and human inflammatory bowel disease[3], probiotics have been used to modify the bacterial flora of the gut. Lactobacillus plantarum is isolated from Kimchi, a traditional Korean food made from fermented vegetables[9]. L. plantarum attenuates intestinal inflammation in the interleukin (IL) 10 gene-deficient mouse model, which spontaneously develops enterocolitis[10].
The mechanisms of action of probiotics include improvement of epithelial barrier function and immunoregulatory effects[11]. Each probiotic species may have an individual mechanism of action. The combination probiotic, VSL3 contains L. plantarum and enhances human intestinal epithelial barrier function[12]. Intestinal epithelial cells release potent neutrophil attractant chemokines such as IL-8 when stimulated by TNF-α. Secretion of IL-8 by epithelial cells has been suggested to be important in the pathogenesis of inflammatory bowel diseases, because IL-8 induces migration of inflammatory cells into the mucosa. Some lactobacilli inhibit the induction of IL-8 production by TNF-α in human intestinal epithelial cells[13-15]. TNF-α-stimulated IL-8 secretion by intestinal epithelial cells is mediated by extracellular signal-regulated kinase (ERK) and nuclear factor κB (NF-κB)[16].
The aim of this study was to determine whether L. plantarum reverses the deleterious effects of TNF-α on intestinal epithelial cells. We performed an in vitro study in which Caco-2 cells were treated with TNF-α alone or with TNF-α plus L. plantarum. We investigated the effect of L. plantarum on TNF-α-induced alteration of epithelial barrier function, IL-8 production, and ERK/NF-κB pathway dynamics.
Caco-2 cells, an established cell line model for mature differentiated enterocytes, were obtained from the American Type Culture Collection (ATCC). Cell lines were cultured in 25 mmol/L glucose-Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum, 100 U/mL penicillin, 100 μg/mL streptomycin, 1% nonessential amino acids, and 4 mmol/L glutamine. Cultures were maintained at 37°C in an incubator containing an atmosphere of 5% CO2. Cells were used within 14 d of seeding or within five days of confluency. The Caco-2 cell culture medium was replaced with antibiotic-free culture medium 24 h before experiments.
L. plantarum (ATCC 8014) was incubated in Lactobacillus MRS broth at 37°C for 24 h, then diluted in MRS broth to a density of 0.5 absorbance units at a wavelength of 600 nm. Then, 1 × 107 colony-forming units of L. plantarum per mL were added at a multiplicity of 10:1 to the Caco-2 cells. Untreated cells were used as controls in all experiments.
Caco-2 cells were grown as polarized monolayers on 6.5 mm transwell plates (0.4 μm pores; Corning Incorporated, Acton, MA, USA). Caco-2 monolayers with epithelial resistance greater than 500 Ωcm2 were used, and L. plantarum was added apically to the polarized monolayers. TNF-α (10 ng/mL) was simultaneously added to the basolateral side of the cell monolayers. Electrical resistance across the monolayers was measured at various times using an epithelial volt-ohm meter (World Precision Instruments, Sarasota, FL, USA). Measurements were expressed in Ωcm2 after subtracting mean values for resistance obtained from cell-free inserts.
TNF-α (10 ng/mL) and L. plantarum were added simultaneously to Caco-2 cells and incubated for 5 h. Culture medium was collected and centrifuged for 10 min to pellet residual bacteria. The supernatant was collected for determination of IL-8 concentration using an ELISA (Pierce, Rockford, IL, USA). Cytokine concentrations were determined using 96-well plates as described by the manufacturer.
TNF-α (10 ng/mL) and L. plantarum were added simultaneously to Caco-2 cells. The treated and untreated cells were washed with PBS and scraped into cell lysis buffer (20 mmol/L HEPES, 0.1% SDS, 1% Triton X-100, phosphatase inhibitor and protease inhibitor cocktail). Thirty minutes after treatment, the lysate was centrifuged at 15 000 r/min for 15 min at 4°C. The protein content of the supernatant was determined using Bio-Rad DC reagents (Bio-Rad, Hercules, CA, USA). For western blotting, equal amounts of cellular lysate protein were mixed with Laemmli sample buffer and separated by SDS-PAGE. Separated proteins were transferred to PVDF membranes, which were blocked and then immunoblotted with anti-phospho-ERK, anti-ERK and anti-IκB-α (Santa Cruz Biotechnology, Santa Cruz, CA, USA). The blot was then developed using horseradish peroxidase-conjugated secondary antibodies and enhanced chemiluminescence.
All data are expressed as means ± SD. Data comparisons were made with Student’s t test. Differences were considered significant at P < 0.05.
