Published online Apr 7, 2007. doi: 10.3748/wjg.v13.i13.1906
Revised: October 15, 2006
Accepted: October 25, 2007
Published online: April 7, 2007
Idiopathic portal hypertension is one of the interesting causes of portal hypertension. Even in very developed medical centers, this disorder is still one of the most important misdiagnoses of clinical practice. To inexperienced physicians, presenting esophageal varices and upper gastrointestinal bleeding usually prompt an unfortunate diagnosis of cirrhosis. A heterogenous clinical presentation and progression of this disorder should be recognized by physicians, and management should be directed towards some specific problems confined to this disorder. Although a genetic basis and other factors are implicated in its pathogenesis, exact underlying mechanism(s) is (are) unknown. In this review, we discuss the heterogeneity of idiopathic portal hypertension, its etiopathogenesis, clinical presentation and management issues. With the expectation of an excellent prognosis, a practicing gastroenterologist should be aware that “not all varices mean cirrhosis”.
- Citation: Harmanci O, Bayraktar Y. Clinical characteristics of idiopathic portal hypertension. World J Gastroenterol 2007; 13(13): 1906-1911
- URL: https://www.wjgnet.com/1007-9327/full/v13/i13/1906.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i13.1906
Non-cirrhotic portal hypertension is a constellation of liver disorders in which liver cirrhosis is not present and the main clinicopathologic findings are encountered in the portal venous system. Portal vein thrombosis, portal vein cavernous transformation, parasitic diseases (schistosomiasis), peliosis hepatis, nodular regenerative hyperplasia of the liver, congenital hepatic fibrosis and veno-occlusive liver disease constitute the major sub-categories. The most confusing and complex disorder is idiopathic portal hypertension (IPH). IPH is characterized by non-pathognomonic pathological changes (with the absence of cirrhosis) of the liver in addition to findings of portal hypertension. Study groups (mostly originating from Japan and India) have not reached a conclusion on the nomenclature for IPH. Indian study groups prefer to use the term non-cirrhotic portal fibrosis, while Japanese researchers call the disorder IPH. American study groups named the disorder as hepatoportal sclerosis[1]. Regardless of the nomenclature used, IPH is a distinct clinical entity with characteristic features including interesting and complicated pathogenetic mechanisms.
In this review we will discuss the pathogenesis, clinicopathologic findings, treatment and prognosis of this disorder under the light of current knowledge.
IPH has a non-sporadic character in some parts of the world. It is one of the most important causes of portal hypertension and esophageal variceal bleeding. The latter case is encountered in Indian continent. In India, IPH causes up to 25% of all esophageal variceal bleeding[2]. Such a heterogeneous distribution is most probably due to possible underlying etiological factors. The pathogenesis of this disorder is still not understood and there is some controversy about this subject.
Although there are some theories on the pathogenesis of IPH, unfortunately none have proved to be a single factor fully explaining the pathogenesis. These theories include the trace element-chemical theory, autoimmunity theory, infection theory, thrombosis theory and genetic theory.
IPH seems to be a multifactorial disease in which two or more etiological factors may play a role.
Chronic exposure to arsenic[3] or vinyl chemicals[4] has been incriminated in the development of IPH. Exposure to these chemical substances for a long time may result in histological findings resembling hepatoportal sclerosis. Although it is tempting to incriminate these chemicals as primary etiological factors, most patients never report such exposure. Some drugs and pharmaceutical agents may also result in clinicopathologic findings mimicking IPH. Vitamin A toxicity, methotrexate and 6-mercaptopurine may result in such a clinical picture[5].