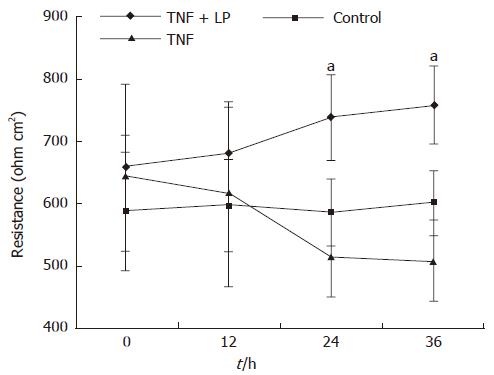
To determine the effect of L. plantarum on TNF-α-induced epithelial barrier dysfunction, Caco-2 cells were basolaterally incubated with TNF-α alone or with TNF-α plus L. plantarum, which was administered apically. Transepithelial electrical resistance was monitored for 36 h. The monolayer resistance of TNF-α treated cells did not change until 12 h had elapsed. TNF-α caused a decline in transepithelial resistance 24 h after treatment. L. plantarum inhibited TNF-α-induced decrease in transepithelial electrical resistance at 24 h and 36 h after treatment (P < 0.05) (Figure 1). The epithelial barrier function of TNF-α-stimulated Caco-2 cells was thus preserved by L. plantarum.
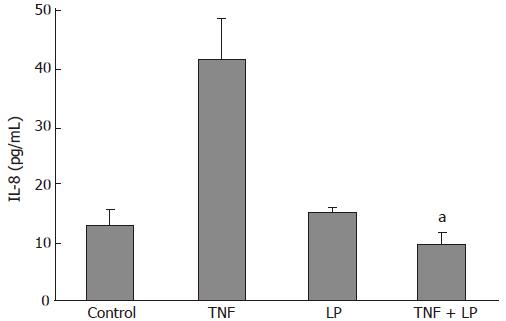
The secretion of IL-8 into culture medium was measured to determine the effect of L. plantarum on the inflammatory response of Caco-2 cells to TNF-α. IL-8 concentrations in media of Caco-2 cells cultured with L. plantarum were not significantly different from those of the controls. When TNF-α (10 ng/mL) was incubated with the cells for 5 h, IL-8 secretion was increased to 41.5 ± 7.2 pg/mL. IL-8 secretion was reduced to 9.5 ± 2.1 pg/mL (P < 0.05) when TNF-α was cocultured with L. plantarum (Figure 2). These data showed that L. plantarum inhibited TNF-α-induced IL-8 secretion.
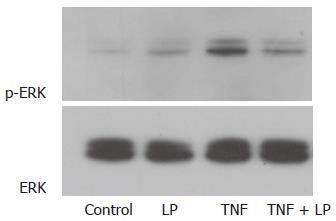
The effect of L. plantarum on TNF-α–induced ERK pathway activity was investigated. Treatment of Caco-2 cells with TNF-α induced phosphorylation of ERK-1 and ERK-2. The amount of p-ERK in L. plantarum-treated cells was not significantly different from that of the control. Phosphorylation of ERK-1 and ERK-2 in TNF-α-treated cells was decreased by L. plantarum. Nonphosphorylated forms of ERK showed the presence of same amounts of these proteins. L. plantarum thus inhibited TNF-α-induced activation of the ERK pathway (Figure 3).
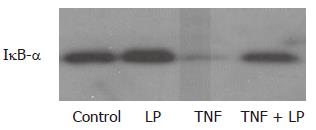
To study the effect of L. plantarum on the NF-κB pathway, the level of IκB-α was determined using western blotting. NF-κB activation involves the phosphorylation of IκB-α and subsequent degradation of IκB-α, resulting in the translocation of NF-κB to the nucleus. Treatment with TNF-α caused degradation of IκB-α. Coincubation with TNF-α and L. plantarum inhibited TNF-α-induced degradation of IκB-α (Figure 4).
Ma et al[17] demonstrated that TNF-α decreases transepi-thelial electrical resistance of Caco-2 cells after 24 and 48 h. We also observed a decrease in transepithelial electrical resistance after 24 h. We showed that the TNF-α-induced decrease in transepithelial electrical resistance was inhibited by L. plantarum. Saccharomyces boulardii prevented a decrease in transepithelial electrical resistance in enteropathogenic E. coli-infected T84 cells[18]. Intestinal mucosal permeability is decreased by VSL3 in IL-10 gene-deficient mice[12]. All these findings support the contention that probiotics enhance epithelial barrier function.