Based on several reports in the literature, there is some evidence that IPH may be related to autoimmune reactions and immunological abnormalities. It is not known whether this immunity connection is the primary underlying disorder or a coincidental phenomenon. However, it is widely accepted that autoimmune diseases (especially connective tissue diseases) increases the prevalence of IPH in certain patient groups. The most important IPH associated diseases are mixed connective tissue disease (6 cases[6]), systemic sclerosis (20 cases[7]) and systemic lupus erythematosus (16 cases[8]). In addition, a Japanese survey[9] found that hypergammaglobulinemia is the most common presenting autoimmune dysfunction (26% of all included IPH patients), along with chronic thyroiditis as the most common autoimmune disease. Although there may be a coincidental relationship between these disorders, some evidence supports autoimmunity having some effect over IPH in selected patients. For example, platelet-derived growth factor (PDGF), connective tissue growth factor (CTGF) and other cytokines are found to play role in the development of such disorders. These cytokines may result in pathological changes resembling IPH[6], such as peri-biliary fibrosis in the portal tracts and increased myofibroblastic spindle cell activity in the portal tracts. As previously noted by Nakanuma et al[10], the increased expression of adhesion molecules in portal venous endothelial lining cells may also reflect the secondary effects of these autoimmune diseases.
Chronic or recurrent infections are incriminated as an etiological factor for the development of IPH. The insidious clinical nature of IPH supports this theory. Chronic exposure to antigenemia of intestinal origin may result in mild portal inflammation. When repetitive antigenemia occurs for a long time, these successive inflammatory reactions in portal tracts may trigger the pathological changes to eventually result in IPH. The cornerstone studies that first showed the importance of portal antigenemia in the development of IPH-like pathological changes were animal studies performed in rabbits and dogs[11-13]. The profound high prevalence of IPH in India is attributed to increased prevalence of abdominal and intestinal infections[14].
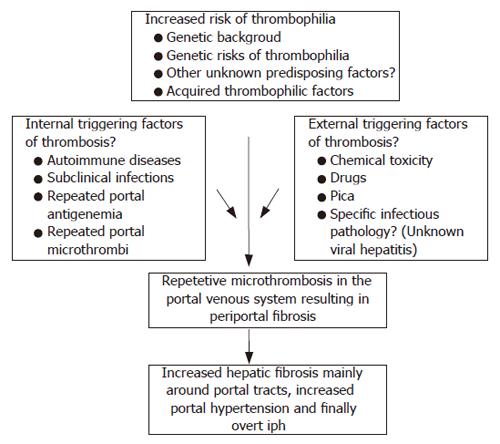
The most controversial issue in IPH is the contribution of portal tract thrombosis in the pathogenesis of IPH. As stated previously by Okuda[15], the Japanese Research Committee on IPH argues against the thrombosis theory based on the following: (1) the insidious onset of IPH, (2) increased splenic blood flow in IPH (splenic blood flow is expected to decrease in portal vein thrombosis), (3) no evidence of increased thrombophilia in IPH cases, and (4) biopsy examinations of IPH cases[16] reveal a very low percentage of portal vein thrombosis (2, 3%). However, some of these points conflict. In our opinion, the thrombosis theory must be expanded as “repetitive micro-thrombosis theory”. We believe that in the very early stages, clinically undetectable micro-thrombosis in the small intrahepatic branches of the portal vein eventually result in periportal fibrosis-like reconstruction. The disease then becomes clinically evident when portal hypertension develops, either as splenomegaly (related to anemia or found incidentally) or variceal bleeding. In the very late stages, overt portal vein thrombosis in the major branches is observed. These suggestions are supported by other studies (discussed later). In our own unpublished data, the prevalence of thrombophilic factors (either genetic or acquired) is found to be increased. In our IPH patient population, protein C-S deficiencies were found in 13%, the factor-II mutation was found in 4.3%, the factor-V Leiden mutation in 13%, paroxysmal nocturnal hemoglobinuria in 4.3% and systemic lupus erythematosus in 4.3% of all IPH patients. We believe that the increased prevalence of thrombophilic factors in this patient population is beyond the point of coincidence.