In our study, TNF-α-induced IL-8 secretion was inhibited by L. plantarum. This indicates that L. plantarum attenuates the epithelial inflammatory response to TNF-α. McCracken et al[19] showed that L. plantarum decreased TNF-α-induced IL-8 secretion in HT-29 cells in which IL-8 mRNA levels were elevated. In contrast, Lactobacillus reuteri and L. GG inhibited TNF-α-induced IL-8 secretion and IL-8 mRNA expression[14,15]. The level of IL-8 expression is correlated with disease activity in patients with inflammatory bowel disease. A number of Lactobacillus and Bifidobacterium species, including L. plantarum[10], L. reuteri[19], VSL3[12], L. salivarius and B. infantis[20], attenuate experimental colitis in IL-10 knockout mice.
ERK and p38 mitogen-activated protein (MAP) kinase contribute to TNF-α-stimulated IL-8 secretion by intestinal epithelial cells via a posttranscriptional mechanism[16]. Yan et al[21] showed that L. GG prevents cytokine-induced apoptosis in intestinal epithelial cells by inhibition of TNF-α-induced p38 MAP kinase activation. Jijon et al[22] demonstrated that VSL3 inhibits IL-8 secretion and reduces p38 MAP kinase activation. The effect of L. plantarum on TNF-α-stimulated ERK activation had not been investigated. We demonstrated that L. plantarum inhibited ERK activation in TNF-α-treated intestinal epithelial cells. ERK signaling is involved in IL-8 production because ERK inhibitors attenuate IL-8 secretion induced by TNF-α[23]. In our study, L. plantarum inhibited TNF-α-induced ERK activation, suggesting that L. plantarum may inhibit IL-8 secretion, at least partially, through the ERK pathway. NF-κB regulates IL-8 transcription, and some lactobacilli have been shown to inhibit TNF-α-induced NF-κB translocation to the nucleus and IκB-α degradation[13,14]. We also showed that L. plantarum inhibited the degradation response of IκB-α to TNF-α. In contrast, L. GG did not affect TNF-α-induced ERK activation or IκB-α degradation[21]. Probiotics may exert anti-inflammatory responses by modifying the signal transduction pathway. The mechanisms involved may depend on the species of probiotics.
Epithelial barrier functions are modulated by the NF-κB and MAP kinase pathways. A TNF-α-induced increase in intestinal tight junction permeability was shown to be mediated by NF-κB activation[17]. The increase in transepithelial resistance induced by VSL3 is mediated in part via the ERK pathway[24]. The effect of L. plantarum on monolayer resistance appears to be mediated by NF-κB and the ERK pathway. Although in vitro models are useful for evaluating mechanisms by which probiotics exert beneficial effects and provide a rationale for the therapeutic use of probiotics, the beneficial health effects of probiotics should also be determined by double- blinded placebo-controlled trials.
In summary, L. plantarum inhibits epithelial barrier dysfunction, IL-8 secretion, ERK activation, and IκB-α degradation in TNF-α-stimulated Caco-2 cells. Our findings suggest that probiotics may preserve epithelial barrier function and inhibit the inflammatory response by affecting the signal transduction pathway in human intestinal epithelium.
S- Editor Liu Y L- Editor Alplini GD E- Editor Chin GJ
| 1. | Sullivan A, Nord CE. Probiotics and gastrointestinal diseases. J Intern Med. 2005;257:78-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 113] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 2. | Guandalini S, Pensabene L, Zikri MA, Dias JA, Casali LG, Hoekstra H, Kolacek S, Massar K, Micetic-Turk D, Papadopoulou A. Lactobacillus GG administered in oral rehydration solution to children with acute diarrhea: a multicenter European trial. J Pediatr Gastroenterol Nutr. 2000;30:54-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 339] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 3. | Fedorak RN, Madsen KL. Probiotics and prebiotics in gastrointestinal disorders. Curr Opin Gastroenterol. 2004;20:146-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 80] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Gionchetti P, Rizzello F, Venturi A, Brigidi P, Matteuzzi D, Bazzocchi G, Poggioli G, Miglioli M, Campieri M. Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119:305-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1077] [Cited by in RCA: 956] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 5. | Braegger CP, Nicholls S, Murch SH, Stephens S, MacDonald TT. Tumour necrosis factor alpha in stool as a marker of intestinal inflammation. Lancet. 1992;339:89-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 500] [Cited by in RCA: 481] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 7. | Söderholm JD, Streutker C, Yang PC, Paterson C, Singh PK, McKay DM, Sherman PM, Croitoru K, Perdue MH. Increased epithelial uptake of protein antigens in the ileum of Crohn's disease mediated by tumour necrosis factor alpha. Gut. 2004;53:1817-1824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 114] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 8. | Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, Rachmilewitz D, Wolf DC, Olson A, Bao W. Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet. 2002;359:1541-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2987] [Cited by in RCA: 3055] [Article Influence: 132.8] [Reference Citation Analysis (0)] |