Therefore, extrahepatic portal vein obstruction secondary to thrombosis is a distinct clinical entity and should not be confused with the thrombosis theory. Secondly, there are studies that suggest thrombosis of the portal vein occurs during the course of IPH. In 2005, Matsutani et al[17] showed that 2 of 22 IPH patients had partial portal vein thrombosis at the time of IPH diagnosis, and another 7 patients (who were free of thrombosis at the time of IPH diagnosis) developed portal vein thrombosis in a mean period of 10.5 years. The authors believe that frequent follow up ultrasonography screening may be helpful in identifying thrombosis attacks. The IPH patients with portal vein thrombosis in this study were found to have a significantly higher degree of decreased liver functions, refractory ascites, hypersplenism and poor general status. In 2002, Hillaire et al[18] investigated a group of IPH patients for prothrombotic conditions. They initially included 42 patients with predominant pathological findings of IPH but excluded 11 patients with concurrent portal vein thrombosis. Additionally, during follow up of the final 28 patients studied, 13 of the patients developed portal vein thrombosis and 9 of them were also positive for an overt prothrombotic condition. The authors concluded that in 50% of the IPH cases, a prothrombotic disorder was found (which is similar to our patient population) and patients with portal vein thrombosis were given a poor prognosis, indicating advanced disease and poor liver capacity.
These two studies were performed after the comprehensive review by Nakanuma et al[10] who suggested a staging system for IPH cases. They proposed that 4 stages are observed and identifiable in IPH. Stage-1 indicates the earliest stage of IPH where the liver is free of apparent atrophy, and stage-4 consistes of advanced liver atrophy together with portal vein thrombosis, which is clinically detectable and indicates a poor prognosis. This classification system also defines the stages of the natural history of IPH. We believe that undetectable micro-portal venule thrombosis is one of the major contributors to the development of IPH and detectable macro-portal vein thrombosis is an indicator of advanced IPH with poor prognosis. This topic is very controversial and is open for prospective follow-up studies to investigate the contribution of thrombosis in the pathogenesis of IPH. Additionally, there is no prospective study in the literature that shows clinicopathologic and histologic progression of a total thrombosis in the main portal vein or intrahepatic portal vein branches to IPH.
In the literature, there is a lack of genetic studies in IPH. In 1987, Sarin et al[19] found the association of a high degree of HLA-DR3 aggregation in family members with IPH. Interestingly, in 2005 and 2006, two case reports[20-22] associated IPH with a genetic syndrome called “Adams-Oliver” syndrome. It is characterized by skeleton abnormalities (i.e. limp and extremity malformations, brachydactyly, poly and oligodactyly, hypoplastic nails, scalp and skull defects) and congenital heart defects. Extrahepatic portal vein thrombosis is not accepted as a component of the syndrome. This syndrome is mostly inherited in an autosomal dominant fashion and is related to undefined genes that regulate vascular development. Among these 4 cases, two of them were found to have portal vein thrombosis along with a diagnosis of IPH. The authors believe that this vascular genetic disorder predisposes to thrombosis and IPH. Interestingly, the only genetic link to IPH also seems to be dependent on the thrombosis theory.
The literature includes conflicting studies about the etiopathogenesis of IPH, which has resulted in controversy. We believe that this is a multifactorial disease that includes a possible genetic background and onset may occur at any time in life. Thrombosis throughout the portal system probably plays a key role in both the initiation and progression of the disease. Our proposal about the etiopathogenesis of IPH is shown in Figure 1.
The pathological findings in IPH are very heterogeneous and have been studied in detail by various groups[10,18,23,24]. This heterogeneity is most probably due to changes occurring with the progression of the disease and changes in hepatic blood flow dynamics.
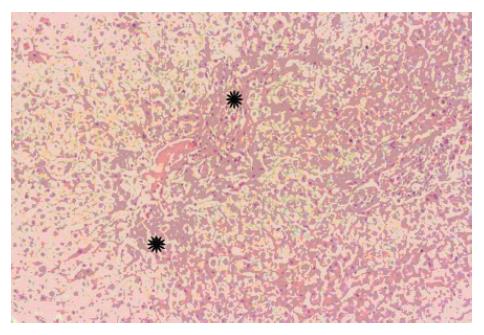
Grossly, the liver may be atrophic and/or nodular. Liver atrophy is a later finding in the course of the disease due to collapse of the peripheral liver architecture, along with ischemia related hepatocyte drop-out via apoptosis. In histological examination (Figure 2), the classical findings of IPH can be divided into two aspects. The primary finding directly related to IPH is intimal fibroelastic thickening of medium and small branches of the intrahepatic portal vein. The second aspect, resulting secondarily to obliterated portal branches, are aberrant neo-vascular formations, sinusoidal dilatation and hepatocellular nodular hyperplasia. (NOTE: Neo-vascular formations are sometimes termed “herniation of portal vein” and these neo-vessels are thought to arise from vasa septalis or inlet venules. These vessels play an important role in shunting blood flow from the obliterated portal segment towards unaffected sites).
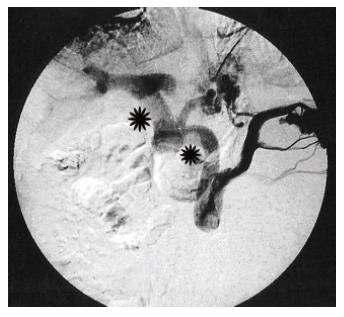
At autopsy, there is a high degree of thrombosis in medium to large portal branches. Indian and Japanese authors are in agreement on this topic. In advanced cases, liver examination at autopsy revealed this common finding in the previously mentioned studies. Additionally, at various anatomic segments of the portal tract, depending on the biopsy site (e.g. wedge biopsy, needle biopsy and autopsy findings), thrombosis in the portal venous system was also found[10,18,25]. These findings support the staging system suggested by Nakanuma et al[10] in which grossly evident portal thrombosis is accepted as an advanced stage finding. In Figure 3, splenoportography of a patient with IPH (who was diagnosed and followed in our hospital) is shown. The patient developed a portal vein thrombosis during follow up. Although there are 2 major sites of thrombosis in the major portal veins, the portal vein at the hepatic hilum is found to be dilated despite a severely occluded portal vein. This finding is very suggestive of concomitant IPH (Figures 3 and 4).
A clinical definition of IPH is “a state of portal hypertension in which no liver disease is found”. Thus IPH is usually the final diagnosis of an exclusion process. Usually IPH patients are in good condition, have near normal or normal liver functions (including normal albumin levels and prothrombin time) but present with variceal bleeding that is detected in investigations of hypersplenism. Ascites is almost always a finding of advanced cases in which the liver atrophies and residual capacity is limited.
There is no known male or female preponderance of IPH; both sexes are believed to be equally affected. There is a slight female preponderance in some studies[23,26] and this may be related to autoimmune disease, which is a condition mostly observed in the female population.
Clinical presentation has been studied in two large Indian studies. Sarin et al[14] reported that 13.5% of patients had splenomegaly, 84.5% of patients had a history of upper gastrointestinal bleeding, 92% of patients had esophageal varices, and 22.3% of patients had gastric varices. When they compared these patients with portal vein thrombosis patients, the IPH group was found to have larger spleens but lower prevalence of ascites, gastric varices, history of upper gastrointestinal bleeding and almost no jaundice. In another Indian study, Dhiman et al[23] reported that 96.7% of patients had splenomegaly and 64.9% of patients had a history of upper gastrointestinal bleeding. Interestingly, the durations of patient presentation to a medical unit after onset of symptoms were within 12 mo (36.4%), 13-60 mo (27.2%), 61-120 mo (20.5%) and more than 120 mo (15.9%). This is possibly a reflection of medical care problems in a developing country. Another study by Okuda et al[26] reported different results. In 1984, they reported 86 cases of IPH. The main presenting symptom of these patients were anemia related symptoms (26.2%), hematemesis (23.7%) and splenomegaly (18.4% of all patients, mostly detected and referred by a physician). They detected varices in 84% of all cases. The clear difference in presentation patterns suggests that there are two, non homogenous IPH groups in these countries. But we believe that this important clue has a very important and basic message: the Indian IPH population is formed of more chronic and advanced IPH cases than the Japanese and Western cases. We believe this is one of the main causes of conflict in the IPH literature.