| 9. | Rhee CH, Park HD. Three glycoproteins with antimutagenic activity identified in Lactobacillus plantarum KLAB21. Appl Environ Microbiol. 2001;67:3445-3449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Schultz M, Veltkamp C, Dieleman LA, Grenther WB, Wyrick PB, Tonkonogy SL, Sartor RB. Lactobacillus plantarum 299V in the treatment and prevention of spontaneous colitis in interleukin-10-deficient mice. Inflamm Bowel Dis. 2002;8:71-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 245] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 11. | Mack DR, Lebel S. Role of probiotics in the modulation of intestinal infections and inflammation. Curr Opin Gastroenterol. 2004;20:22-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Madsen K, Cornish A, Soper P, McKaigney C, Jijon H, Yachimec C, Doyle J, Jewell L, De Simone C. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology. 2001;121:580-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 771] [Cited by in RCA: 742] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 13. | Bai AP, Ouyang Q, Zhang W, Wang CH, Li SF. Probiotics inhibit TNF-alpha-induced interleukin-8 secretion of HT29 cells. World J Gastroenterol. 2004;10:455-457. [PubMed] |
| 14. | Ma D, Forsythe P, Bienenstock J. Live Lactobacillus rhamnosus [corrected] is essential for the inhibitory effect on tumor necrosis factor alpha-induced interleukin-8 expression. Infect Immun. 2004;72:5308-5314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 196] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 15. | Zhang L, Li N, Caicedo R, Neu J. Alive and dead Lactobacillus rhamnosus GG decrease tumor necrosis factor-alpha-induced interleukin-8 production in Caco-2 cells. J Nutr. 2005;135:1752-1756. [PubMed] |
| 16. | Jijon HB, Panenka WJ, Madsen KL, Parsons HG. MAP kinases contribute to IL-8 secretion by intestinal epithelial cells via a posttranscriptional mechanism. Am J Physiol Cell Physiol. 2002;283:C31-C41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 104] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 17. | Ma TY, Iwamoto GK, Hoa NT, Akotia V, Pedram A, Boivin MA, Said HM. TNF-alpha-induced increase in intestinal epithelial tight junction permeability requires NF-kappa B activation. Am J Physiol Gastrointest Liver Physiol. 2004;286:G367-G376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 613] [Cited by in RCA: 714] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 18. | Czerucka D, Dahan S, Mograbi B, Rossi B, Rampal P. Saccharomyces boulardii preserves the barrier function and modulates the signal transduction pathway induced in enteropathogenic Escherichia coli-infected T84 cells. Infect Immun. 2000;68:5998-6004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 128] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 19. | McCracken VJ, Chun T, Baldeón ME, Ahrné S, Molin G, Mackie RI, Gaskins HR. TNF-alpha sensitizes HT-29 colonic epithelial cells to intestinal lactobacilli. Exp Biol Med (Maywood). 2002;227:665-670. [PubMed] |
| 20. | McCarthy J, O'Mahony L, O'Callaghan L, Sheil B, Vaughan EE, Fitzsimons N, Fitzgibbon J, O'Sullivan GC, Kiely B, Collins JK. Double blind, placebo controlled trial of two probiotic strains in interleukin 10 knockout mice and mechanistic link with cytokine balance. Gut. 2003;52:975-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 301] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 21. | Yan F, Polk DB. Probiotic bacterium prevents cytokine-induced apoptosis in intestinal epithelial cells. J Biol Chem. 2002;277:50959-50965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 378] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 22. | Jijon H, Backer J, Diaz H, Yeung H, Thiel D, McKaigney C, De Simone C, Madsen K. DNA from probiotic bacteria modulates murine and human epithelial and immune function. Gastroenterology. 2004;126:1358-1373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 202] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 23. | Yu Y, Zeng H, Lyons S, Carlson A, Merlin D, Neish AS, Gewirtz AT. TLR5-mediated activation of p38 MAPK regulates epithelial IL-8 expression via posttranscriptional mechanism. Am J Physiol Gastrointest Liver Physiol. 2003;285:G282-G290. [PubMed] |
| 24. | Otte JM, Podolsky DK. Functional modulation of enterocytes by gram-positive and gram-negative microorganisms. Am J Physiol Gastrointest Liver Physiol. 2004;286:G613-G626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 274] [Article Influence: 13.0] [Reference Citation Analysis (0)] |