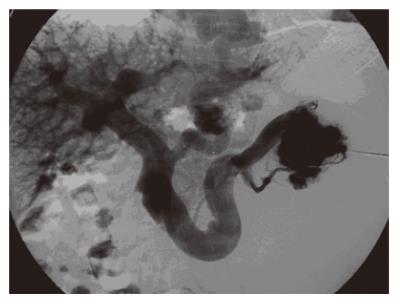
The major complications of IPH can be summarized as esophageal varices and hypersplenism. Although portal hypertension is very evident (Figure 5), the absence of liver dysfunction (except for very chronic and late stages of the disease) is a protective factor against these aforementioned complications. Ascites, hepatic encephalopathy, abnormal liver function tests and jaundice are rarely observed and all of these findings are transient if occurring at all. Interestingly, in contrast to this judgment, the studies from India state that 70% of IPH patients present with a history of major variceal bleeding[27]. This might not be confusing because, as previously stated, the Indian patient population harbors chronologically older IPH disease and patients present in later stages.
The esophageal varices (EV) that form during the course of IPH have some distinctive features. The walls of these variceal veins are relatively thicker than the varices observed in cirrhosis. They rarely harbor “red-spots” that herald a variceal bleeding. They are simply dilated veins that rarely complicate, and are relatively easy to treat compared to cirrhosis. Varices with varying degrees are found in almost all of the patients with IPH. In some surveys, the EV is reported to be found in 90% of the IPH patients[2].
One of the important issues in management of EV in IPH is the mode of treatment. The portal hypertension that occurs in IPH is not accompanied by liver synthetic dysfunction. The mechanisms that play a major role in cirrhotic patients are not observed in IPH due to a liver that remains functioning. The hyper-dynamic mesenteric circulation and imbalance in vasoactive mediators are not observed in IPH. Therefore, conventional medical treatment regimens, such as beta-blockers and nitrates, can be accepted as ineffective due to the lack of these mentioned changes. This is supported by a recent study by Sarin et al[28] in which medical treatment was found to be inferior to endoscopic variceal ligation (EVL) in patients with IPH in reducing the rebleeding rate. One of the major disadvantages of this report is the low number of patients studied. Another uncontrolled study from India found that variceal sclerotherapy reduces the monthly rebleeding rate 14-fold[27].
Another management problem is the development of portal hypertensive gastropathy and gastric varices. Although these two conditions are rarely observed in IPH cases, EVL may have serious adverse effects in that EVL may induce their formation. Once formed, gastric varices should be treated by intravariceal glue-injection therapy, which is an effective modality of eradication. Portal gastropathy in IPH cases are usually transient. The formation of new spontaneous shunts after a successful eradication program is an expected finding and is protective against varices formation.
Levels of all blood elements begin to decrease as the condition worsens and severe hypersplenism is now considered as one of the most important indications for splenectomy in this group of patients. In our personal experience, it may be good practice to select patients according to their co-morbid conditions, degree of hypersplenism and the condition of varices. Also splenectomy has been found to be more complicated in patients with massive splenomegaly, and these patients must be followed carefully after this surgery.
The overall prognosis for IPH is almost always excellent. This is mostly related to and dependent on preserved liver synthetic functions. The most important factor is the follow up of EV and management of EV by EVL or sclerotherapy. One important follow-up study from Japan reported very important findings about the prognosis of these patients[29]. Thirty per cent of all deaths were due to esophageal bleeding while 25% were due to hepatic insufficiency. The major adverse prognostic factor were sex and age of onset. Male patients showed a shorter life-span than females, and the patients who had onset of disease before 40 years of age showed poorer prognoses. These data (despite the small size of the study) suggest that early onset of disease results in earlier completion of the natural course. The disease resembles a cascade in which liver atrophy is the main rate-limiting step in the course of IPH. The overall prognosis can be placed between the normal population and cirrhotic patients.
IPH is a heterogenous disorder with varying clinical pictures, including asymptomatic splenomegaly and recurrent variceal bleeding. Its etiology is still unknown, but some evidence and epidemiological studies suggest that it is a multifactorial disease with a genetic basis. Current knowledge indicates that some triggers are intra-abdominal infections, autoimmune processes, and chemical toxicity. Although the prognosis is excellent, careful follow up and management of patients, with extra attention to treatment esophageal varices, is required. Further studies are needed in order to clarify the issues of etiology and possible genetic background.
S- Editor Liu Y L- Editor Lutze M E- Editor Zhou T
| 1. | Mikkelsen WP, Edmondson HA, Peters RL, Redeker AG, Reynolds TB. Extra- and intrahepatic portal hypertension without cirrhosis (hepatoportal sclerosis). Ann Surg. 1965;162:602-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 204] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 2. | Sarin SK, Aggarwal SR. Idiopathic portal hypertension. Digestion. 1998;59:420-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Huet PM, Guillaume E, Cote J, Légaré A, Lavoie P, Viallet A. Noncirrhotic presinusoidal portal hypertension associated with chronic arsenical intoxication. Gastroenterology. 1975;68:1270-1277. [PubMed] |
| 4. | Thomas LB, Popper H, Berk PD, Selikoff I, Falk H. Vinyl-chloride-induced liver disease. From idiopathic portal hypertension (Banti's syndrome) to Angiosarcomas. N Engl J Med. 1975;292:17-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 99] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Sarin SK. Non-cirrhotic portal fibrosis. Gut. 1989;30:406-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Rai T, Ohira H, Fukaya E, Abe K, Yokokawa J, Takiguchi J, Shishido S, Sato Y. A case of merged idiopathic portal hypertension in course of mixed connective tissue disease. Hepatol Res. 2004;30:51-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Takagi K, Nishio S, Akimoto K, Yoshino T, Kawai S. A case of systemic sclerosis complicated by idiopathic portal hypertension: case report and literature review. Mod Rheumatol. 2006;16:183-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Inagaki H, Nonami T, Kawagoe T, Miwa T, Hosono J, Kurokawa T, Harada A, Nakao A, Takagi H, Suzuki H. Idiopathic portal hypertension associated with systemic lupus erythematosus. J Gastroenterol. 2000;35:235-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Saito K, Nakanuma Y, Takegoshi K, Ohta G, Obata Y, Okuda K, Kameda H. Non-specific immunological abnormalities and association of autoimmune diseases in idiopathic portal hypertension. A study by questionnaire. Hepatogastroenterology. 1993;40:163-166. [PubMed] |
| 10. | Nakanuma Y, Tsuneyama K, Ohbu M, Katayanagi K. Pathology and pathogenesis of idiopathic portal hypertension with an emphasis on the liver. Pathol Res Pract. 2001;197:65-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 70] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Sugita S, Ohnishi K, Saito M, Okuda K. Splanchnic hemodynamics in portal hypertensive dogs with portal fibrosis. Am J Physiol. 1987;252:G748-G754. [PubMed] |
| 12. | Kono K, Ohnishi K, Omata M, Saito M, Nakayama T, Hatano H, Nakajima Y, Sugita S, Okuda K. Experimental portal fibrosis produced by intraportal injection of killed nonpathogenic Escherichia coli in rabbits. Gastroenterology. 1988;94:787-796. [PubMed] |
| 13. | Kathayat R, Pandey GK, Malhotra V, Omanwar S, Sharma BK, Sarin SK. Rabbit model of non-cirrhotic portal fibrosis with repeated immunosensitization by rabbit splenic extract. J Gastroenterol Hepatol. 2002;17:1312-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Sarin SK. Non-cirrhotic portal fibrosis. J Gastroenterol Hepatol. 2002;17 Suppl 3:S214-S223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Okuda K. Non-cirrhotic portal hypertension: why is it so common in India. J Gastroenterol Hepatol. 2002;17:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Okudaria M. Report of the subcommittee for the study of intrahepatic portal thrombosis in IPH. 1994 Report of the Research Committee on Aberrant Portal Hemodynamics. Japan: Japanese Ministry of Health and Welfare 1995; 69-70. |
| 17. | Matsutani S, Maruyama H, Akiike T, Kobayashi S, Yoshizumi H, Okugawa H, Fukuzawa T, Kimura K, Saisho H. Study of portal vein thrombosis in patients with idiopathic portal hypertension in Japan. Liver Int. 2005;25:978-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Hillaire S, Bonte E, Denninger MH, Casadevall N, Cadranel JF, Lebrec D, Valla D, Degott C. Idiopathic non-cirrhotic intrahepatic portal hypertension in the West: a re-evaluation in 28 patients. Gut. 2002;51:275-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 206] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 19. | Sarin SK, Mehra NK, Agarwal A, Malhotra V, Anand BS, Taneja V. Familial aggregation in noncirrhotic portal fibrosis: a report of four families. Am J Gastroenterol. 1987;82:1130-1133. [PubMed] |
| 20. | Girard M, Amiel J, Fabre M, Pariente D, Lyonnet S, Jacquemin E. Adams-Oliver syndrome and hepatoportal sclerosis: occasional association or common mechanism. Am J Med Genet A. 2005;135:186-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Pouessel G, Dieux-Coeslier A, Wacrenier A, Fabre M, Gottrand F. Association of Adams-Oliver syndrome and hepatoportal sclerosis: an additional case. Am J Med Genet A. 2006;140:1028-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Swartz EN, Sanatani S, Sandor GG, Schreiber RA. Vascular abnormalities in Adams-Oliver syndrome: cause or effect. Am J Med Genet. 1999;82:49-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 23. | Dhiman RK, Chawla Y, Vasishta RK, Kakkar N, Dilawari JB, Trehan MS, Puri P, Mitra SK, Suri S. Non-cirrhotic portal fibrosis (idiopathic portal hypertension): experience with 151 patients and a review of the literature. J Gastroenterol Hepatol. 2002;17:6-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 149] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 24. | Abramowsky C, Romero R, Heffron T. Pathology of noncirrhotic portal hypertension: clinicopathologic study in pediatric patients. Pediatr Dev Pathol. 2003;6:421-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Willner IR, Waters B, Payton JD. Hepatoportal sclerosis: clinical features and association with hypercoagulable states. Hepatology. 1997;26:204A. |
| 26. | Okuda K, Kono K, Ohnishi K, Kimura K, Omata M, Koen H, Nakajima Y, Musha H, Hirashima T, Takashi M. Clinical study of eighty-six cases of idiopathic portal hypertension and comparison with cirrhosis with splenomegaly. Gastroenterology. 1984;86:600-610. [PubMed] |
| 27. | Chawla YK, Dilawari JB, Dhiman RK, Goenka MK, Bhasin DK, Kochhar R, Singh K, Kaur U. Sclerotherapy in noncirrhotic portal fibrosis. Dig Dis Sci. 1997;42:1449-1453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Sarin SK, Wadhawan M, Gupta R, Shahi H. Evaluation of endoscopic variceal ligation (EVL) versus propanolol plus isosorbide mononitrate/nadolol (ISMN) in the prevention of variceal rebleeding: comparison of cirrhotic and noncirrhotic patients. Dig Dis Sci. 2005;50:1538-1547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Ichimura S, Sasaki R, Takemura Y, Iwata H, Obata H, Okuda H, Imai F. The prognosis of idiopathic portal hypertension in Japan. Intern Med. 1993;32:441-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |